Journal of Applied Mathematics and Physics
Vol.04 No.05(2016), Article ID:66664,9 pages
10.4236/jamp.2016.45097
Mathematical Model of a Bacteria-Immunity System with the Influence of Quorum Sensing Signal Molecule
Zhiwen Zhang
School of Sciences, Xi’an University of Science and Technology, Xi’an, China

Copyright © 2016 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 30 March 2016; accepted 20 May 2016; published 23 May 2016
ABSTRACT
A recent study illustrates an exciting new element of bacteria-host interactions in which the opportunistic pathogen Pseudomonas aeruginosa quorum sensing signal molecule can modulate the host immune system. In this paper, on the basic of a model which describes the competition between bacteria and immune system, a mathematical model is proposed to investigate the modulation of quorum sensing signal molecule on immune response. It is shown that the modulation leads to a backward bifurcation and some more new dynamical behaviors. By numerical simulations, we find a fold bifurcation of equilibria, which induces three patterns of dynamical behaviors including the bistable phenomenon. Our model quantifies the modulation of quorum sensing signal molecule on the immune response, which may provide a therapy for bacteria infections.
Keywords:
Pseudomonas aeruginosa, Quroum Sensing, Modulation, Bifurcation

1. Introduction
Pseudomonas aeruginosa (abbr. P. aeruginosa) is an increasingly prevalent opportunistic human pathogen capable of causing a broad range of acute and chronic infections. It is a common gram-negative bacterium in nosocomial infection, and commonly found in soil and water. P. aeruginosa can cause severe disease in animals and humans and infect the hosts with defective immune system function, such as those with burns, neutropenic cancer and bone marrow transplant patients [1] . These infections are difficult to treat using conventional antibiotic therapies and often result in considerable morbidity and mortality [2] . One of the reasons that P. aeruginosa produces such diverse, often overwhelming infections is due to an array of virulence factors, including elastase, alkaline protease, exotoxin A, lipase, pyocyanin, and rhamnolipid, which are capable of causing tissue damage and promoting systemic dissemination and interfering with immune clearance mechanisms. Several studies in models of burn wound infection and pneumonia have demonstrated that the virulence factors are regulated in a cell density dependent process termed quorum sensing [3] . Recently, studies have shown that P. aeruginosa quorum sensing signal molecule (abbr. QSSM) can not only help bacteria adapt to the environment but also they contribute to the pathogenesis of P. aeruginosa infections by acting as virulence factors per se. Apart from this, previous data have demonstrated that the quorum sensing signal molecules themselves have immunomodulatory activity, which means that P. aeruginosa can tailor the host immune response [4] . Particular, 3-O-C12-HSL stimulates various host signaling pathways to inhibit or activate immune cell responses, and recently, Bortolotti et al. [5] observe that 3-O-C12-HSL is able to induce HLA-G expression in human monocytes and T cells to control the host immune response.
The theoretical study of bacteria-immune dynamics in the aspect of immunology has a long history. Majority mathematical models have been formulated to study the bacteria-immune dynamics behavior. For example, Fergola et al. [6] firstly constructed an ODEs model to characterize the competition between bacteria and innate immune system, and found that the quorum sensing term in the model significantly influenced the stability of the meaningful steady-state solutions. Zhang et al. [7] [8] formulated delay differential equations (abbr. DDEs) to find that Hopf bifurcation occurred for a long enough time delay, and formulated ODEs by identifying the nonlinear function to characterize quorum sensing to study the intervention of quorum in the competition between bacteria and the immune system.
The purpose of the present paper is to develop a mathematical model to depict the influence of quorum sensing signal molecule on the competition between bacteria and immune system. We provide theoretical analysis and numerical simulations to investigate the influence of QSSM on bacteria infection. We find that the influence of QSSM leads to backward bifurcation, multiple positive equilibrium and patterns of bistability, which may be helpful for immunotherapy.
For this paper, it is organized as follows. We introduce the mathematic model in Section 2, and explore the analysis of the model for mathematical and biological implications in Section 3. We present the numerical simulation in Section 4. Section 5 provides the conclusion and discussion.
2. Model Formulation
Denote the concentrations of the bacteria, quorum sensing signal molecule, and immune cells, as 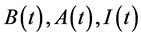 respectively. Suppose the dynamic relations among them are as the following: the bacteria grow logistically at rate a with the effective carrying capacity of environment k, and it is cleared by immunity (mass action term
respectively. Suppose the dynamic relations among them are as the following: the bacteria grow logistically at rate a with the effective carrying capacity of environment k, and it is cleared by immunity (mass action term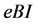 ). The immune cells have a source term and a half-life term denoted by
). The immune cells have a source term and a half-life term denoted by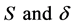 , and S represents that the memory cells are present and includes a wide range of cells involved in the first wave of defense of the host. The immune cells are increased by signals captured by the bacteria load.
, and S represents that the memory cells are present and includes a wide range of cells involved in the first wave of defense of the host. The immune cells are increased by signals captured by the bacteria load.
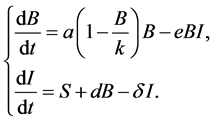 (1)
(1)
The background production of quorum sensing signal molecule is 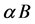 and the natural degeneration rate is
and the natural degeneration rate is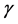 . Considering the effect of quorum sensing on the competition between bacteria and innate immune system, we have been known that quorum sensing signal molecule could modulate immune response. Here, similar with the methods in [9] , we introduce the following:
. Considering the effect of quorum sensing on the competition between bacteria and innate immune system, we have been known that quorum sensing signal molecule could modulate immune response. Here, similar with the methods in [9] , we introduce the following:
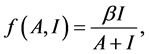
which represents the modulation of quorum sensing signal molecule between bacteria and immune response.
Taking all of the above into considerations, we obtain the following model.
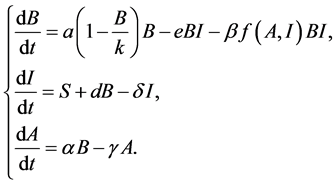 (2)
(2)
As we know, only a certain threshold concentration of quorum sensing signal molecule is exceeded, the bacteria can change their behavior by gene transcription induction. Then, the bacteria have ability to infect host. So we assume the quorum sensing signal molecule to be in a quasi-steady state such that 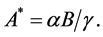 Then, we could obtain the competition model between bacteria and the immune system.
Then, we could obtain the competition model between bacteria and the immune system.
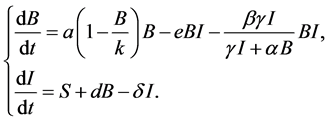 (3)
(3)
The basic reproduction number of (3) is
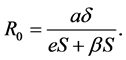
Setting 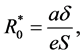 then
then 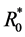 is the basic reproduction number of (1). It is shown in [6] that the dynamical beha-
is the basic reproduction number of (1). It is shown in [6] that the dynamical beha-
viors of system (1) are completely determined by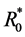 , specifically, the boundary equilibrium
, specifically, the boundary equilibrium 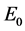 is globally asymptotically stable if
is globally asymptotically stable if

