Open Journal of Acoustics
Vol.04 No.03(2014), Article ID:49733,6 pages
10.4236/oja.2014.43013
Ultrasonic investigation of Elastic anomalies in Lithium Sodium Sulphate Hexahydrate single crystal
George Varughese1, Santhosh Kumar2
1Department of Physics, Catholicate College, Pathanamthitta, India
2SPAP, Mahatma Gandhi University, Kottayam, India
Email: gvushakoppara@yahoo.co.in
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 22 July 2014; revised 15 August 2014; accepted 9 September 2014
ABSTRACT
An extensive study of the thermal properties of Lithium Sodium Sulphate Hexa hydrate (LSSW) single crystal, with Trigonal structure, has been carried out using ultrasonic Pulse Echo Overlap (PEO) technique, Differential Thermal Analysis (DTA) and Thermo Gravimetric Analysis (TGA). The temperature variation of elastic constants of LiNa3(SO4)2∙6H2O single crystal have been reported for the first time. The second order elastic stiffness constants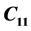 ,
, 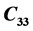 ,
, 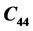 , along the various directions in the crystal have been determined in the temperature range 300 - 330 K. The change in velocity with temperature with respect to the room temperature value has been measured using PEO technique. Significant anomalies were observed in
, along the various directions in the crystal have been determined in the temperature range 300 - 330 K. The change in velocity with temperature with respect to the room temperature value has been measured using PEO technique. Significant anomalies were observed in 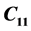 and
and 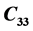 at 316 K. The elastic constant
at 316 K. The elastic constant 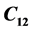 has shown no variation in the temperature range 300 - 319 K. A minor deviation for
has shown no variation in the temperature range 300 - 319 K. A minor deviation for 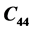 at 305 K following a parabolic change has been observed. The minor anomalies observed in the elastic constants of LSSW may be due to its dehydration of water of crystallization in the range 304 - 319 K. DTA studies showed an appreciable endothermic change in the range 309 K-369.79 K. TGA curve exhibited a decrease in weight of 1.687 mg in the temperature range 304 K-360 K. The minor anomalies observed in the elastic constants of LSSW may be due to loosing of its water of crystallization in the range 309 - 319 K. On loosing water there will not be any change in chemical structure but there will be physical change associated with loosing of water molecule.
at 305 K following a parabolic change has been observed. The minor anomalies observed in the elastic constants of LSSW may be due to its dehydration of water of crystallization in the range 304 - 319 K. DTA studies showed an appreciable endothermic change in the range 309 K-369.79 K. TGA curve exhibited a decrease in weight of 1.687 mg in the temperature range 304 K-360 K. The minor anomalies observed in the elastic constants of LSSW may be due to loosing of its water of crystallization in the range 309 - 319 K. On loosing water there will not be any change in chemical structure but there will be physical change associated with loosing of water molecule.
Keywords:
Ultrasonics, Single crystal, Elastic properties, Pulse Echo Overlap Technique

