Journal of Power and Energy Engineering
Vol.04 No.05(2016), Article ID:66751,6 pages
10.4236/jpee.2016.45004
Simulation and Analysis of Hydrogen Production by Dimethyl Ether Steam Reforming for PEMFC
Zhenguo Luo, Cong Li
Shanghai University of Engineering Science, Shanghai, China
Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 May 2016; accepted 22 May 2016; published 25 May 2016
ABSTRACT
Energy crisis has become a serious global problem, and Proton exchange membrane fuel cell (PEMFC) has played an important role in the solution of the energy crisis. The hydrogen production process by dimethyl ether steam reforming for PEMFC was studied. The yield of H2 and energy efficiency of system with different mass ratios (0.3:0.7, 0.35:0.65, 0.4:0.6) of dimethyl ether (DME) and steam were analyzed, and both of yield of H2 and energy efficiency of system increased with the increase of the mass ratio of DME and steam. The energy efficiency of hydrogen production system using reactor as heat source and hydrogen production system using engine exhaust gas as heat source is compared, and energy efficiency of using reactor as heat source (57.96117%, 63.89651%, 69.0002%) is higher than that using engine exhaust gas as heat source (54.4913%, 60.11311%, 66.25342%).
Keywords:
Steam Reforming, Dimethyl Ether, Proton Exchange Membrane Fuel Cell

1. Introduction
An increase in energy consumption that results from human-related activities causes the depletion of fossil fuel and the global warming problem. Thus, searching for clean and sustainable energy source is necessary for the future. Hydrogen is an important alternative fuel that is expected to replace fossil fuels because it is clean and environmentally friendly energy source [1] . Currently, hydrogen is commonly used as a reactant in chemical industries. In addition, hydrogen-based fuel cells have the advantages of zero emission, high energy conversion efficiency, low noise, etc. It will become a significant fuel in the near future [2] [3] .
Dimethyl ether (DME) is an excellent resource for hydrogen production with its high H/C ratio and high- energy volume density. DME does not contain harmful materials and it burns without producing NOx, and particulates. DME was liquid in the low pressure similar to liquefied petroleum gas (LPG), thus could be stored and transported using the facilities providing LPG. DME is an ideal vehicle fuel, but also has capability of chemical hydrogen storage. Therefore, many scholars have been studied to the DME reforming processes. Steam Reforming (SR) is the most commonly used process of hydrocarbon reforming techniques of DME because it provides high yields of hydrogen production. However, it also requires a large amount of external heat source. The exhaust gas of the vehicle is wasted after clarification by an after-treatment system, but the heat resource and steam from the exhaust gas can be efficient by SR reaction [4] - [9] .
PEMFC exhibits special advantages including fast start-up, low working temperature, high specific energy density, simple structure, convenient operation and great durability [3] . These characteristics are precisely that the automotive engine needs to be satisfied, so the PEMFC is recognized as the main source of energy of electric vehicles in the future. The working principle of proton exchange membrane fuel cell is same as the ordinary fuel cell. Platinum carbon or platinum ruthenium as an electro catalyst, hydrogen as fuel, air or pure oxygen as the oxidant and graphite or surface of the gas flow passage change of sheet metal is a bipolar plate.
In this paper, the hydrogen production system for PEMFC was studied. The hydrogen production process and operating parameters were calculated and simulated by using commercial process simulation software Aspen Plus. The effects of mass ratio of DME and steam and energy efficiency of the PEMFC power system are analyzed.
2. Simulation
2.1. Reactions of System
The system of hydrogen production mainly includes the following reactions:
Overall reaction of steam reforming of DME:
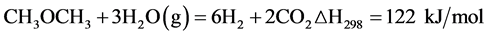 (Equation 1)
(Equation 1)
And steam reforming of DME process involves following four main reactions:
Water-gas shift:
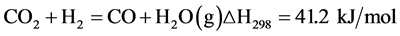 (Equation 2)
(Equation 2)
DME hydrolysis:
 (Equation 3)
(Equation 3)
Steam reforming of methanol:
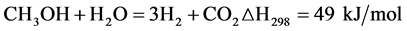 (Equation 4)
(Equation 4)
Methanol decomposition:
 (Equation 5)
(Equation 5)
In addition, Partial oxidation reaction (PROX):
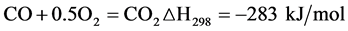 (Equation 6)
(Equation 6)
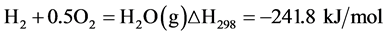 (Equation 7)
(Equation 7)
2.2. Describe of Model
Figure 1 shows the flowchart of hydrogen production system for PEMFC. The correspond steam and abbreviations are defined in Table 1. And H2/CO indicate H2 and CO produced by steam reforming, H2_rest indicates rest H2 of PEMFC, Electric Power represent power generated by PEMFC, Q_rest indicates rest energy of engine exhaust gas, Q_lost indicate energy consumption of engine work.
