Advances in Materials Physics and Chemistry
Vol.4 No.2(2014), Article ID:42945,10 pages DOI:10.4236/ampc.2014.42006
Effect of rf Plasma Carbonitriding on the Biocompatibility and Mechanical Properties of AISI 321 Austenitic Stainless Steel
1Physics Department, Faculty of Science, Sohag University, Sohag, Egypt
2Zoology Department, Faculty of Science, Sohag University, Sohag, Egypt
Email: mraaif@daad-alumni.de
Copyright © 2014 F. M. El-Hossary et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property F. M. El-Hossary et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received January 10, 2014; revised February 11, 2014; accepted February 17, 2014
KEYWORDS
Plasma Carbonitriding; Surface Energy; Wear; Corrosion Resistance; Blood Culture; Protein Adsorption
ABSTRACT
AISI 321 austenitic stainless steel was treated using rf plasma carbonitriding with the intention of use low-cost orthopedic implant material in biomedical applications. The treatment process was carried at low working gas pressure of 0.075 mbar in nitrogen-acetylene gaseous mixture to form a superficial carbonitrided layer. The samples were treated using rf inductively coupled at a fixed plasma-processing power of 500 W and for a processing time varied from 4 to 20 minutes. The microstructural, mechanical and tribological properties of the untreated and treated samples were studied. The surface hardness is improved by rf plasma carbonitriding to a maximum of 1468 HV0.1 for plasma-processing time of 16 min. To evaluate the biocompatibility performance, the blood was cultured in RPMI media to test the adhesion of blood cells on the untreated and treated samples. It has been found that the blood adhesion on the treated samples is enhanced with increasing the plasmaprocessing time. The contact angle of the carbonitrided surfaces is decreased to lower values compared to that of the untreated surface. Furthermore, the carbonitrided layer in-vitro corrosion was tested in Ringer’s solution. A degradation in the corrosion resistance was observed for the sample carbonitrided at low plasma processing time of 4 min. However, the corrosion resistance increased to a maximum value at a plasma-processing time of 8 min then gradually decreased with further increase of plasma processing time.
1. Introduction
AISI 321 is a stabilized austenitic stainless steel alloy containing low amount of titanium to minimize inter-granular attack under certain service conditions [1,2]. Accordingly, the improvement in the inter-granular corrosion resistance makes this austenitic grade very motivating for high temperature applications such as industrial heat exchangers and nuclear power plants [1,3-5]. Furthermore, it is considered as an interesting biomedical material in the fabrication of the economical orthopedic implants [6,7]. However, the poor surface hardness leads to degradation in tribological properties which frustrate many of these industrial and biomedical applications
[1,8]. Therefore, different plasma surface treatment techniques are applied to develop the mechanical performance and to increase the service life of this alloy for various applications [9]. Among these techniques, rf plasma carbonitriding with nitrogen/acetylene gaseous mixture has been previously used for surface modification of different austenitic stainless steel alloys including AISI 321 grade [1,10,11]. It has been succeeded to achieve an anticorrosive and wear-resistant superficial top layer with low friction coefficient [1,12]. The superior improvement of the tribological properties was ascribed to formation of hard nitrides and carbides phases and the existence of superficial carbonitrided top layer [1,13]. Contact angle and surface energy measurements were fundamentally conducted to recognize the biocompatibility of the metallic and modified metallic alloys such as surface ability to protein adsorption and cells adhesion [14]. Directed Cell adhesion has been considered as an important criterion of implant and tissue engineering technology [15]. Generally, the surface energy is proportional to cellular adhesion strength [15]. Cellular adhesion on metals demonstrated a linear correlation with surface energy. Materials of higher surface energy have higher cellular adhesion.
This work was initiated to obtain a thick carbonitrided layer into the top of AISI 321 substrate with high tribological, mechanical and biocombatibility performance for low-cost orthopedic implants. The biocompatibility tests including blood culture and protein adsorption are applied to the carbonitrided AISI 321 substrates. Furthermore, the carbonitrided layer in-vitro corrosion was tested in Ringer’s solution. This is to improve the level of in-vitro biocompatibility assessment required for the specific use of the treated austenitic substrates in the human body.
2. Experimental Work
2.1. Sample Preparation
The austenitic stainless steel AISI 321 consisted of 1.7 mm thick rolled sheet, cut into pieces with small dimensions of 10 mm × 10 mm × 1.7 mm. The chemical composition of AISI 321 in wt% is꞉ 0.042 C, 1.10 Mn, 17.50 Cr, 10.70 Ni, 2.06 Mo, 0.23 Ti, 0.39 Si, 0.005 P, 0.01 S and Fe balance. The samples were ultrasonically cleaned in acetone bath for 15 min before they were inserted into the plasma reactor tube. The samples were carbonitrided using radio frequency (rf) plasma inductively coupled operated in continuous mode. Details of the carbonitriding system can be found somewhere else [16,17]. In brief, the rf plasma system is comprised of a quartz reactor tube with length of 500 mm and a diameter of 41.5 mm and it was evacuated to a base pressure of 1.0 × 10−2 mbar by a two-stage rotary pump. A gas mixture of 85% nitrogen and 15% acetylene was introduced and the gas flow rates were adjusted to establish a total gas pressure of 7.5 × 10−2 mbar, as measured by a capacitance manometer. The induction copper coil, energized by a 13.65 MHz rf power generator (model HFS 2500 D) via a tunable matching network. The samples were supported on a water-cooled copper sample holder and the water cooling rate of the substrate was adjusted to be 3200 cm3/min. The sample temperature was around 500˚C and measured during the rf plasma process by a Chromel-Alumel thermocouple, which was placed close to the surface of the sample. The samples were treated at a fixed plasmaprocessing power of 500 W and for a processing time varied from 4 to 20 minutes. It is important to state that, this treatment process was performed without using any external source of heating. After carbonitriding process, the samples were allowed to cool slowly to the room temperature in the evacuated reactor plasma tube.
2.2. Samples Testing and Characterization
Different techniques have been used to test and characterize the untreated and carbonitrided AISI 321 samples. X-ray diffraction (XRD) using Philips-PW1710 diffractometer with Co Kα radiation of λ = 1.78896 Å was used to characterize the crystallographic configuration of the samples. The XRD scan was run between 40˚ and 100˚, with step interval of 0.02˚ and scan rate of 2˚/min. The treated samples were exposed to the standard metallographic procedure including sectioning, mounting, grinding, polishing and etching. The surface and cross-section morphologies of the untreated and carbonitrided AISI 321 samples were investigated using Olympus BX51 optical microscope.
Vickers microhardness measurements of the untreated and carbonitrided AISI 321 samples were carried out using a Leitz Durimet microhardness tester with a contact load of 100 gmf. The microhardness measurements were performed according to ASTM E384-11 standard test method at temperature of 25˚C ± 3˚C [18]. The microhardness tester has been accredited according to ISO/ IEC 17025:2005 requirements. The wear measurements were performed at room temperature in air atmosphere with humidity of 35% - 40% using an oscillating ball-ondisk type tribometer wear tester without lubrication. The wear measurements were performed according to ASTM G 133-10 standard test method (linearly reciprocating ball-on flat sliding wear). The 3 mm ball of cobalt tungsten carbide moves at a mean sliding speed of 30 mm/s with a normal load of 2 N has been used. During the wear measurements, the friction coefficient was continuously measured by using a force sensor. The oscillating ball-ondisk type tribometer wear tester is accredited according to ISO/IEC 17025:2005 requirements. The surface roughness of the investigated samples was performed using a Form Talysurf 50 which has been accredited according to ISO/IEC 17025:2005 requirements.
The water contact angle measurement, at room temperature, was performed using Phoenix 300 (Contact Angle Analyzer manufactured by S.E.O Co. Ltd). The Phoenix 300 utilized a precision camera and advanced PC technology to capture the static droplet image and calculate the contact angle measurement by Sessile Drop method. The Electrochemical experiments were performed in Ringer’s solution using the potentiodynamic technique at temperature of 25˚C ± 3˚C and humidity of 38% ± 5%. The effective area of samples exposed to corrosive solution was fixed at 0.36 cm2. The test was performed using three-electrodes; silver-silver chloride saturated electrode as a reference electrode, platinum as a counter electrode and the investigated sample as a working electrode. The potential–current corrosion curve is recorded and plotted with potential scan rate of 1 mV/s using Gill AC instrument and ACM program version 5.
In order to investigate the biocompatibility of the untreated and carbonitrided AISI 321 samples, the blood culture and protein adsorption tests have been performed. The blood culture test is performed in RPMI media. Initially, the investigated samples were cleaned by alcohol 90% and sterilized in autoclave (KGemmy FA-260MA) for 40 minutes. The untreated and carbonitrided samples were immersed in the blood in RPMI media for 3 days at temperature of 37˚C. After that, the samples were washed in Phosphate buffer solutions to be prepared for blood culture investigations. For protein adsorption test, the untreated and carbonitrided AISI 321 were immersed in bovine serum albumin (BSA) soluble in phosphate buffer solution at constant PH of 7.4 for one day. The blood culture and protein adsorption for the samples were investigated using Olympus BX51 optical microscopy.