1. Introduction
Lithium Sodium Sulphate single crystal is an extensively studied super ionic crystal, which exhibits piezoelectric and pyro-electric properties. It is also non-centrosymmetric. In this family a new crystal LiNa3(SO4)2∙6H2O (LSSW) is synthesized. This new sulphate salt characterized by the presence of water molecule in the unit cell, and it was reported [1] . The crystal structure was examined by X ray diffraction (XRD) pattern and compared it
with Joint Committee Powder Diffraction Scan (JCPDS) file [2] . The crystal belongs to space group 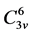 with
with
6 molecules per unit cell, Trigonal in symmetry and lattice parameters 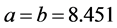 Å and
Å and 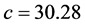 Å. The Fourier Transform Infrared spectrum (FTIR) of a sample is highly characteristic and hence it is widely used to identify substances. The presence of hydroxyl [OH] in the sample was confirmed by group in the FTIR spectrum of the sample [3] . Raman studies already reported in the literature have investigated phase transition in the temperature range 12 K - 300 K. They haven’t noticed any anomalies in that range of temperature [1] . For each site of Li+ ion, there are two ions bound to three oxygen atoms of three different water molecules and an oxygen atom of the sulfates producing a perfect tetrahedron. There are 4 hydrogen bonds formed by water molecule with the oxygen atom of
Å. The Fourier Transform Infrared spectrum (FTIR) of a sample is highly characteristic and hence it is widely used to identify substances. The presence of hydroxyl [OH] in the sample was confirmed by group in the FTIR spectrum of the sample [3] . Raman studies already reported in the literature have investigated phase transition in the temperature range 12 K - 300 K. They haven’t noticed any anomalies in that range of temperature [1] . For each site of Li+ ion, there are two ions bound to three oxygen atoms of three different water molecules and an oxygen atom of the sulfates producing a perfect tetrahedron. There are 4 hydrogen bonds formed by water molecule with the oxygen atom of 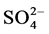 and
and  ions, namely 1) One connecting the oxygen of the H2O molecule to the top oxygen of the
ions, namely 1) One connecting the oxygen of the H2O molecule to the top oxygen of the 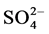 tetrahedron; 2) Another connecting the oxygen of the H2O molecule to the top oxygen of the
tetrahedron; 2) Another connecting the oxygen of the H2O molecule to the top oxygen of the 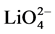 tetrahedron and 3) the remaining two are connecting the oxygen of the H2O molecule only to the base oxygen of the
tetrahedron and 3) the remaining two are connecting the oxygen of the H2O molecule only to the base oxygen of the 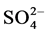 tetrahedron. No investigation on phase transition has been conducted so far in the above room temperature range by any other method. Hence aim of this investigation is to investigate any anomalies in the elastic properties of the crystal by studying the variation of elastic properties of LSSW with temperature by ultrasonic Pulse Echo Overlap technique [4] .
tetrahedron. No investigation on phase transition has been conducted so far in the above room temperature range by any other method. Hence aim of this investigation is to investigate any anomalies in the elastic properties of the crystal by studying the variation of elastic properties of LSSW with temperature by ultrasonic Pulse Echo Overlap technique [4] .
2. Experimental
2.1. Sample preparation
Li2CO3 and NaHSO4∙H2O was mixed in equi molar ratio in triply distilled water. Large single crystals can be grown by Slow evaporation technique for a duration of 60 days. At 308 K Lithium Sodium Sulphate Hexahydrate crystal (LSSW) and at 323 K Lithium Sodium Sulphate single crystal were synthesized (LSS). The optical quality of the crystal can be enhanced by re-crystallization from the solution for several times, and with the usage of triply distilled water. Temperature of the bath is kept constant by using an efficient temperature controller having 0.5 K stability. Powder XRD of these crystals has been reported [3] . Size of the grown single crystal LSSW was (30 × 30 × 25) mm3 and depicted in figure 1. The natural faces of the sample have been identified by comparing the measured interfacial angles and the computed stereographic projection of the crystal planes. The stereographic net of the crystal about a- and c-axes are made with the help of the computer programme “Jcrystal” by giving the values of lattice parameters, crystal system and space group of the crystal [5] . Bulk samples have been cut using a slow speed diamond wheel saw so as to have propagation direction along [100] and [001] axes of the crystal [6] . The cuttings are made very accurately and the mis-orientation in cutting is less than 1°. The thickness of the sample crystals along the measurement direction are in the range 0.8 - 1.2 cm. The samples are well polished by using water paper of grade 1500 and Cerium oxide powder. This enables one to get proper bonding of transducer.
2.2. Ultrasonic Velocity measurements
A Rhombohedral crystal has three-fold axis of symmetry and three mirror planes. The expression for the velocity
of elastic waves in different symmetry directions are derived. It is found that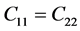 ,
, 

and 

elastic constant 
at 




The elements of determinantal equation are defined by elastic constants and the direction cosines of the direction of propagation [6] . Non zero elastic constants are shown in the detrmininent.
Figure 1. Grown crystal of Lithium Sodium Sulphate Hexahydrate (LSSW).
Here
If the cosines and the velocity in crystallographic directions are known, the adiabatic elastic constants can be determined [3] . The velocity of ultrasonic waves was measured for ultrasonic longitudinal and transverse waves in the specimen using 

The McSkimin 
It has the following six second order elastic stiffness constants









tionship with the suitable ultrasonic mode velocity given by 





Here,




where




Here, 



2.3. Variation of 
The temperature variation of 




2.4. Differential Thermal Analysis (DTA) & Thermo Gravimentric Analysis (TGA) spectrum of LSSW
The DTA and TGA scans have been depicted in Figure 5. An appreciable endothermic dip at 369.79 K. There
Figure 2. Variation of elastic constants 