Figure 1. Flowchart of hydrogen production system.
Table 1. Steam and block abbreviations.
Q_rest can provide heat for reaction of dimethyl ether steam reforming, and H2_rest can be reused by PEMFC. It indicates that the system has a good energy cycle, and the energy can be used to the maximum extent. This can also improve the energy efficiency of the whole system.
Figure 2 shows the simulation diagram of hydrogen production process by Aspen Plus software. Figure 2(a) represents model A that illustration a hybrid system by using engine exhaust gas as heat source for hydrogen production. Figure 2(b) represents model B which indicates a hydrogen production system using reactor as heat source. The model A and model B is based on a zero-dimensional approach with steady and isothermal operation condition and all working fluid are simulated by using the Peng-Robinson equation.
The hydrogen production process mainly consists of three parts: SR part, purification part and supply part. SR part is the core part of the hydrogen production process, it mainly include DME stream reforming reaction (Equation 1) and water-gas reaction (Equation 2). These two reactions occur simultaneously in the reactor and their conversions are considered to be close the equilibrium. H2O and DME are preheated by heater 1 and heater 2.
Their temperature must be up to the temperature of the SR reaction. The SR reactor temperature is constant, so the external heat supply should be maintain isothermal. Purification part includes high-temperature water-gas reaction (HTWGS), low-temperature water-gas reaction (LTWGS) and PROX (Equation 6-7). HTWGS and LTWGS use the Gibbs reactor and PROX uses the Stoic reactor. Those three reactions also should be maintained constant temperature. The isothermal conditions mean that the heat produced by the reactions must be removed and can be used as hot streams for energy recovery [10] . CO is converted to H2 and CO2 during the HTWGS and LTWGS reactions, with outlet streams containing about 5% - 12% and 0.5% - 1.0% CO, respectively. The high CO concentration can cause the deactivation of PEMFC catalysts. This means a further CO


Figure 2. Simulation diagram of hydrogen production process: (a) model A: hybrid power by using engine exhaust gas as heat source, (b) model B: using reactor as heat source.
reduction unit needed to decrease CO to levels below 10 ppm [11] - [13] . In the Supply part, the purified hydrogen by Purification part is supplied directly to the PEMFC through the heater and separator.
2.3. Parameters
Table 2 shows the parameters initial value of this simulation. Some of the parameters of hydrogen production system are based on typical parameters reported for literature. SR block was used in Yield reactor, the reaction temperature is 750 K. And kinetic data were measured by the experiment, as shown in Table 3.
3. Result and Analysis
Table 4 illustrates the yield of H2 of system while the inlet flow rate of DME is 0.30 kg/s, 0.35 kg/s, 0.4 kg/s. It indicates that the yield of H2 increases with the increase of the mass ratio of DME and steam, because the increasing in DME can promote the production of H2. The yield of H2 achieves a maximum (186.878333 kmol/hr) while mass ratio is 4:6 of DME and steam.
Table 5 shows the heat duty of hydrogen production process for PEMFC of model A and model B. In order to evaluate the performance of the DME steam reforming system for PEMFC, the energy efficiency of the system were defined by the relationship:
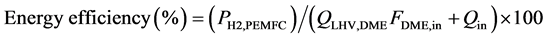 (Equation 8)
(Equation 8)
FDME,in is the molar flow rates of DME at the inlet. QLHV,DME is the low heat values of DME. PH2,PEMFC is the energy produced of PEMFC with supplying hydrogen.
Selection of a certain type of 1.8 L engine, power 80 kW, indicated thermal efficiency of 35%, fuel consumption 8.0 L/100 km. And Selection of ChaoYue 3 PEMFC fuel cell vehicle, power 50 KW, fuel consumption 1.12 kg/100 km.
The calculated results are as shown in Table 6. It indicates that the energy efficiency is also increased with the increase of the mass ratio of DME and steam. And the efficiency of model B (57.96117%, 63.89651%,
Table 2. Parametervalues in calculations.
Table 3. Kinetic data of DME steam reforming reaction.
Table 4. Yield of H2.
Table 5. Heat duty of hydrogen production process.
Table 6. Energy efficiency of hydrogen production process for PEMFC.
69.0002%) is higher than that of model A (54.4913%, 60.11311%, 66.25342%), because the engine has energy losses of model A, while model B has no energy loss by engine working.