3. Equilibria and Bifurcation
There always has a bacteria free equilibrium 

tem (3) at the bacteria free equilibrium 
thus, by the Routh-Hurwitz criterion, we have
Theorem 1. The bacteria free equilibrium 



Next let us discuss the existence and stability of positive equilibria. Let 

From second equation in Equation (4), it follows that 


in which
Note that 

For positive equilibria to exist, the solutions of Equation (5) must be positive. Note that 



Next, we put our attention on finding positive equilibria of system (3) when


Let


fore, when


Theorem 2.
1) There exists a unique positive equilibrium 





2) There are two positive equilibria 



3) There not exists any positive equilibrium if one of the following conditions holds:
i)



where 


Remark 1. From the expression of

Then system (3) changes to the system (1), where we could see that a forward bifurcation exhibits in system (3) when





Theorem 2 indicates that system (3) exhibits multiple positive equilibria via a backward bifurcation. Then we have the following:
Theorem 3. There exhibits a backward bifurcation at 

Proof: Let
Choosing 


at the bacteria free equilibrium 

then, the characteristic roots are
Denote


Furthermore, the left eigenvector 




and their cross derivatives are coincide. Moreover,
and the other second-order partial derivatives of 

According to coefficients 

and
Obviously, the coefficient 


model (3) undergoes a backward bifurcation at 

So a backward bifurcation occurs at 




4. Numerical Simulation
In this section, we implement numerical simulations based on the theoretical results above for the purpose of clear understanding of the complex dynamical behavior of system (3) and explore more patterns of dynamical behaviors of model (3).
Example 1. When we choose




A forward bifurcation is shown in Figure 1, where 

Choosing 



Figure 1.A forward bifurcation diagram.
Table 1. Values for the parameters of the ODE system (3).
point (LP) at
Hence, from Figure 2 we conclude that if we increase 
Firstly, if





Secondly, if







Thirdly, if





5. Conclusion and Discussion
In this paper, we have developed a mathematical model based on the model which describes the competition between bacteria and immune system to illustrate the influence of quorum sensing signal molecule on the competition between bacteria and immune system. In order to simplify the model, we make an assumption that quorum sensing signal molecules have reached a steady state when they modulate the immune response during the bacteria infection. By combing qualitative analysis and numerical simulations, comparing with the results in
Figure 2.Bifurcation diagram (LP: limit point, BP: branch point).
Figure 3.A numerical solution of system (3) tends to the bacteria free equilibrium as time tends to infinity when
Figure 4.A numerical solution of system (3) tends to the bacteria free equilibrium as time tends to infinity when
model (1), we find our model can exhibit some new and complicated dynamical behaviors. We observe that if we neglect the modulation of quorum sensing signal molecule to immune response, that is,


Figure 5.A numerical solution of system (3) tends to the positive equilibrium as time tends to infinity when
Figure 6.A stable bacteria-free equilibrium 


Figure 7.A numerical solution of system (3) tends to the positive equilibrium as time tends to infinity when
molecule on the competition between bacteria and immune system, bacteria infection becomes complicated.
From our model, we find some interesting results that the model can exhibit three different patterns of dynamical behaviors if we choose 


Table 2. Three typical patterns of dynamical behaviors of system (3).