Figure 3. Variation of elastic constants 

Figure 4. Differential Thermal Analysis and Thermo Gravimetric spectra of LSSW.
are also two small dips at 526.62 K and 765.04 K. The energy associated with the transition can be evaluated [12] from area enclosed by dip. The peak areas in differential thermo grams depend upon the mass of the sample, “m”, and enthalpy/unit mass is 

where “


3. Results and Discussions
For the Trigonal class system, there are six elastic constants





of four second order elastic stiffness constants



lies were observed in 


ature range 300 - 319 K. A minor deviation for 
The minor anomalies observed in the elastic constants


of water of crystallization in the range 304 - 319 K. The shape of the curves is shown in figure 2 and figure 3. One could not investigate beyond 330 K because of the opaqueness developed in the crystal. This may be due the exclusion of water molecule associated with the crystal between 316 K and 320 K.
4. Conclusion
The structural discrepancies found in the crystal grown at 323 K and 308 K have been solved to an extent using the ultrasonic velocity measurements. Temperature variations of four second order elastic stiffness constants






The elastic constant 

Table 1. Velocity of ultrasonic modes in LSSW at 300 K. L, T, QL, QT represent longitudinal, transverse, quasi-longitudinal and quasi transverse modes respectively. The relations between mode velocities and elastic constant are also given in [3] .
where 

Figure 5. Symmetry axis and mirror planes of Trigonal crystals.
associated with loosing of water molecule. Differential Thermal Analysis Scan showed a large endothermic dip during the range 309 K - 369.79 K. The energy associated with the sample is estimated as 2.566 J/g. Thermo Gravimetric Analysis curve exhibited a reduction in mass of 1.687 mg in the temperature range 304 K - 373 K.
Acknowledgements
The author (G.V.) is grateful to UGC Delhi for a Teacher Fellowship.
References
- Filho, J.M., Paiva, R.O., Freire, P.T.C. and Melo, F.E. (1999) X-Ray and Temperature Polarized Raman Spectroscopic Characterization of the LiNa3(SO4)2·6H2O Crystal. Journal of Raman Spectroscopy, 30, 289. http://dx.doi.org/10.1002/(SICI)1097-4555(199904)30:4<289::AID-JRS375>3.0.CO;2-F
- JCPDS File Card No. 712172.
- George, V., Kumar, A.S. and Louis, G. (2009) Anisotropy in Elastic Properties of Lithium Sodium Sulphate Hexa Hydrate Single Crystal—An Ultrasonic Study. Bulletin of Materials Science, 32, 621. http://dx.doi.org/10.1007/s12034-009-0096-7
- Papadakis, E.P. (1967) Ultrasonic Pulse Velocity by the Pulse-Echo Overlap Method Incorporating Diffraction Phase Correlation. The Journal of the Acoustical Society of America, 42, 1045. http://dx.doi.org/10.1121/1.397777
- Godfrey, L. and Philip, J. (1994) Elastic Constants and High Temperature Anomalies Near 425 K in Lithium Hydrazeniumsulphate. Journal of Applied Physics, 75, 2393. http://dx.doi.org/10.1063/1.356260
- Truell, R., Elbaum, C. and Chick, B.B. (1969) In: Ultrasonic Methods in Solid State Physics, Academic Press, New York, 370.
- McSkimin, H.J. (1964) In: Mason, W.P., Ed., Physical Acoustics, Part A, Academic Press, New York, Vol. I, 271.
- Papadakis, E.P. (1976) Ultrasonic Velocity and Attenuation: Measurement Methods with Scientific and Industrial Applications. In: Mason, W.P. and Thurston, R.N., Eds., Physical Acoustics, Academic Pressm New York, Vol. XII, 227.
- Nye, N.F. (1957) Physical Properties of Crystals. Oxford University Press, London, 145.
- May Jr., J.E. (1958) Circuit Theory; Ultrasonic Engineering. IRE.Natl.Conv.Rec., 6, Part 2, 134.
- McSkimin, H.J. (1961) Pulse Superposition Method for Measuring Ultrasonic Wave Velocities in Solids. The Journal of the Acoustical Society of America, 33, 12. http://dx.doi.org/10.1121/1.1908386
- Skoog, D.A., Holler, F.J. and Nieman, T.A. (1997) In: Principles of Instrumental Analysis, Harcourt Asia PTE Ltd., Singapore.











