Open Journal of Soil Science
Vol.3 No.2(2013), Article ID:32585,7 pages DOI:10.4236/ojss.2013.32011
Response of Nitrous Oxide Flux to Addition of Anecic Earthworms to an Agricultural Field
![]()
Laboratory of Soil Ecology and Microbiology, 024 Coastal Institute, University of Rhode Island, Kingston, USA.
Email: jamador@uri.edu
Copyright © 2013 José A. Amador, Edward J. Avizinis. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 29th, 2013; revised March 4th, 2013; accepted March 12th, 2013
Keywords: Lumbricus terrestris; Anecic Earthworms; Fertilizer Nitrogen; Nitrous Oxide Flux; Denitrification
ABSTRACT
The burrowing and feeding activities of earthworms may have a strong effect on the flux of N2O from agricultural soils. As such, shifts to agricultural management practices that increase the number of earthworms require an understanding of the role of earthworms in N2O dynamics. We conducted a field experiment to examine the effects of addition of anecic earthworms (Lumbricus terrestris) on N2O flux in a field previously planted with corn (Zea mays) in southern Rhode Island, USA. Plots were amended with (15NH4)2SO4 and either 0 (CTL) or 48 L. terrestris m−2 (EW). The flux of N2O, 15N2O and 15N2 was measured over 28 days between October and November 2008. The EW treatment had a significantly higher flux of N2O and 15N2O 1 - 3 days after 15NH4 addition. No treatment effects were observed on 15N2 flux. The addition of earthworms significantly increased (Day 1) and decreased (Day 12) the mole fraction of N2O relative to the CTL. Our results suggest that anecic earthworm additions can increase N2O flux from inorganic fertilizer N amendments, but the effects appear to short-lived.
1. Introduction
Upland agricultural soils account for ~28% of the global N2O budget [1]. Anecic earthworms play a key role in upland agroecosystems [2], where they translocate crop residues, accelerate decomposition and nutrient mineralization, increase inorganic N, change the physical properties of soil (e.g. pore size distribution, water retention, gas diffusion), and alter the size, composition and activities of microbial and faunal communities. These effects overlap with known controls on N2O flux in soil [3], including availability of precursors, inhibitors, electron donors and acceptors, the size and spatial distribution of relevant microbial populations, and the establishment of conditions necessary for their activities.
Increasing the number of earthworms in agricultural soils is a key component of programs that promote soil quality and sustainability [4,5], such as reduced and conservation tillage. Conservation tillage practices were used on more than 109 million acres of farmland in the US in 2000, with a three-fold increase in no-till acreage between 1990 and 2000 [6]. These practices are also increasingly being adopted in Europe [7] and Africa [8].
Adoption of farming practices that retain plant residues, minimize soil disturbance and rely more extensively on foodweb interactions for plant nutrition is expected to ameliorate the impact of agriculture on greenhouse gases [9,10]. However, the positive impact of these practices may be negated by unintended effects on N2O flux [9], some of which may be associated with larger earthworm population densities. The number of earthworms can increase 5 - 10 fold in no-till relative to plowed fields [11,12], and no-till practices preferentially benefit anecic, deep-burrowing species like Lumbricus terrestris [12-14].
Recent reviews suggest that earthworms have varying effects on N2O flux in soil [15,16]. For example, Rizhiya et al. [17] reported that inoculation of soil mesocosms containing crop residues with Aporrectodea longa, an anecic earthworm, increased emissions of N2O nearly four-fold relative to uninoculated soil over the course of 90 days. In another soil mesocosm experiment, Bertora et al. [18] observed only an initial, transient increase in N2O flux for treatments inoculated with A. longa relative to an uninoculated control. More recently Evers et al. [19] reported that soil mesocosms with a high moisture content inoculated with L. terrestris at high population densities had significantly higher N2O emissions than uninoculated controls. Epigeic and endogeic earthworms have also been shown to increase N2O emissions in soil mesocosms [20]. The results of these studies point to the need for field-scale evaluation of the effects of earthworms on the flux of N2O from agricultural soils.