According to the mathematical analysis and numerical simulations of the model proposed in this paper, it may provide new insights to fight the bacteria infection. It is interesting to find a way to inhibit the modulation, so that we can find an available way to defeat bacteria infection. We act this work as our future goal.
Cite this paper
Zhiwen Zhang, (2016) Mathematical Model of a Bacteria-Immunity System with the Influence of Quorum Sensing Signal Molecule. Journal of Applied Mathematics and Physics,04,888-896. doi: 10.4236/jamp.2016.45097
References
- 1. Rahme, L.G., Stevens, E.J., Wolfort, S.F., et al. (1995) Common Virulence Factors for Bacterial Pathogenicity in Plants and Animals. Science, 268, 1899-1902.
http://dx.doi.org/10.1126/science.7604262 - 2. Wagner, V.E., Frelinger, J.G., Barth, R.K. and Iglewski, B.H. (2006) Quorum Sensing: Dynamic Response of Pseudomonas aeruginosa to External Signals. Trends in Microbiology, 14, 55-58.
http://dx.doi.org/10.1016/j.tim.2005.12.002 - 3. Miller, M. and Bassler, B. (2001) Quorum Sensing in Bacteria. Annual Review of Microbiology, 55, 165-199.
http://dx.doi.org/10.1146/annurev.micro.55.1.165 - 4. Wu, L., Estrada, O., Zaborina, O., et al. (2005) Recognition of Host Immune Activation by Pseudomonas aeruginosa. Science, 309, 774-777.
http://dx.doi.org/10.1126/science.1112422 - 5. Bortolotti, D., Lemaoult, J., Trapella, C. et al. (2015) Pseudomonas aeruginosa Quorum Sensing Molecule N-(3-Oxododecanoyl)-l-Homoserine-Lactone Induces HLA-G Expression in Human Immune Cells. Infection and Immunity, 83, 3918-3925.
- 6. Fergola, P., Zhang, J., Cerasuolo, M. and Ma, Z. (2008) On the Influence of Quorum Sensing in the Competition between Bacteria and Immune System of Invertebrates. AIP Conference Proceedings, 1028, 24-28.
http://dx.doi.org/10.1063/1.2965091 - 7. Zhang, Z.H., Suo, Z.H., Zhang, J. and Peng, J.G. (2009) A Bacteria-Immunity System with Delayed Quorum Sensing. Journal of Applied Mathematics and Computing, 30, 201-217.
http://dx.doi.org/10.1007/s12190-008-0167-8 - 8. Zhang, Z.H., Suo, Y.H. and Zhang, J. (2015) Stability and Bifurcations in a Model of Bacteria Immunity with Quorum Sensing. Mathematical Population Studies, 22, 209-233.
http://dx.doi.org/10.1080/08898480.2014.999498 - 9. Smith, A.M., Mccullers, J.A. and Adler, F.R. (2011) Mathematical Model of a Three-Stage Innate Immune Response to a Pneumococcal Lung Infection. Journal of Theoretical Biology, 276, 106-116.
http://dx.doi.org/10.1016/j.jtbi.2011.01.052 - 10. Chavez, C.C. and Song, B.J. (2014) Dynamical Models of Tuberculosis and Their Applications. Mathematical Biosciences and Engineering, 1, 361-404.
http://dx.doi.org/10.3934/mbe.2004.1.361 - 11. Dhooge, A., Govaerts, W., Kuznetsov, Y.A., et al. (2008) New Features of the Software Matcont for Bifurcation Analysis of Dynamical Systems. Mathematical and Computer Modelling of Dynamical Systems, 14, 145-175.
http://dx.doi.org/10.1080/13873950701742754 - 12. Kirsschner, D. and Panetta, J.C. (1998) Modeling Immunotherapy of the Tumor-Immune Interaction. Journal of Mathematical Biology, 37, 235-252.
http://dx.doi.org/10.1007/s002850050127 - 13. Dockery, J. and Keener, J. (2001) A Mathematical Model for Quorum Sensing in Pseudomonas aeruginosa. Bulletin of Mathematical Biology, 63, 95-116.
http://dx.doi.org/10.1006/bulm.2000.0205




