3. Results and Discussion
3.1. Layer Thickness and Carbonitriding Rate
Figure 1 shows the variation of treated layer thickness and carbonitriding rate as a function of plasma-processing time. The carbonitriding thickness is calculated as average value taken from five different positions on the cross-section image. The carbonitriding rate is calculated using the formula of d2/t, where d is the average thickness of the treated layer in μm, and t is the plasma processing time in sec. It is demonstrated from this figure that with an increase in processing time from 4 to 8 min, a significant increase in the layer thickness from 6.9 to 23.3 µm is observed. Afterword, the thickness is slowly increased with a further increase of processing time. The
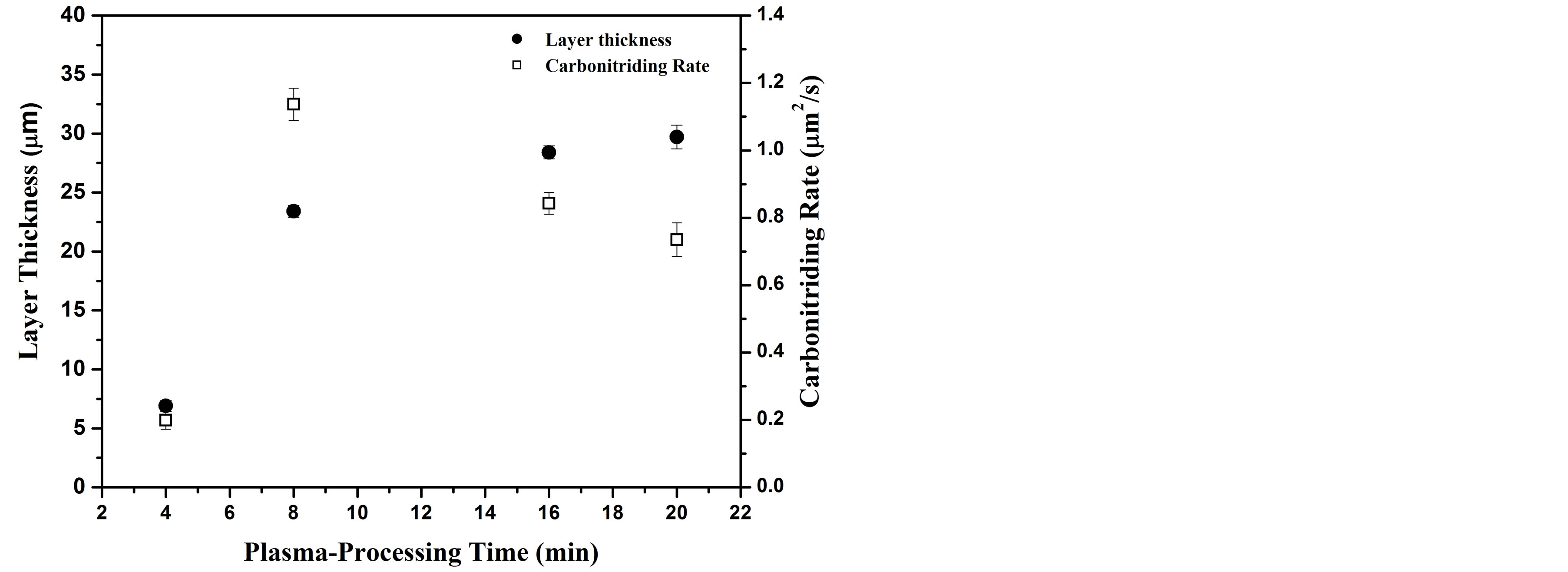
Figure 1. Carbonitriding layer thickness and carbonitriding rate as a function of plasma-processing time.
significant increase in layer thickness is attributed to the large variation in carbonitriding rate (from 0.2 µm2/s to 1.13 µm2/s). Comparable results and carbonitriding mechanism discussion are reported in previous work of plasma surface treatment [1,19,20]. Concentration gradient diffusion, surface porous and microcracks were suggested for interpretation such high rate of carbonitriding. Plasma species diffuses into the bulk substrate and the activated surface creates microcracks and porous (microdefects) [21,22]. These surface microdefects serve as effective channels for the incorporation of reactant plasma species into the surface immersed in plasma environment [19]. Further, the fast incorporation of plasma species through the grain boundaries is also considered as a natural diffusion path. Plasma processing for 8 min, was enough to overcome the barrier of native oxide layer. Moreover, the carbonitriding layer was not thick and dense enough to block the nitrogen-carbon plasma species through the microdefects. However, at longer processing plasma times, the wide and dense carbonitrided layer decreases the effect of microdefects hence the carbonitriding rate should depends mainly on the diffusion process.
3.2. Microstructure Analysis
The XRD patterns of the carbonitrided samples and the as-received substrate are presented in Figure 2. The patterns confirm that the untreated substrate consists mainly of fcc γ-austenitic phase and bcc α-ferritic phase [11,23, 24]. After plasma carbonitriding for 4 min, broaden peak of austenitic phase is observed which results from the interstitial dissolution of carbon atoms in the austenite lattice [25,26]. This phase is identified as carbon expanded austenite (γC) phase, which is observed with a maximum peak shift less than 1%. A similar peak shift of γC-phase
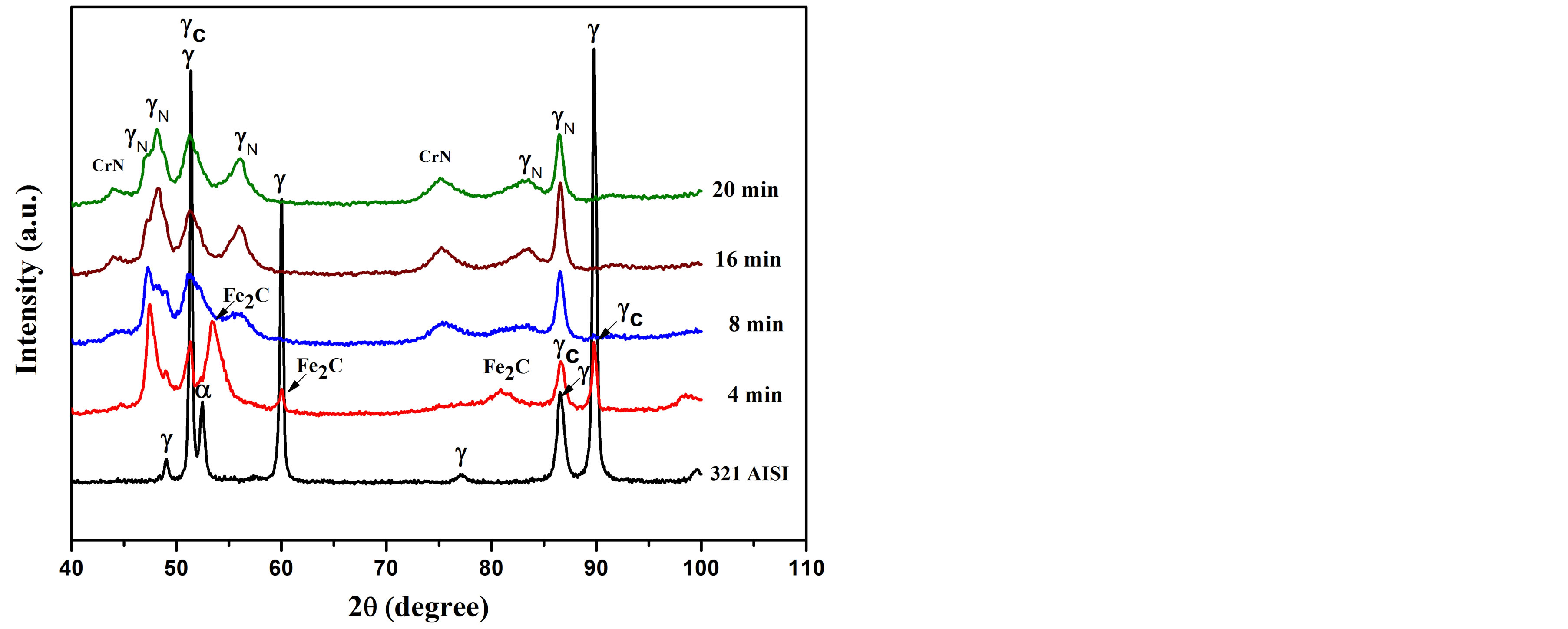
Figure 2. X-ray diffraction patterns of the untreated and carbonitriding AISI 321 using rf plasma for different processing times.