In a previous study we reported that inoculation of mesocosms with L. terrestris appeared to increase nitrogenous gas losses from 15N-labeled corn litter [21]. In the present study we determined the effects of inoculation with L. terrestris on the flux of N2O in corn field plots amended with (15NH4)2SO4. The use of 15N-labeled ammonium allowed us to examine the extent to which anecic earthworms affect production of nitrogenous gas emissions from a common inorganic fertilizer.
2. Materials and Methods
2.1. Site Description
Our experiment was conducted at a University of Rhode Island research farm in Kingston, Rhode Island, USA. The soil at the site is an Enfield silt loam (Coarse-silty over sandy or sandy-skeletal, mixed, active, mesic Typic Dystrudepts) [22]. The soil (top 10 cm; n = 6) had a pH of 5.9 and an organic matter content of 18.1 g·kg−1. Levels of extractable ammonium and nitrate were 9.9 and 4.2 mg N·kg−1 soil, respectively. The field was used to grow corn using conventional management practices and the corn had been harvested prior to our experiment. The field had a mean (n = 6) earthworm population density of 44 individuals m−2, and an earthworm biomass of 26.8 g fresh weight m−2. No anecic earthworms were found in the area.
2.2. Experimental Design
Six square plots (0.25 m2) were established in a straight line, 1.0 m apart on-edge, parallel to a row. One of two earthworm addition rates was assigned randomly to each plot: 0 (CTL) or 48 earthworms m−2 (EW), with each rate replicated three times. An additional plot was used to record soil temperature. Every plot was surrounded by a fiberglass screen (20 cm buried below the surface, 26 cm above) to prevent loss of corn litter, limit earthworm migration, and allow for unaltered hydrologic connections. After the screens were installed, 150 g (600 g·m−2) of air-dried, aged corn leaf litter (C/N = 18.9) gathered from the experimental field (cut into ~2.0 cm pieces) was applied to the plots on 30 September 2008. Plots were inoculated with adult anecic earthworms (L. terrestris; obtained from a commercial outlet) on 7 October 2008. The earthworms had a mean (n = 10) fresh weight of 5.3 ± 0.9 g.
On 8 October 2008 the corn litter was removed quantitatively from each plot and stored, and 572 mg 15N·m−2 (as (15NH4)2SO4; 60 At.%) was applied to each plot in 100 mL (~0.04 cm) of water using a pressurized dispersion apparatus that delivered the solution at a rate of 20 mL·s−1. This amount of water and application rate resulted in quick infiltration into the soil, preventing pooling and runoff. The corn litter was placed back on the plots after application of (15NH4)2SO4.
2.3. Gas Sampling and Analysis
A cylindrical plastic chamber (20-cm dia., 16-cm high) fitted with a rubber septum on the top was used to measure the flux of N2O. At the time of sampling the chamber was placed randomly within the plot and pushed ~2.5 cm into the soil. A sample of the gases within the chamber was obtained initially and within 30 - 45 min after chamber placement with a 20-mL gas-tight syringe. The syringe was pumped three times to mix the gases in the headspace prior to sample collection, and the sample transferred immediately to a 15-mL evacuated tube (Vacutainer®; BD, Franklin Lakes, NJ). The concentration of N2O and the 15N enrichment of 15N2O and 15N2 were determined at the Stable Isotope Facility, University of California, Davis, CA, USA. Isotope ratios were measured using a SerCon Cryoprep trace gas concentration system interfaced to a PDZ Europa 20 - 20 isotope ratio mass spectrometer (SerCon Ltd., Cheshire, UK). Gas fluxes were calculated as described by Mosier and Schimel [23]. The total mass of N2O-N, 15N2O-15N and 15N2- 15N emitted from the plots over the course of the 28-day experiment was estimated from the area under each curve.
2.4. Additional Measurements
Soil temperature was measured with a digital thermometer at a depth of 10 cm in a separate uninoculated plot treated in an identical manner as the other experimental plots. Soil pH, OM and extractable N content, and the C/N ratio of corn litter were determined as described in Savin et al. [24]. At the end of the experiment (28 days after addition of (15NH4)2SO4, the leaf litter was removed quantitatively from each plot and dried to a constant weight at 60˚C. The dried leaf litter was separated from soil using tweezers and the dry mass of litter recorded for each plot. Soil samples (top 10 cm, one per plot) were obtained after litter removal.