4. Conclusion
In this study, the simulation of hydrogen production via dimethyl ether steam reforming for PEMFC was studied by using Aspen Plus software. It shows that the whole system has good energy cycle utilization. Both of yield of H2 and energy efficiency of system increased with the increase of the mass ratio of DME and steam, and the energy efficiency of hydrogen production system using reactor as heat source is higher than that hydrogen production system using engine exhaust gas as heat source. Finally, this article can provide important theoretical references to the hydrogen production process for PEMFC.
Cite this paper
Zhenguo Luo,Cong Li, (2016) Simulation and Analysis of Hydrogen Production by Dimethyl Ether Steam Reforming for PEMFC. Journal of Power and Energy Engineering,04,25-30. doi: 10.4236/jpee.2016.45004
References
- 1. Arpornwichanop, A., Isuleewan, M., Patcharavorachot, Y. and Assabumrungrat, S. (2011) Investigation of a Dual-Bed Autothermal Reforming of Methane for Hydrogen Production. Chemical Engineering Transactions, 25, 929-934.
- 2. Suthida, A., Pounyaporn, A., Yaneeporn, P. and Amornchai, A. (2014) Theoretical Analysis of a Biogas-Fed PEMFC System with Different Hydrogen Purifications: Conventional and Membrane-Based Water Gas Shift Processes. Energy Conversion and Management, 86, 60-69.
http://dx.doi.org/10.1016/j.enconman.2014.04.093 - 3. Chen, B., et al. (2015) Operation Characteristics and Carbon Corrosion of PEMFC (Proton Exchange Membrane Fuel Cell) with Dead-Ended Anode for High Hydrogen Utilization. Energy, 91, 799-806.
http://dx.doi.org/10.1016/j.energy.2015.08.083 - 4. Wang, B. and Sun, Q. (2014) Steam Reforming of Dimethyl Ether by Gliding Arc Gas Discharge Plasma for Hydrogen Production. Chinese Journal of Chemical Engineering, 22, 104-112.
http://dx.doi.org/10.1016/S1004-9541(14)60020-3 - 5. Feng, D.M., Wang, J.F. and Wang, D.Z. (2007) Production of Hydrogen from Dimethyl Ether over Catalysts. Sciencepaper Online.
- 6. Li, C., Wang, Y.S. and Fan, P.Q. (2012) Numerical Analysis and Experimental Study of Hydrogen Production from Dimethyl Ether Steam Reforming. Science China Chemistry, 55, 1982-1987.
http://dx.doi.org/10.1007/s11426-012-4603-0 - 7. Zheng, Z.L. and Li, S.L. (2013) Thermodynamics Analysis of Hydrogen Production in Vehicle DME Steam Reforming Reaction System. Transactions of the Chinese Society for Agricultural Machinery, 9, 1-6.
- 8. Luo, Z.G. and Li, C. (2016) Thermodynamic Model Study and Analysis of Dimethyl Ether Steam Reforming Reaction. International Journal of Research in Engineering and Science, 4, 1-6.
- 9. Li, C., Yu, Y. and Wu, C. (2015) Thermodynamic and Experimental Studies of Hydrogen Production from Dimethyl Ether Steam Reforming Utilization of Exhaust Gas. Journal of Energy Engineering, 141, 4.
http://dx.doi.org/10.1061/(asce)ey.1943-7897.0000250 - 10. Lei, J., Yue, H.R., Tang, H. and Liang, B. (2015) Heat Integration and Optimization of Hydrogen Production for a 1 kW Low-Temperature Proton Exchange Membrane Fuel Cell. Chemical Engineering Science, 123, 81-91.
http://dx.doi.org/10.1016/j.ces.2014.10.036 - 11. Di Bona, D., Jannelli, E., Minutillo, M. and Perna, A. (2011) Investigations on the Behavior of 2 kW Natural Gas Fuel Processor. International Journal of Hydrogen Energy, 36, 7763-7770.
http://dx.doi.org/10.1016/j.ijhydene.2011.01.110 - 12. Kamarudin, S., Daud, W., Som, A.M., Takriff, M., Mohammad, A. and Loke, Y. (2004) Design of a Fuel Processor Unit for PEM Fuel Cell via Short Cut Design Method. Chemical Engineering Journal, 104, 7-17.
http://dx.doi.org/10.1016/j.cej.2004.07.007 - 13. Sandhu, S., Saif, Y. and Fellner, J. (2005) A Reformer Performance Model for Fuel Cell Applications. Journal of Power Sources, 140, 88-102.
http://dx.doi.org/10.1016/j.jpowsour.2004.08.013