has been observed elsewhere [1,23,26]. Additionally, the treated surface contains a further phase of iron carbide (Fe2C) which is completely disappeared after increasing the plasma-processing times. However, for plasma processing time ≥ 8 min, carbonitriding process induced much peak broadening in the austenite phase. This is owed to the interstitial dissolution of nitrogen atoms instead of carbon in austenite lattice and the phase is called nitrogen expanded austenite (γN). It is combined with a lattice expansion of about 6% compared to the as-received substrate, which was in agreement with that estimated by others [1,23,27]. It has been observed that the intensity of nitrided phases for the sample treated at processing time of 16 min is high. The treated layer formed at 16 and 20 min provides traces amount of CrN phase with a less intense broad peaks compared to previous studies [1,23,28]
3.3. Mechanical and Tribological Properties
3.3.1. Surface Microhardness
Hardness is a material property that is interesting for mechanical applications. It is correlated with wear resistance and other tribological properties. Figure 3 shows the surface microhardness of the untreated and carbonitrided AISI 321 at different plasma-processing times. From this figure one can observe, the microhardness of the untreated sample is 196 HV0.1. After carbonitriding process, the microhardnes of AISI 321 increases incessantly as the plasma-processing time increases up to 16 min to reach a value of 1468 HV0.1. Afterward the microhardness decreases to reach a value of 1141 HV0.1 at a plasmaprocessing time of 20 min. The formation of Fe2C, γN, γC and the precipitation of CrN on the grain boundaries are the main reasons for the increment in the hardness. The sample that was treated at a plasma-processing time of 16 min recorded high intensity of carbide and nitride phases. The low hardness for the sample that was treated
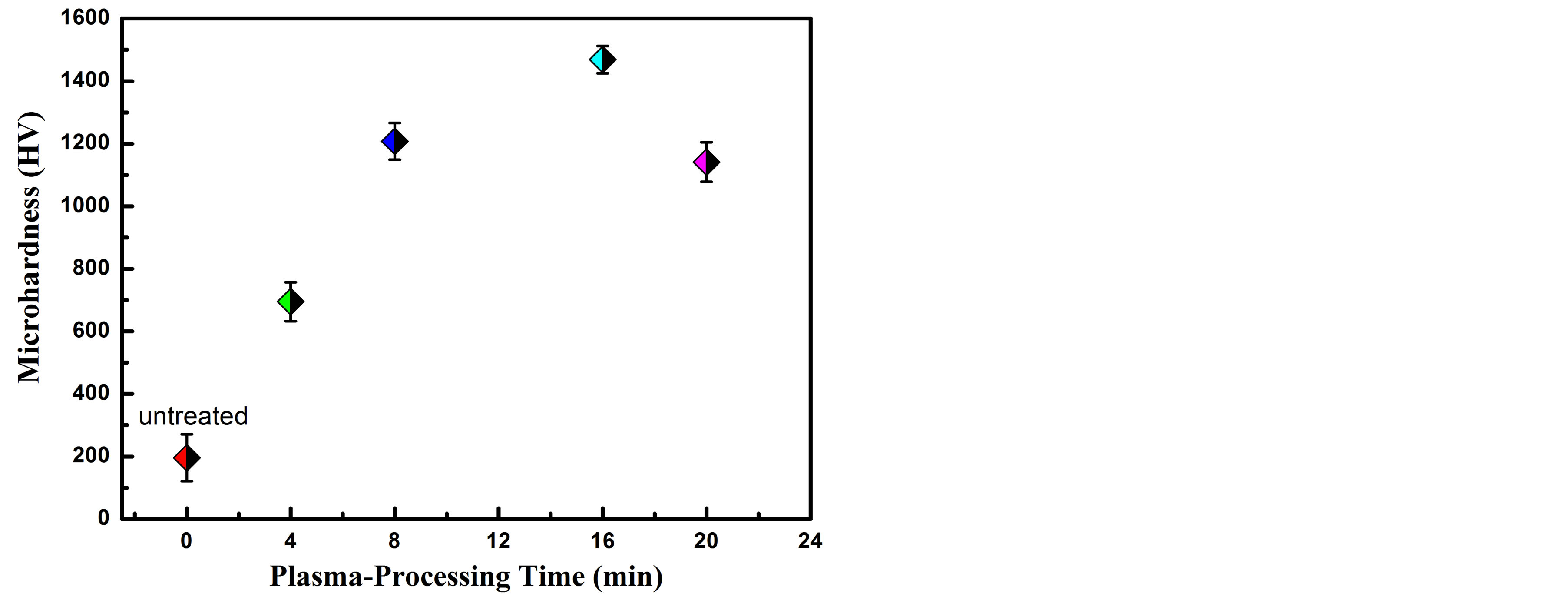
Figure 3. Surface microhardness, 100 gf, versus carbonitriding plasma-processing time.
at a plasma-processing time of 20 min is ascribed to the low intensity of γN and γC phases. The expansion of nitride and carbide phases might block the formed microcracks in the treated layer. Therefore, the penetration rate of nitrogen and carbon species through these microcracks decreases. Consequently, the nitrogen and carbon concentration in the far depth region of the compound layer decreases and the microhardness reduces to lower values [29].
3.3.2. Wear Performance
Figure 4 shows the optical micrograph of the wear track of the untreated and carbonitrided AISI 321 samples at different plasma-processing times. Generally, it has been observed that the track width of carbonitrided samples is narrower than that of the untreated sample, demonstrating the augmentation in wear resistance for the carboni-
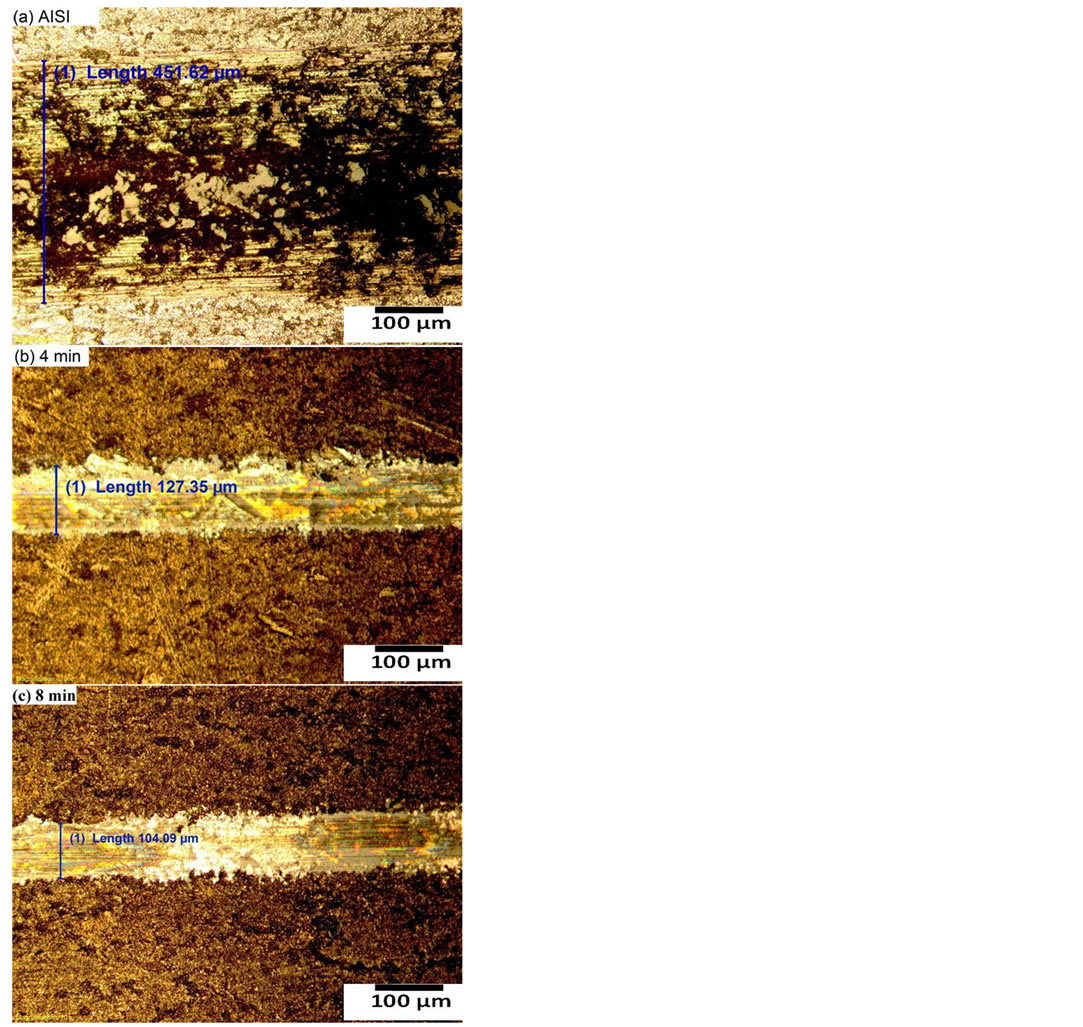
Figure 4. Wear track of (a) untreated and carbonitriding samples for plasma-processing time (b) 4 min and (c) 8 min.
trided samples. It has been reported that, the improvement of mechanical and tribological properties is ascribed to the surface strengthening resulting from the formation of carbon/nitrogen solid solution hard phases and CrN precipitates in the near-surface region [30]. The high hardness of these phases combining with high ductility imparts considerable strength to the surface and accordingly enrichment in wear resistance. During the wear measurements, the recording of the friction coefficient was incessantly measured by using a force sensor. Figure 5 represents the friction coefficient of the untreated and carbonitrided AISI 321 samples at different plasma-processing times. From this figure one can observe that, the friction coefficient for the carbonitrided samples decreases from nearly 0.5 for the untreated sample to nearly 0.25 for all carbonitrided samples; representing a reduction of 50%. The subsistence of a large volume fraction of nitrogen and carbon species modifies the surface composition that accompanied by a reduction in coefficient of friction and enhancement in wear resistance.