2.5. Statistical Analysis
Student’s t-test was used to examine treatment differences for gas and isotope data (P < 0.05). A one-way analysis of variance was used to examine differences in mass of litter and soil properties.
3. Results
At the end of the experiment the amount of litter (g dry weight m−2) remaining in both treatments was significantly lower relative to the initial amount, and was significantly lower in the EW than the CTL treatment, with mean (s.d.) values of 232 ± 26 and 395 ± 19 for the EW and CTL treatments, respectively. No significant differences were observed between initial and final levels of soil NH4 and NO3 for either treatment (data not shown).
Addition of earthworms significantly increased the flux of N2O relative to the CTL treatment 1 day after addition of (15NH4)2SO4 to the plots (Figure 1). No significant differences in flux were observed subsequently between EW and CTL treatments, and the lowest flux values for both treatments were observed between Day 12 and Day 15. The flux of N2O increased in both treatments from Day 15 until the end of the experiment, coinciding with a number of precipitation events that yielded
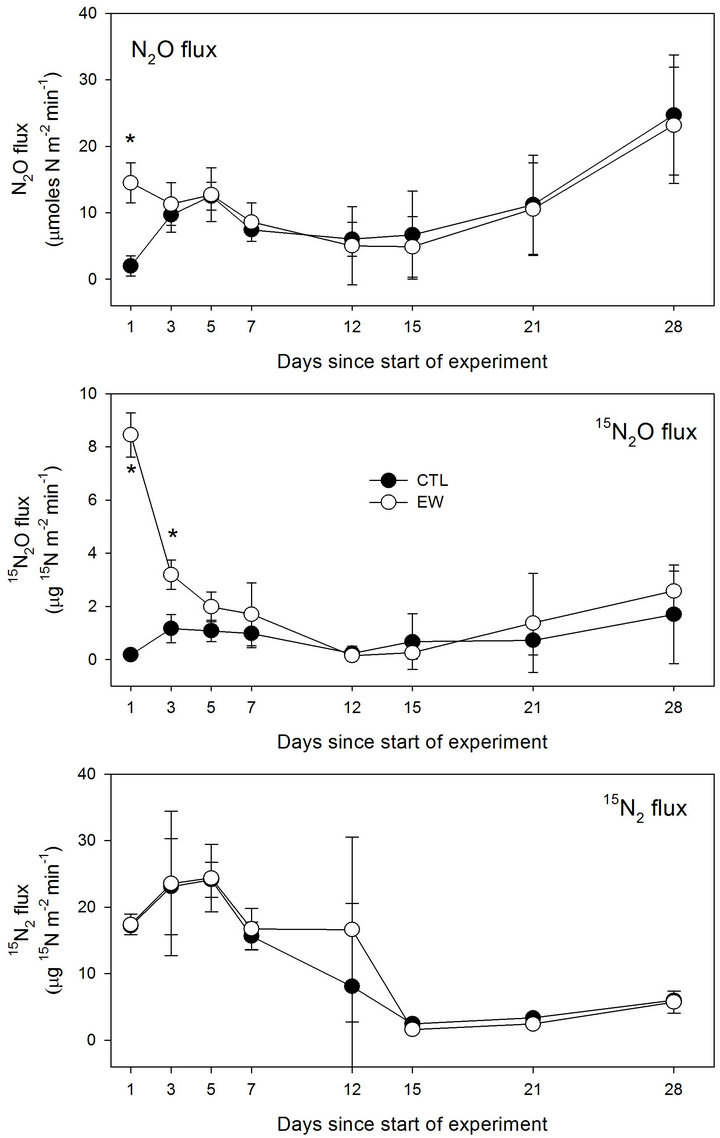
Figure 1. Flux of N2O, 15N2O and 15N2 as a function of time for field plots amended with 0 (CTL) or 48 Lumbricus terrestris m−2 (EW). Values are means (n = 3); bars represent one standard deviation. (*) indicates significant difference (P < 0.05).
a total of ~3.5 cm of rain during the last 13 days of the experiment (Figure 2). No significant differences were observed in total mass of N2O-N emitted over 28 days (Table 1).
The flux of 15N2O was significantly higher for the EW treatment on Day 1 and Day 3 (Figure 1). No significant differences were observed among treatments between Day 5 and Day 28, and the lowest flux was observed between Day 12 and Day 15. The 15N2O flux increased steadily in both treatments after Day 15 until the end of the experiment. The total amount of 15N emitted as 15N2O over 28 days was significantly higher for the EW than for the CTL treatment (Table 1). The yield of 15N2O from 15NH4 initially added was 5.8% for CTL and 11.6% for the EW treatment (Table 1).
There were no significant differences in 15N2 flux between treatments on any sampling day (Figure 2). The flux was highest on Day 3, lowest on Day 15, and increased subsequently until the end of the experiment. There were no significant differences in the total amount of 15N emitted as 15N2 over 28 days (Table 1). The yield of 15N2 from 15NH4 was 17.9 % and 15.2% for the CTL and EW treatments, respectively (Table 1). The yield of 15N-labeled gases was lowest for the CTL (23.7%) and highest for the EW treatment (26.8%).
Significant differences in the mole fraction of 15N2O between treatments were observed on Day 1 and Day 12 (Figure 3). On Day 1, the mole fraction in the EW treatment was 0.87, with a value of 0.16 observed for the CTL treatment (0.16). On Day 12 the highest mole fraction (0.56) was observed for the CTL treatment, whereas the EW treatment had a value of 0.18.
4. Discussion
The dynamics of 15N2O and 15N2 production were generally similar for both treatments, suggesting a synchrony
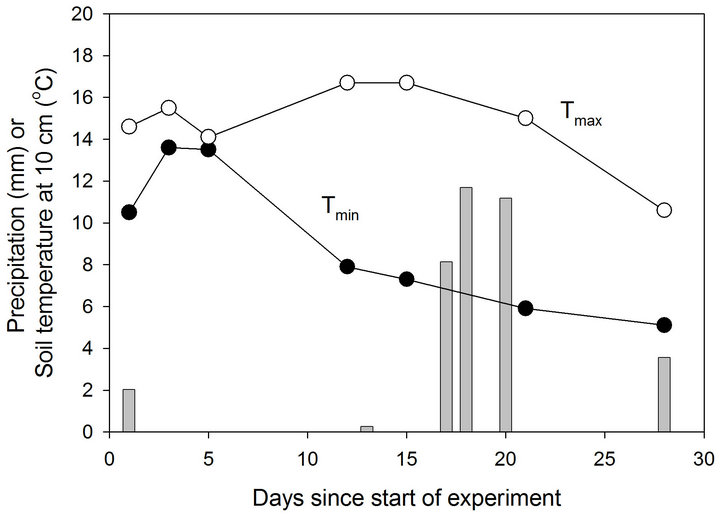
Figure 2. Precipitation and maximum and minimum soil temperature at a depth of 10 cm in field plots during the course of the experiment.
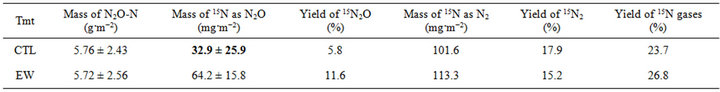
Table 1. Total mass of N2O-N, N2O-15N, and N2-15N over the course of 28 days, and yield of 15N2O, 15N2 and 15N2 gases from added 15NH4 in uninoculated plots (CTL) and plots inoculated with 48 L. terrestris m−2 (EW). Values in bold within a column were significantly different (P < 0.05).
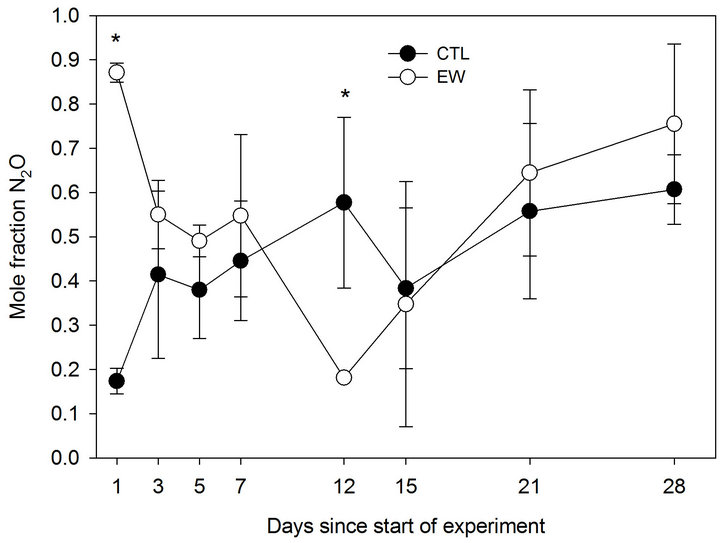
Figure 3. Mole fraction of 15N2O as a function of time for field plots amended with 0 (CTL) or 48 Lumbricus terrestris m−2 (EW). Values are means (n = 3); bars represent one standard deviation. (*) indicates significant difference (P < 0.05).