3.3.3. Surface Roughness
Figure 6 shows the roughness average (Ra) for the untreated and carbonitrided AISI 321 samples at different plasma-processing time. One can see from this figure, the as–received sample recorded an initial surface roughness of approximately 0.35 µm. The Ra decreased to approximately 0.26 µm for the carbonitrided samples. The formation of CN amorphous layer on the top surface of the carbonitrided samples could be the reason for the decrease in the surface roughness. This layer is previously reported by Abd El-Rahman [1] and acts as a mask for the irregularities on the top surface.
3.3.4. Surface Energy
Figure 7 shows the surface energy and the contact angle
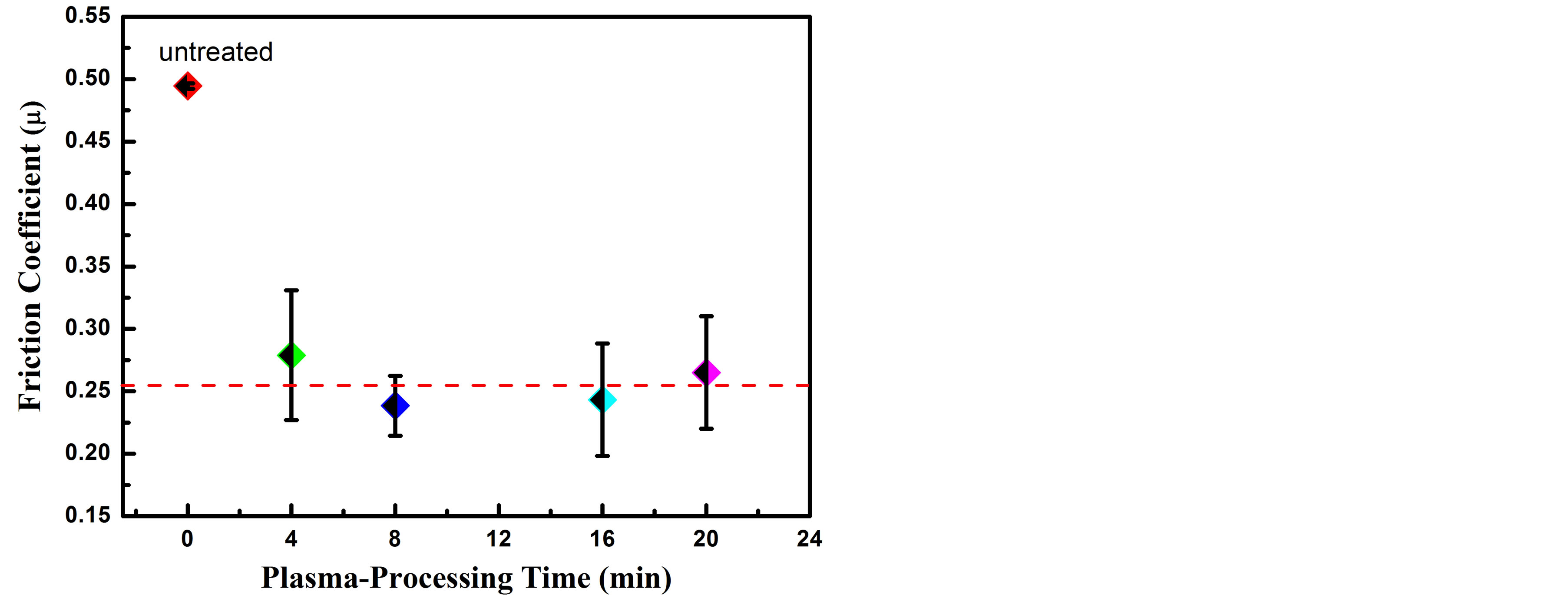
Figure 5. Friction coefficient variation with plasma-processing time of untreated and carbonitrided AISI 321.
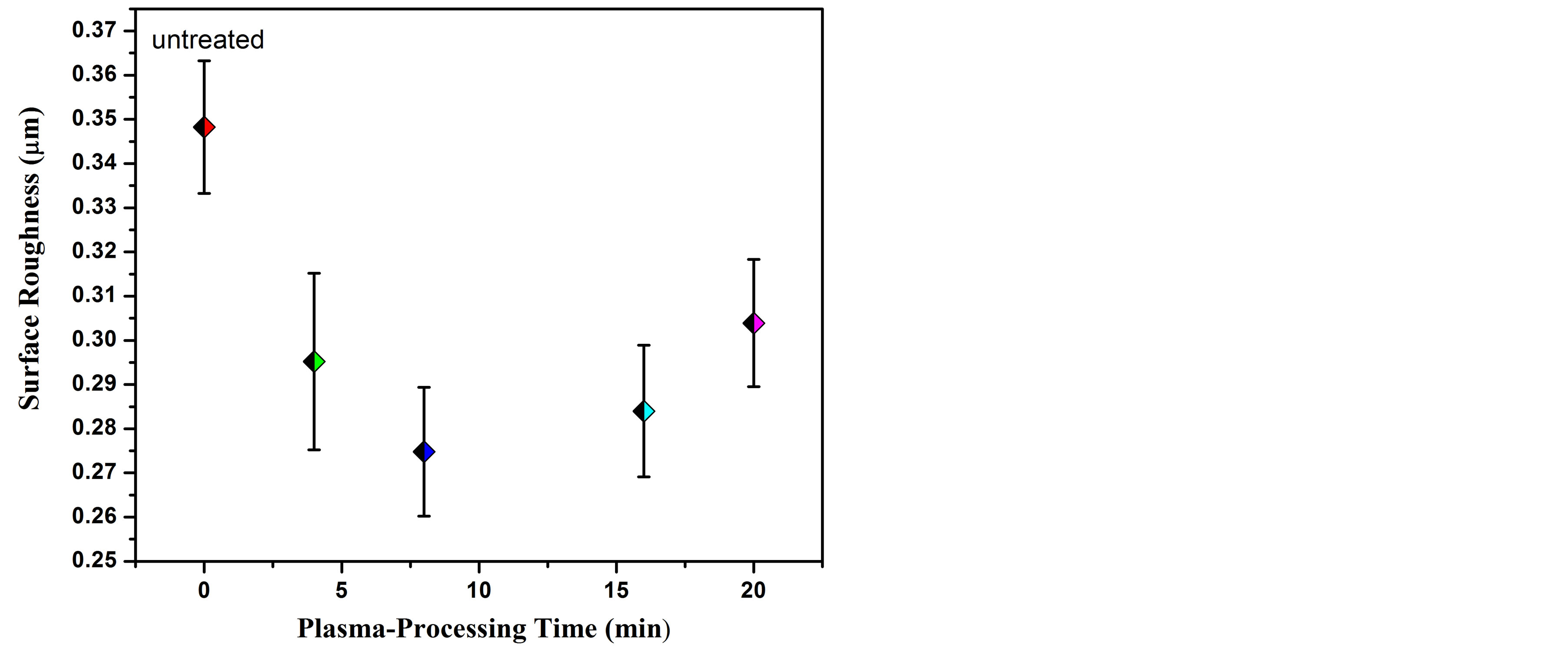
Figure 6. Diagram representing average roughness (Ra) for untreated and treated AISI 321 samples.
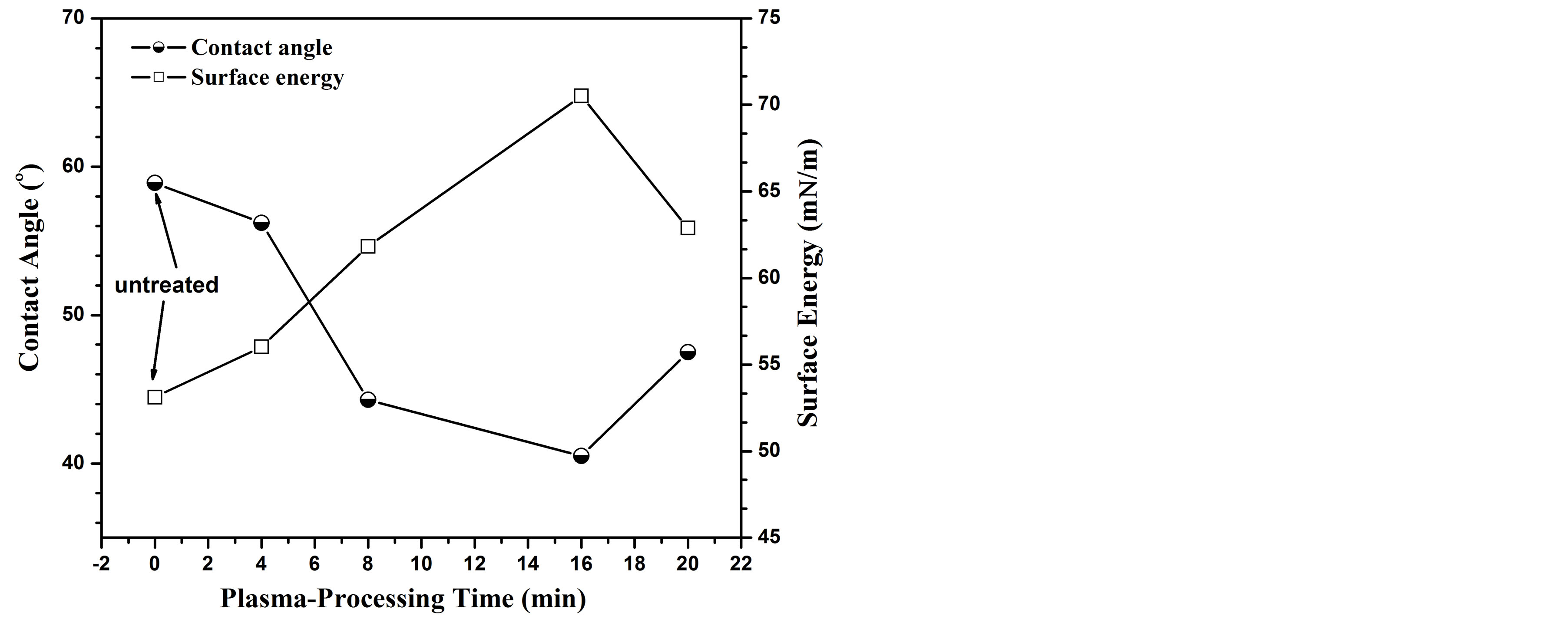
Figure 7. Contact angle and surface energy variation as a function of plasma-processing time.