driven by external factors common to all plots, such as soil moisture and temperature (Figure 2). In particular, the timing of precipitation events appeared to exert an important control on N2O flux. This is consistent with previous reports that N2O flux responds positively to increases in soil moisture [25].
The N2O flux values in the present study ranged from 0.23 to 5.7μg N2O-N m−2·s−1, higher than those reported by Molodovskaya et al. [26], who found average flux values in a corn field of 0.059 μg N2O-N m−2·s−1, with a maximum flux of 0.76 μg N2O-N m−2·s−1 observed during a spring thaw event. The fraction of available N converted to N2O for soils planted to grain crops has been reported by Tonitto et al. [27] to vary from less than 1 to about 4% on a seasonal scale, whereas our values ranged from 5.8% to 11.6%. The high values for flux and greater fraction of added N converted to N2O in our study may be due to the high soil moisture content and presence of high amounts of plant litter, both of which promote N2O production in agricultural soils [3]. The absence of a plant sink for NO3 may also have contributed to higher than expected flux values. Relative losses of 15N in gaseous forms in the present study for the EW treatment (Table 1) were comparable to those estimated by Amador and Görres [21] for mesocosms inoculated with earthworms at a rate of 127 m−2 (16.8% to 39.8%), but losses in the CTL treatment were higher than for mesocosms without earthworms in that study (1.9% to 7.4%). The differences are likely due to differences in soil moisture and temperature that favored denitrification under field conditions.
The effects of earthworm additions on N2O flux under field conditions in the present study are in general agreement with those observed by others. For example, Borken et al. [28] reported a 57% increase in N2O emissions from field mesocosms containing forest soil and inoculated with L. terrestris at a population density of 113 individuals m−2. Evers et al. [19] observed that inoculation of mesocosms containing a sandy loam from a forested area with L. terrestris at population densities greater than 300 m−2 had N2O emission rates that were significantly higher than uninoculated mesocosms. Furthermore, the effects of L. terrestris depended on soil moisture, with maximum values at 35%. However, the effects of L. terrestris were not observed when inoculated at densities representative of field values (90 - 270 m−2). The addition of an anecic earthworm (Aporrectodea longa) in soil mesocosms with a high moisture content at a population density of 100 m−2 significantly increased the flux of N2O relative to soil without earthworms over the first 12 days of incubation, but the flux was significantly lower between days 44 and 62 in the earthworm treatment [18]. Rizhiya et al. [17] observed that additions of A. longa (~100 m−2) to soil mesocosms containing grass residues significantly increased N2O flux relative to a control without earthworms. They suggested that the role of earthworms consisted of mixing residues into the soil, switching residue decomposition from aerobic to anaerobic, and from low denitrification to high denitrification and N2O production. By contrast, in a field study of the effects of anecic earthworm additions (L. terrestris and A. longa) on N2O flux during the growing season in an unfertilized cornfield, Speratti and Whalen [29] were unable to conclude that earthworms affected N2O flux. Although they observed generally higher N2O fluxes in enclosures amended with earthworms, poor survival of introduced earthworms and seasonal variability yielded flux values that were not significantly different [29].
Direct production of N2O by earthworms has been reported [30,31], but the flux is generally very low compared to total flux [18]. The positive effects of earthworms on denitrification are generally attributed to indirect mechanisms [26,27], including increased soil moisture in burrow soil and increased availability of organic carbon through translocation and greater physical contact of plant detritus with soil. Earthworms have also been shown to increase rates of nitrification in soil [26].
The effects of earthworm amendment were most apparent at the outset of the experiment, with significant differences disappearing after 5 days. The short time frame and dynamics of the effects suggest that these may be associated with activities such as burrowing, which took place within a day after inoculation. Van den Heuvel et al. [32] observed that soil disturbance resulted in considerably higher N2O emissions from soils in a riparian area, an effect they attributed to increased diffusion of O2. Denitrifiers decrease reduction of N2O to N2 as O2 levels increase [33]. This may explain the initial increase in N2O flux and higher N2O mole fraction in the EW treatment.
The presence of earthworms resulted in a significant increase (Day 1) and decrease (Day 12) in the mole fraction of 15N2O relative to the CTL (Figure 3). Firestone et al. [3] have shown that the mole fraction of N2O is a function of a number of variables, including soil pH, concentration of NO3 and O2, and time under anaerobiosis. Positive effects of earthworm additions on mole fraction of 15N2O may be due to higher concentrations of NO3 in earthworm burrows and casts [27,34], possibly as a result of increased O2 diffusion into soil [35]. By contrast, greater retention of moisture (and consequently lower O2 concentration) in casts and burrow soil due to shifts in pore size distribution [36] relative to bulk soil may have resulted in significantly lower mole fraction of 15N2O on Day 12.
5. Conclusion
We have shown that addition of L. terrestris to an agricultural soil at a rate of 48 earthworms m−2 significantly increased the flux of N2O and 15N2O in the early stages of the 28-day experimental period, as well as the total mass of 15N2O-N emitted relative to an uninoculated control. Earthworms had no significant effect on the flux of 15N2. Our results need to be interpreted with caution, given the short duration of the experiment and the fact that it was conducted at the end of the growing season— when temperature, moisture and plant detritus were likely optimal for earthworm activity and N2O emissions. As such, our data may represent an upper limit for effects of L. terrestris on 15N2O flux. Nevertheless, our results suggest that L. terrestris can enhance N2O emissions from a corn field temporarily. These results point to a need for better understanding of the mechanisms by which anecic earthworms affect trace gas fluxes as we move towards more sustainable, foodweb-based management of nutrients in agroecosystems.
6. Acknowledgements
This research was funded by an grant-in-aid from the University of Rhode Island’s Provost’s Office to E.J. Avizinis, by a grant from the USDA-AFRI program to J.A. Amador, and by private funds of the authors. This is contribution no. 5327 from the Rhode Island Agricultural Experiment Station.
REFERENCES
- J. P. Schimel and E. A. Holland, “Global Gases,” In: J. J. Fuhrman, D. M. Sylvia, P. G. Hartel and D. A. Zuberer, Eds., Principles and Applications of Soil Microbiology, 2nd Edition, Pearson-Prentice Hall, Upper Saddle River, 2005, pp. 491-509.
- C. A. Edwards, “The Importance of Earthworms as Key Representatives of the Soil Fauna,” In: C. A. Edwards, Ed., Earthworm Ecology, 2nd Edition, CRC Press, Boca Raton, 2004, pp. 3-11. doi:10.1201/9781420039719.pt1
- M. K. Firestone, R. B. Firestone and J. M. Tiedje, “Nitrous Oxide from Soil Denitrification: Factors Controlling Its Biological Production,” Science, Vol. 208, 1980, pp. 749-751. doi:10.1126/science.208.4445.749
- A. J. Tugel and A. M. Lewandowski, “Soil Biology Primer,” NRCS Soil Quality Institute, Ames, 1999.
- FAO (Food and Agriculture Organization of the United Nations), “Manual on Integrated Soil Management and Conservation Practices,” No. 8, Rome, 2000.
- C. Sandretto, “Conservation Tillage Firmly Planted in U.S. Agriculture,” Agricultural Outlook: Economic Research Service—USDA, 2001, pp. 5-6.