for the untreated and carbonitrided AISI 321 samples at different plasma-processing times. One can observe from this figure that the surface energy increases as the plasma-processing time increases up to 16 min to reach a value of 70.2 mN/m. After that it decreases to reach a value of 62 mN/m for a plasma processing time of 20 min. The surface wettability has the same behavior of surface energy. The data trend of surface energy has been correlated with the surface microhardness. There are numerous reports demonstrated that the surface energy increases with increasing the surface microhardness [29,31-33]. The formation of Fe2C, γN, γC and CrN hard phases increase the surface strengthening and consequently increase the surface energy.
3.3.5. Corrosion Performance
Figure 8 displays open circuit potential (OCP) versus immersion time for untreated and treated samples in a corrosive medium of Ringer’s solution at room tempera-
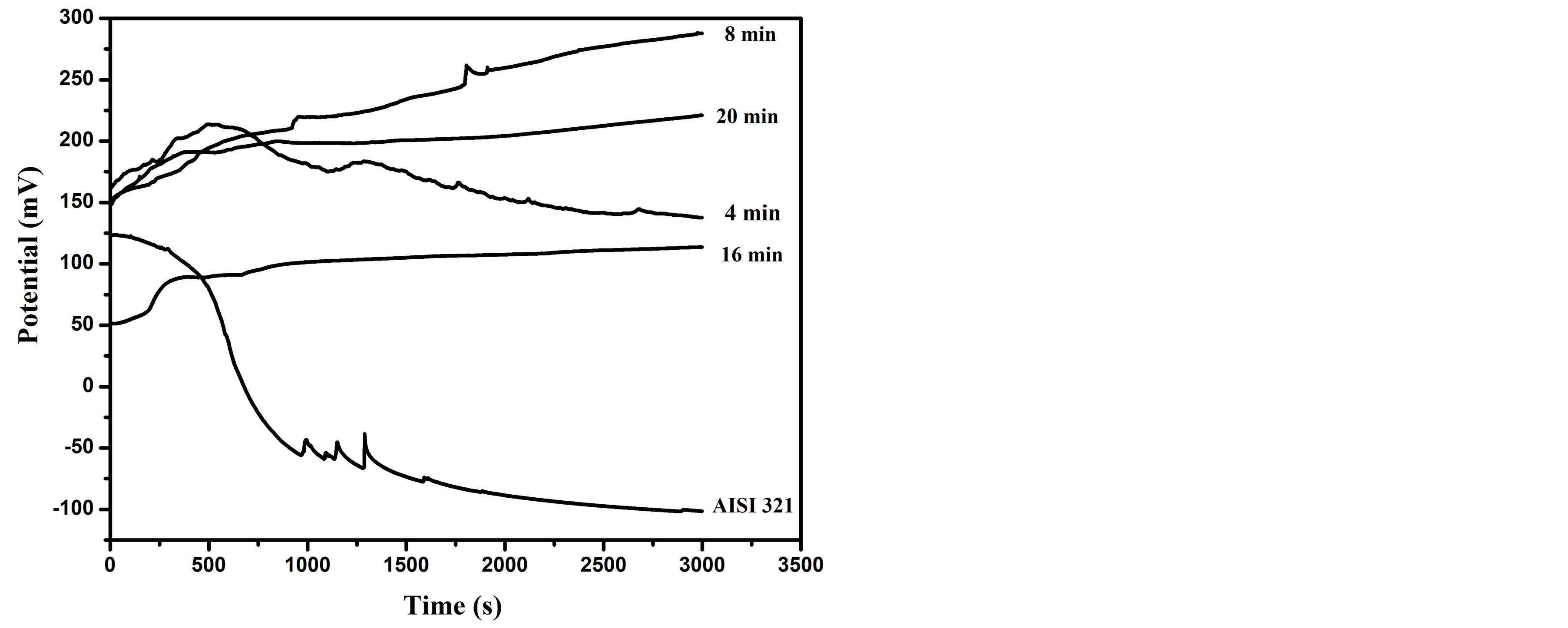
Figure 8. Open circuit potential of untreated and carbonitrided samples; were immersed in Ringer’s solution for 3000 s.
ture. It is measured between the working and reference electrodes when no potential or current is being applied to the cell. The OCP curve is used as a criterion for the characterization of corrosion behaviors. It is presently used to determine approximately the potential at which the metallic surface freely corrodes. The typical value of the corrosion potential can be determined from potentiodynamic polarization tests. As shown in Figure 8, the open circuit potential for the untreated sample decreases gradually with increasing the immersion time up to 400 sec. After that, it rabidly decreases to a negative value of −50 mv. A continues descent can be seen up to a steadystate negative potential of −100 mv with further increase of the immersion time. The rapid decay is owing to the dissolution of a passive film, which is thin (order of nanometer) oxide layer that form on the metal surface [34]. However, reaching a steady potential is an indicator for the formation of a passive film on the surface; giving a protective character to the AISI 321 steel [35]. For the sample treated at processing time of 4 min, the OCP increases up to about 205 mv followed by a decrease with similar behavior to that of the untreated, but with a positive potential around 150 mv. On the other hand, the OCP of the samples treated at higher processing time ˃ 4 min increase with further increase of the immersion time and tend to reach a higher steady state positive potential compared with the untreated one. The higher positive potential values reflecting the formation of strong passive layer with the existence of dense carbonitriding layer consists of nitrogen and carbon solid solutions [36,37]. Moreover, the OCP profile of the sample treated at processing time of 8 min has higher positive shift (136 mv) while the samples treated for 16 and 20 min, have 62 and 70 mv, respectively. Consequently, the sample surface has more noble effect and leads to low corrosion rate and high corrosion resistance.
Potentiodynamic polarization curves of untreated and treated AISI 321 samples immersed in Ringer’s solution are shown in Figure 9. Potentiodynamic polarization curve expresses anodicand cathodic-polarization reactions that can take place on the immersed surface. The corrosion potential and current for all investigated samples are summarized in Table 1. The measured corrosion potentials for the AISI 321 treated samples show more positive values than the untreated, which means that the treated layer needs more energy to initiate the corrosion reaction [38]. The sample treated at processing time of 8 min shows a maximum corrosion potential and a minimum corrosion current in comparison with the other samples. Wen-Ta Tsai and Shyan-Liang Chou have reported that the nitrogen has beneficial effect on improving the corrosion potential [39]. Further, the solid solution γC and γN phases have more passivity than the austenite phase [36,40,41]. It is also observed that the untreated sample suffers from pitting corrosion at high potential of 1000 mv but sample carbonitrided at processing time of 8 min reveals a clear improve in the passive range and achieves high pitting corrosion resistance. On the other hand, traces of CrN phase are detected in the samples treated for 16 and 20 min, leading to a degrada-
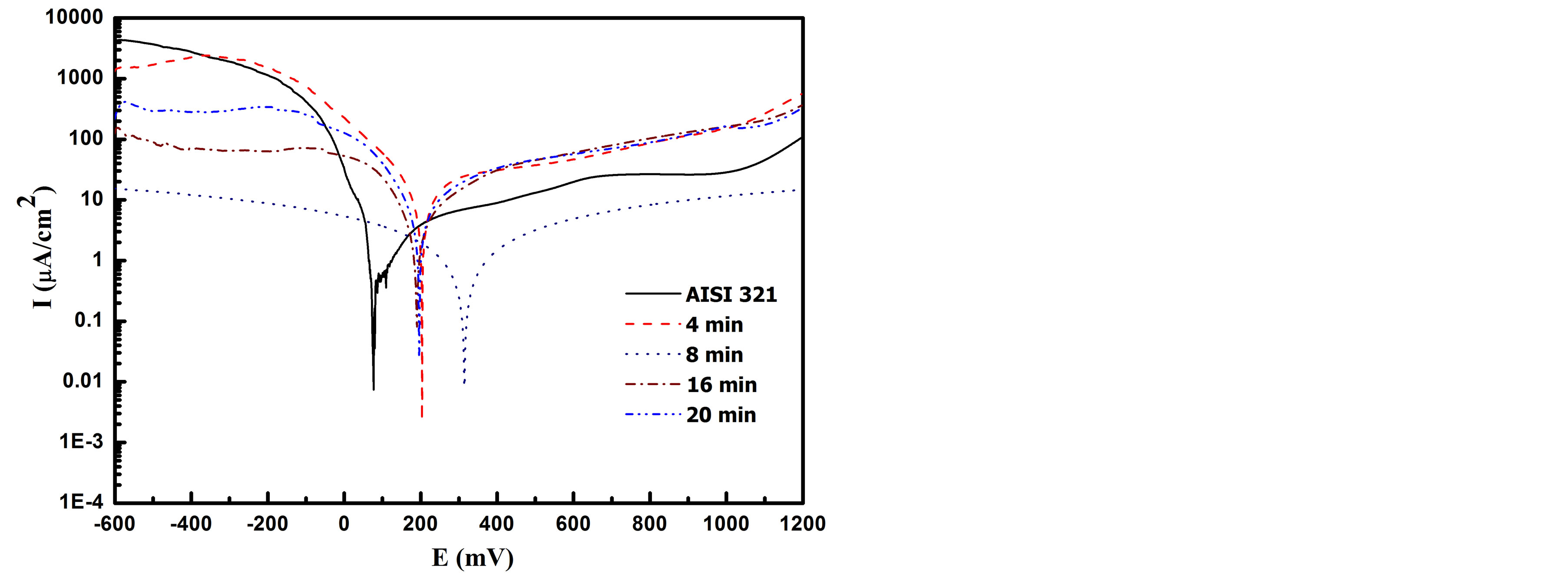
Figure 9. Tafel curves of the untreated and carbonitrided samples for different plasma-processing times measured in Ringer’s solution.