- J. M. Holland, “The Environmental Consequences of Adopting Conservation Tillage in Europe: Reviewing the Evidence,” Agriculture, Ecosystems & Environment, Vol. 103, No. 1, 2004, pp. 1-25. doi:10.1016/j.agee.2003.12.018
- R. Fowler and J. Rockstrom, “Conservation Tillage for Sustainable Agriculture: An Agrarian Revolution Gathers Momentum in Africa,” Soil and Tillage Research, Vol. 61, No. 1-2, 2001, pp. 93-107. doi:10.1016/S0167-1987(01)00181-7
- E. G. Gregorich, P. Rochette, A. J. VandenBygaart and D. A. Angers, “Greenhouse Gas Contributions of Agricultural Soils and Potential Mitigation Practices in Eastern Canada,” Soil and Tillage Research, Vol. 83, No. 1, 2005, pp. 53-72. doi:10.1016/j.still.2005.02.009
- R. Lal, J. M. Kimble, R. F. Follett and C. B. Cole, “The Potential of U.S. Cropland to Sequester Carbon and Mitigate the Greenhouse Effect,” CRC Press, Boca Raton, 1998.
- C. A. Edwards and J.R. Lofty, “The Effect of Direct Drilling and Minimal Cultivation on Earthworm Populations,” Journal of Applied Ecology, Vol. 19, 1982, pp. 723-734. doi:10.2307/2403277
- W. Ehlers, “Observations on Earthworm Channels and Infiltration on Tilled and Untilled Loess Soil,” Soil Science, Vol. 119, 1975, pp. 242-249. doi:10.1097/00010694-197503000-00010
- W. M. Edwards, M. J. Shipitalo, L. B. Owens and L. D. Norton, “Effect of Lumbricus terrestris L. Burrows on Hydrology of Continuous No-Till Corn Fields,” Geoderma, Vol. 46, No. 1-3, 1990, pp. 73-84. doi:10.1016/0016-7061(90)90008-W
- V. Nuutinen, “Earthworm Community Response to Tillage and Residue Management on Different Soil Types in Southern Finland,” Soil and Tillage Research, Vol. 23, No. 3, 1992, pp. 221-239. doi:10.1016/0167-1987(92)90102-H
- C. A. Edwards, “Can Earthworms Harm the Planet?” BioCycle, Vol. 49, 2008, p. 53.
- J. G. Ehrenfeld, “Ecosystem Consequences of Biological Invasions,” Annual Review of Ecology, Evolution, and Systematics, Vol. 41, 2010, pp. 59-80. doi:10.1146/annurev-ecolsys-102209-144650
- E. Rizhiya, C. Bertora, P. C. J. van Vliet, P. J. Kuikman, J. H. Faber and J. W. van Groenigen, “Earthworm Activity as a Determinant for N2O Emission From Crop Residue,” Soil Biology & Biochemistry, Vol. 39, No. 8, 2007, pp. 2058-2069. doi:10.1016/j.soilbio.2007.03.008
- C. Bertora, P. C. J. van Vliet, E. W. J. Hummelink and J. W. van Groenigen, “Do Earthworms Increase N2O Emissions in Ploughed Grassland?” Soil Biology & Biochemistry, Vol. 39, No. 2, 2007, pp. 632-640. doi:10.1016/j.soilbio.2006.09.015
- A. K. Evers, T. A. Demers, A. M. Gordon and N. V. Thevathasan, “The Effects of Earthworm (Lumbricus terrestris L.) Population Density and Soil Water Content Interactions on Nitrous Oxide Emissions from Agricultural Soils,” Applied and Environmental Soil Science, Vol. 2010, 2010, Article ID: 737096, 9 p. doi:10.1155/2010/737096
- G. Giannopoulos, M. M. Pulleman and J. W. Van Groenigen, “Interactions netween Residue Placement and Earthworm Ecological Strategy Affect Aggregate Turnover and N2O Dynamics in Agricultural Soil,” Soil Biology & Biochemistry, Vol. 42, No. 4, 2010, pp. 618-625. doi:10.1016/j.soilbio.2009.12.015
- J. A. Amador and J. H. Görres, “Role of the Anecic Earthworm Lumbricus terrestris L. in the Distribution of Plant Residue Nitrogen in a Corn (Zea mays)—Soil System,” Applied Soil Ecology, Vol. 30, No. 3, 2005, pp. 203-214. doi:10.1016/j.apsoil.2005.02.011
- Soil Survey Staff, “Official Soil Series Descriptions.” http://soils.usda.gov/technical/classification/osd/index.html
- A. R. Mosier and D. S. Schimel, “Nitrification and Denitrification,” In: R. Knowles and T. H. Blackburn, Eds., Nitrogen Isotope Techniques, Academic Press, San Diego, 1993, pp. 181-208.
- M. C. Savin, J. H. Görres, D. A. Neher and J. A. Amador, “Biogeophysical Factors Influencing Soil Respiration and Mineral Nitrogen Content in an Old Field Soil,” Soil Biology & Biochemistry, Vol. 33, No. 4-5, 2001, pp. 429- 438. doi:10.1016/S0038-0717(00)00182-6
- P. M. Groffman and J. M. Tiedje, “Denitrification Hysteresis during Wetting and Drying Cycles in Soil,” Soil Science Society of America Journal, Vol. 52, 1988, pp. 1626-1629. doi:10.2136/sssaj1988.03615995005200060022x
- T. B. Parkin and E. C. Berry, “Microbial Nitrogen Transformations in Earthworm Burrows,” Soil Biology & Biochemistry, Vol. 31, No. 13, 1999, pp. 1765-1771. doi:10.1016/S0038-0717(99)00085-1
- T. B. Parkin and E. C. Berry, “Nitrogen Transformations Associated with Earthworm Casts,” Soil Biology & Biochemistry, Vol. 26, No. 9, 1994, pp. 1233-1238. doi:10.1016/0038-0717(94)90148-1
- W. Borken, S. Gründel and F. Beese, “Potential Contribution of Lumbricus terrestris L. to Carbon Dioxide, Methane and Nitrous Oxide Fluxes from a Forest Soil,” Biology and Fertility of Soils, Vol. 32, No. 2, 2000, pp. 142-148. doi:10.1007/s003740000228
- A. B. Speratti and J. K. Whalen, “Carbon Dioxide and Nitrous Oxide Fluxes from Soil as Influenced by Anecic and Endogeic Earthworms,” Applied Soil Ecology, Vol. 38, No. 1, 2008, pp. 27-33. doi:10.1016/j.apsoil.2007.08.009
- P. K. Wust, M. A. Horn, G. Henderson, P. H. Janssen, B. H. A. Rehm and H. L. Drake, “Gut-Associated Denitrification and in Vivo Emission of Nitrous Oxide by the Earthworm Families Megascolecidae and Lumbricidae in New Zealand,” Applied and Environmental Microbiology, Vol. 75, No. 11, 2009, pp. 3430-3436. doi:10.1128/AEM.00304-09
- H. L. Drake and M. A. Horn, “Earthworms as a Transient Heaven for Terrestrial Denitrifying Microbes: A Review,” Engineering in Life Sciences, Vol. 6, No. 3, 2006, pp. 261-265. doi:10.1002/elsc.200620126
- R. N. van den Heuvel, M. M. Hefting, N. C. G. Tan, M. S. M. Jetten and J. T. A. Verhoeven, “N2O Emission Hotspots at Different Spatial Scales and Governing Factors for Small Scale Hotspots,” Science of the Total Environment, Vol. 407, No. 7, 2009, pp. 2325-2332. doi:10.1016/j.scitotenv.2008.11.010
- M. A. Cavigelli and G. P. Robertson, “Role of Denitrifier Diversity in Rates of Nitrous Oxide Consumption in a Terrestrial Ecosystem,” Soil Biology & Biochemistry, Vol. 33, No. 3, 2001, pp. 297-310. doi:10.1016/S0038-0717(00)00141-3
- J. H. Gorres, M. C. Savin and J. A. Amador, “Dynamics of Carbon and Nitrogen Mineralization, Microbial Biomass, and Nematode Abundance Within and Outside the Burrow Walls of Anecic Earthworms (Lumbricus terrestris),” Soil Science, Vol. 162, 1997, pp. 666-671. doi:10.1097/00010694-199709000-00008
- A. Kretzschmar and P. Monestiez, “Physical Control of Soil Biological Activity Due to Endogeic Earthworm Behavior,” Soil Biology & Biochemistry, Vol. 24, No. 12, 1992, pp. 1609-1614. doi:10.1016/0038-0717(92)90158-T
- J. H. Görres, M. C. Savin and J. A. Amador, “Soil Micropore Structure and Carbon Mineralization in Burrows and Casts of an Anecic Earthworm (Lumbricus terrestris),” Soil Biology & Biochemistry, Vol. 33, No. 14, 2001, pp. 1881-1887. doi:10.1016/S0038-0717(01)00068-2