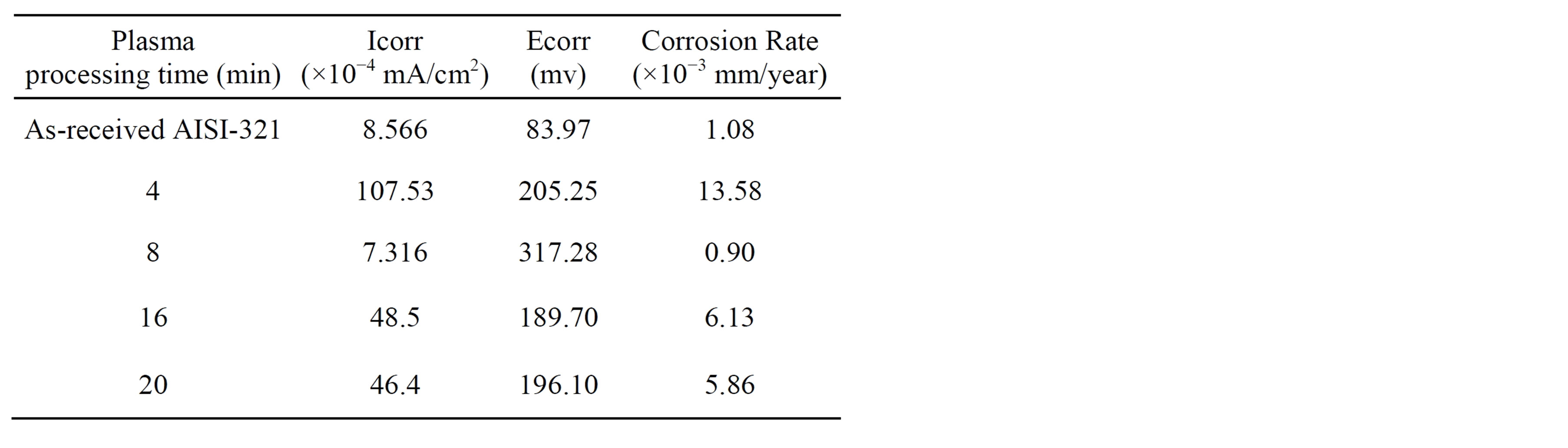
Table 1. Corrosion data for untreated and treated samples investigated in Ringer’s solution.
tion in the corrosion resistance which meets high corrosion current density and low corrosion potential [42,43]. However, the sample treated at 4 min exhibits lowest corrosion resistance which might be ascribed to the difference in the microstructure with the absence of nitrides.
3.4. Biocompatibility Tests
Figures 10 and 11 show the cell culture and protein adsorption on the carbonitrided surfaces for different plasma processing times in comparison with the untreated AISI 321 surface. The untreated surface has not any attracted blood cells or protein adsorption. The low positively surface charge and the relatively low surface energy are the main causes. The surface charge of a solid surface is one of the important factors affecting the adsorption and desorption behaviors of proteins [44]. Once the austenitic substrate is carbonitrided, the surface became more active by increasing its positively surface charge in the phosphate buffer solution; leading to attract more blood cells under the attractive Coulomb force between the positively charged surface and negatively charged blood cells. At these conditions, the modified surface has the ability to capture blood cells from the solution with high adhesion. The same behavior is observed for the protein adsorption as shown in Figure 11.
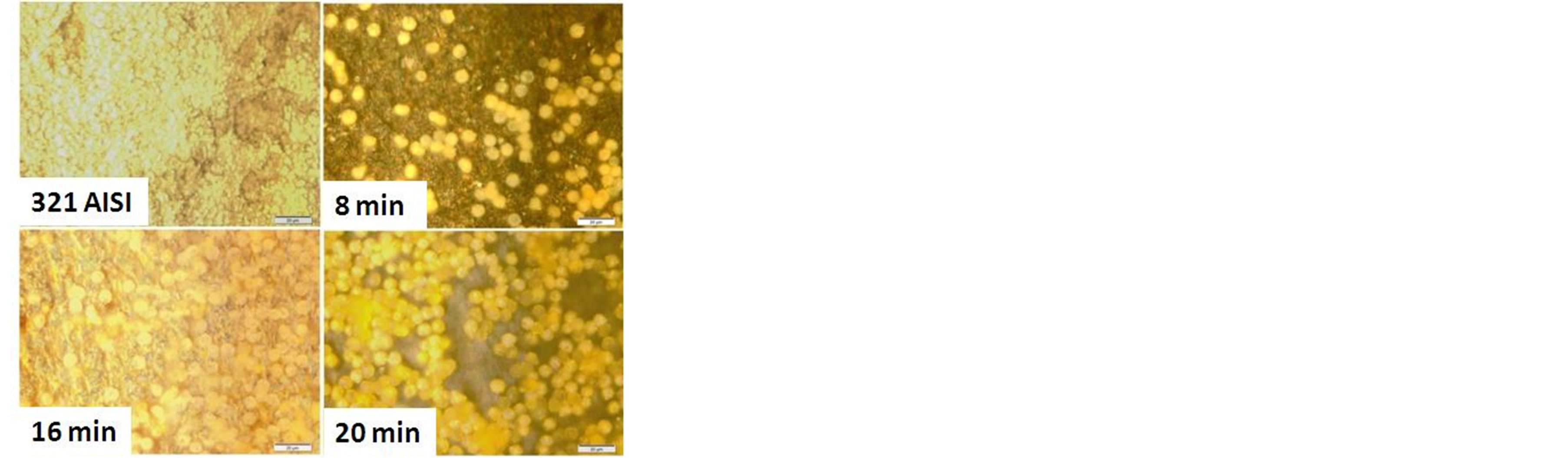
Figure 10. blood cells adhesion on untreated and carbonitrided samples; were immersed in blood culture for 3 days.
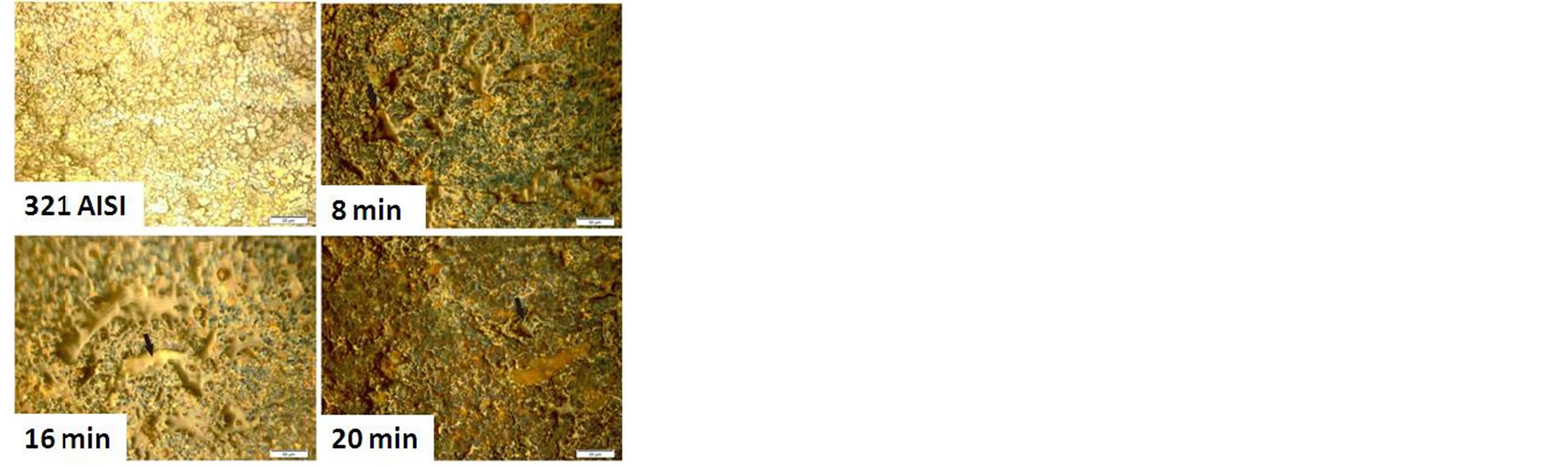
Figure 11. BSA adsorption onto untreated and carbonitrided samples; for one day immersion in phosphate buffer solution at constant PH of 7.4.
As shown in Figure 7, the AISI 321 surface modified for different plasma processing times has a varied contact angles, wettability and surface energy due to changing in the microstructure which provide different manner to cells adhesion and protein adsorption. Further, the existence of CrN precipitates on the surface of the treated samples at higher times lead to increase the number of cell adhesion and density of adsorbed protein [45]. This behavior is typically observed for sample treated at plasma processing time of 16 and 20 min which have higher values of surface energy and wettability compared to other samples.
It is well known that, surface morphology, structure and surface wettability influence on blood cells adhesion and protein adsorption [46]. However, the effect of surface energy has found to be more significant compared to the surface roughness on cellular adhesion strength and proliferation [46]. It means that, samples with high surface roughness not always reflect an improvement in the cell adhesion [47]. Furthermore, the cell adhesion and protein adsorption are found to be much improved by the enhancement of the wettability characteristics of the treated surfaces [46,48].
4. Conclusion
RF plasma carbonitriding achieved a modified surface layer into AISI 321 substrate with a layer thickness varied from 6.5 µm up to 30 µm. It has been found that the nitrogen/carbon solid solutions improve the surface hardness of the treated layer by more than 7 times compared to the untreated one. The tribological performance of the carbonitrided layer has been improved with a significant decrease in the friction coefficient and increase in wear resistance. The contact angle of the modified surface, and thus the wettability can be controlled through the time of the plasma processing. Moreover, the modified microstructure enriched with nitrogen solid solutions led to a relative improvement in the corrosion resistance of sample treated for plasma time of 8 min. Considering the blood cell culture and protein adsorption tests, excellent results were established for the biocompatibility performance of the modified AISI 321 samples in comparison with the untreated one.
Acknowledgements
The work was carried out as part of the research project of “Plasma Technology for Biomedical Applications”. This project was supported financially by the Science and Technology Development Fund (STDF), Egypt, Grant 3894.
REFERENCES
- A. M. Abd El-Rahman, “An Investigation on the Microstructure, Tribological and Corrosion Performance of AISI 321 Stainless Steel Carbonitrided by RF Plasma Process,” Surface and Coatings Technology, Vol. 205, No. 2, 2010, pp. 674-681. http://dx.doi.org/10.1016/j.surfcoat.2010.08.036
- D. A. Moreno, A. M. García, C. Ranninger and B. Molina, “Pitting Corrosion in Austenitic Stainless Steel Water Tanks of Hotel,” Revista De Metalurgia, Vol. 47, No. 6, 2011, pp. 497-506. http://dx.doi.org/10.3989/revmetalm.1146
- K. S. Min and S. W. Nam, “Correlation between Characteristics of Grain Boundary Carbides and Creep-Fatigue Properties in AISI 321 Stainless Steel,” Journal of Nuclear Materials, Vol. 322, 2003, pp. 91-97. http://dx.doi.org/10.1016/S0022-3115(03)00274-5
- Y. F. Liu, J. S. Mu, X. Y. Xu and S. Z. Yang, “Microstructure and Dry-Sliding Wear Properties of TiC-Reinforced Composite Coating Prepared by Plasma-Transferred Arc Weld-Surfacing Process,” Materials Science and Engineering: A, Vol. 458, No. 1-2, 2007, pp. 366-370. http://dx.doi.org/10.1016/j.msea.2006.12.086
- A. K. Roy and V. Virupaksha, “Performance of Alloy 800H for High-Temperature Heat Exchanger Applications,” Materials Science and Engineering: A, Vol. 452- 453, 2007, pp. 665-672. http://dx.doi.org/10.1016/j.msea.2006.11.082
- Y. Okazaki and E. Gotoh, “Metal Release from Stainless Steel, Co-Cr-Mo-Ni-Fe and Ni-Ti Alloys in Vascular Implants,” Corrosion Science, Vol. 50, No. 12, 2008, PP. 3429-3438. http://dx.doi.org/10.1016/j.corsci.2008.09.002
- M. Vallet Regi, I. Izquierdo Barba and F. J. Gil, “Metal Release from Stainless Steel, Co-Cr-Mo-Ni-Fe and Ni-Ti Alloys in Vascular Implants,” Journal of Biomedical Materials Research Part A, Vol. 67A, No. 2, 2003, pp. 674-678. http://dx.doi.org/10.1002/jbm.a.10159
- M. Asgari, A. Barnoush, R. Johnsen and R. Hoel, “Microstructural Characterization of Pulsed Plasma Nitrided 316L Stainless Steel,” Materials Science and Engineering A, Vol. 529, 2011, pp. 425-434. http://dx.doi.org/10.1016/j.msea.2011.09.055
- J. Wang, Y. H. Lin, J. Yan, D. Z. Zen, Q. Zhang, R. B. Huang and H. Y. Fan, “Influence of Time on the Microstructure of AISI 321 Austenitic Stainless Steel in Salt Bath Nitriding,” Surface and Coatings Technology, Vol. 206, No.15, 2012, pp. 3399-3404. http://dx.doi.org/10.1016/j.surfcoat.2012.01.063
- J. Piekoszewski, L. Waliśa and J. Langnerb, “Surface Morphology of Nitrogen-Alloyed Steels Using High Intensity Pulsed Plasma Beams,” Materials Letters, Vol. 32, No. 1, 1997, pp. 49-53. http://dx.doi.org/10.1016/S0167-577X(96)00303-5
- Y. M. Lin, J. Lu, L. P. Wang, T. Xu and Q. J. Xue, “Surface Nanocrystallization by Surface Mechanical Attrition Treatment and Its Effect on Structure and Properties of Plasma Nitrided AISI 321 Stainless Steel,” Acta Materialia, Vol. 54, No. 20, 2006, pp. 5599-5605. http://dx.doi.org/10.1016/j.actamat.2006.08.014
- J. Feugeas, B. Gomez and A. Craievich, “Ion Nitriding of Stainless Steels. Real Time Surface Characterization by Synchrotron X-Ray Diffraction,” Surface and Coatings Technology, Vol. 154, No. 2-3, 2002, pp. 167-175. http://dx.doi.org/10.1016/S0257-8972(02)00017-8
- T. Czerwiec, H. He, S. Weber, C. Dong and H. Michel, “On the Occurrence of Dual Diffusion Layers during Plasma-Assisted Nitriding of Austenitic Stainless Steel,” Surface and Coatings Technology, Vol. 200, No. 18-19, 2006, pp. 5289-5295. http://dx.doi.org/10.1016/j.surfcoat.2005.06.014
- A. Latifi, M. Imani, M. T. Khorasani and M. D. Joupari, “Electrochemical and Chemical Methods for Improving Surface Characteristics of 316L Stainless Steel for Biomedical Applications,” Surface and Coatings Technology, Vol. 221, 2013, pp. 1-12. http://dx.doi.org/10.1016/j.surfcoat.2013.01.020
- N. Hallab, K. Bundy, K. O’Connor, R. L. Moses and J. Jacobs, “Evaluation of Metallic and Polymeric Biomaterial Surface Energy and Surface Roughness Characteristics for Directed Cell Adhesion,” Tissue Engineering, Vol. 7, No. 1, 2001, pp. 55-71. http://dx.doi.org/10.1089/107632700300003297
- F. El-Hossary, N. Z. Negm, S. M. Khalil and A. M. Abd Elrahman, “Formation and Properties of a Carbonitrided layer in 304 Stainless Steel Using Different Radio Frequency Plasma Powers,” Thin Solid Film, Vol. 405, No. 1-2, 2002, pp. 179-185. http://dx.doi.org/10.1016/S0040-6090(01)01729-1
- F. M. El-Hossary, N. Z. Negm, A. M. Abd El-Rahman and M. Hammad, “Duplex Treatment of 304 AISI Stainless Steel Using rf Plasma Nitriding and Carbonitriding,” Materials Science and Engineering: C, Vol. 29, No. 4, 2009, pp. 1167-1173. http://dx.doi.org/10.1016/j.msec.2008.09.049
- ASTM standard E384, ASTM International, West Conshohocken, 2011, PA 19428-2959. http://dx.doi.org/10.1520/E0384-11
- F. M. El-Hossary, “The Influence of Surface Microcracks and Temperature Gradients on the rf Plasma Nitriding Rate,” Surface and Coatings Technology, Vol. 150, No. 2-3, 2002, pp. 277-281. http://dx.doi.org/10.1016/S0257-8972(01)01524-9
- G. G. Tibbetts, “Role of Nitrogen Atoms in Ion-Nitriding,” Journal of Applied Physics, Vol. 45, 1974, p. 5072. http://dx.doi.org/10.1063/1.1663186
- S. Parascandola, O. Kruse and W. Moeller, “The Interplay of Sputtering and Oxidation during Plasma Diffusion Treatment,” Applied Physics Letters, Vol. 75, No. 13, 1999, p. 1851. http://dx.doi.org/10.1063/1.124849
- S. Parascandola, “Nitriding Stainless Steels at Moderate Temperature: Timeand Depth-Resolved Characterization of the Near Surface Composition during the Nitriding Process,” Journal of Vacuum Science & Technology B, Vol. 17, No. 2, 1999, p. 855. http://dx.doi.org/10.1116/1.590650
- A. M. Abd El-Rahman, F. M. El-Hossary, T. Fitz, N. Z. Negm, F. Prokert, M. T. Pham, E. Richter and W. Möller, “Effect of N2 to C2H2 Ratio on r.f. Plasma Surface Treatment of Austenitic Stainless Steel,” Surface and Coatings Technology, Vol. 183, No. 2-3, 2004, p. 268. http://dx.doi.org/10.1016/j.surfcoat.2003.09.057
- C. Blawert, A. Weisheit, B. L. Mordike and R. M. Knoop, “Plasma Immersion Ion Implantation of Stainless Steel: Austenitic Stainless Steel in Comparison to AusteniticFerritic Stainless Steel,” Surface and Coatings Technology, Vol. 85, No. 1-2, 1996, pp. 15-27. http://dx.doi.org/10.1016/0257-8972(96)02880-0
- C. Zhao, C. X. Li, H. Dong and T. Bell, “Low Temperature Plasma Nitrocarburising of AISI 316 Austenitic Stainless Steel,” Surface & Coatings Technology, Vol. 191, No. 2-3, 2005, pp. 195-200. http://dx.doi.org/10.1016/j.surfcoat.2004.03.004
- C. Blawert, B. L. Mordike, G. A. Collins, K. T. Short, Y. Jirásková, O. Schneeweiss and V. Perina, “Characterisation of Duplex Layer Structures Produced by Simultaneous Implantation of Nitrogen and Carbon into Austenitic Stainless Steel X5CrNi189,” Surface and Coatings Technology, Vol. 128-129, 2000, pp. 219-225. http://dx.doi.org/10.1016/S0257-8972(00)00651-4
- A. M. Abd El-Rahman, S. H. Mohamed, M. R. Ahmed, E. Richter and F. Prokert, “Nitrocarburizing of AISI-304 Stainless Steel Using High-Voltage Plasma Immersion Ion Implantation,” Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, Vol. 267, No. 10, 2009, pp. 1792-1796. http://dx.doi.org/10.1016/j.nimb.2009.03.078
- S. Mändl, E. Günzel, E. Richter and W. Möller, “Nitriding of Austenitic Stainless Steels Using Plasma Immersion Ion Implantation,” Surface and Coatings Technology, Vol. 100-101, 1998, pp. 372-376. http://dx.doi.org/10.1016/S0257-8972(97)00651-8
- M. Raaif, F. M. El-Hossary, N. Z. Negm, S. M. Khalil and P. Schaaf, “Surface Treatment of Ti-6Al-4V Alloy by rf Plasma Nitriding,” Journal of Physics: Condensed Matter, Vol. 19, No. 39, 2007, Article ID: 396003. http://dx.doi.org/10.1088/0953-8984/19/39/396003
- V. F. Terent’ev, М. S. Мichugina, A. G. Kolmakov, V. Kvedaras, V. Čiuplys, A. Čiuplys and J. Vilys, “The Effect of Nitriding on Fatigue Strength of Structural Alloys,” Mechanika, Vol. 64, No. 2, 2007, pp. 12-24.
- K. Hirao and M. Tomozawa, “Characterization of Different Materials for Corrosion Resistance under Simulated Body Fluid Conditions,” Journal of the American Ceramic Society, Vol. 70, No. 7, 1987, pp. 497-502. http://dx.doi.org/10.1111/j.1151-2916.1987.tb05683.x
- F. Froehlich, P. Grau and W. Grellmann, “Performance and Analysis of Recording Microhardness Tests,” Physica Status Solidi (A), Vol. 42, No. 1, 1977, pp. 79-89. http://dx.doi.org/10.1002/pssa.2210420106
- G. H. Frischat, “Strength of Inorganic Glass,” Plenum, New York, 1985, p. 135.
- Z. J. Zheng, Y. Gao, Y. Gui and M. Zhu, “Characterization of Different Materials for Corrosion Resistance under Simulated Body Fluid Conditions,” Corrosion Science, Vol. 54, 2012, pp. 60-67. http://dx.doi.org/10.1016/j.corsci.2011.08.049
- I Gurappa, “Characterization of Different Materials for Corrosion Resistance under Simulated Body Fluid Conditions,” Materials Characterization, Vol. 49, No. 1, 2002, pp. 73-79. http://dx.doi.org/10.1016/S1044-5803(02)00320-0
- H. Dong, P. Y. Qi, X. Y. Li and R. J. Llewellyn, “Improving the Erosion-Corrosion Resistance of AISI 316 Austenitic Stainless Steel by Low-Temperature Plasma Surface Alloying with N and C,” Materials Science and Engineering A, Vol. 431, No. 1-2, 2006, pp. 137-145. http://dx.doi.org/10.1016/j.msea.2006.05.122
- D. Starosvetsky and I. Gotman, “Corrosion Behavior of Titanium Nitride Coated Ni-Ti Shape Memory Surgical Alloy,” Biomaterials, Vol. 22, No. 13, 2001, pp. 1853- 1859. http://dx.doi.org/10.1016/S0142-9612(00)00368-9
- C. H. Huang, J. C. Huang, J. B. Li and J. S. C. Jang, “Simulated Body Fluid Electrochemical Response of Zr-Based Metallic Glasses with Different Degrees of Crystallization,” Materials Science and Engineering C, Vol. 33, No. 7, 2013, pp. 4183-4187. http://dx.doi.org/10.1016/j.msec.2013.06.007
- W. T. Tsai and S. L. Chou, “Environmentally Assisted Cracking Behavior of Duplex Stainless Steel in Concentrated Sodium Chloride Solution,” Corrosion Science, Vol. 42, No. 10, 2000, pp. 1741-1762. http://dx.doi.org/10.1016/S0010-938X(00)00029-9
- M. Esfandiari and H. Dong, “The Corrosion and Corrosion-Wear Behaviour of Plasma Nitrided 17-4PH Precipitation Hardening Stainless Steel,” Surface & Coatings Technology, Vol. 202, No. 3, 2007, pp. 466-478. http://dx.doi.org/10.1016/j.surfcoat.2007.06.069
- L. Wang, B. Xu, Z. W. Yu and Y. Q. Shi, “The Wear and Corrosion Properties of Stainless Steel Nitrided by LowPressure Plasma-Arc Source Ion Nitriding at Low Temperatures,” Surface and Coatings Technology, Vol. 130, No. 2-3, 2000, pp. 304-308. http://dx.doi.org/10.1016/S0257-8972(00)00713-1
- A. Fossati, F. Borgioli, E. Galvanetto and T. Bacci, “Corrosion Resistance Properties of Glow-Discharge Nitrided AISI 316L Austenitic Stainless Steel in NaCl Solutions,” Corrosion Science, Vol. 48, No. 6, 2006, pp. 1513-1527. http://dx.doi.org/10.1016/j.corsci.2005.06.006
- L. Wang, “Surface Modification of AISI 304 Austenitic Stainless Steel by Plasma Nitriding,” Applied Surface Science, Vol. 211, No. 1-4, 2003, pp. 308-314. http://dx.doi.org/10.1016/S0169-4332(03)00260-5
- K. Takahashi and S. Fukuzaki, “Cleanability of Titanium and Stainless Steel Particles in Relation to Surface Charge Aspects,” Biocontrol Science, Vol. 13, No. 1, 2008, pp. 9-16. http://dx.doi.org/10.4265/bio.13.9
- K. Bordji, J. Y. Jouzeau, D. Mainard, E. Payan, J. P. Delagoutte and P. Netter, “Evaluation of the Effect of Three Surface Treatments on the Biocompatibility of 316L Stainless Steel Using Human Differentiated Cells,” Biomaterials, Vol. 17, No. 5, 1996, pp. 491-500. http://dx.doi.org/10.1016/0142-9612(96)82723-2
- L. Hao, J. Lawrence, Y. F. Phua, K. S. Chian, G. C. Lim and H. Y. Zheng, “Enhanced Human Osteoblast Cell Adhesion and Proliferation on 316 LS Stainless Steel by Means of CO2 Laser Surface Treatment,” Journal of Biomedical Materials Research-Part B Applied Biomaterials, Vol. 73, No. 1, 2005, pp. 148-156. http://dx.doi.org/10.1002/jbm.b.30194
- G. C. L. B. Neto, M. A. M. da Silva and C. Alves, “In Vitro Study of Cell Behaviour on Plasma Surface Modified Titanium,” Surface Engineering, Vol. 25, No. 2, 2009, pp. 146-150. http://dx.doi.org/10.1179/174329408X271561
- A. Waterhouse, Y. B. Yin, S. G. Wise, D. V. Bax, D. R. McKenzie, M. M. M. Bilek, A. S. Weiss and M. K. C. Ng, “The Immobilization of Recombinant Human Tropoelastin on Metals Using a Plasma-Activated Coating to Improve the Biocompatibility of Coronary Stents,” Biomaterials, Vol. 31, No. 32, 2010, pp. 8332-8340. http://dx.doi.org/10.1016/j.biomaterials.2010.07.062

