Open Journal of Psychiatry
Vol.3 No.2(2013), Article ID:29645,7 pages DOI:10.4236/ojpsych.2013.32020
Comparison of adults with attention-deficit/hyperactivity disorder versus Asperger’s disorder using the WAIS-R
![]()
Fukushima Medical University, School of Medicine, Fukushima, Japan
Email: *mashiko@fmu.ac.jp
Copyright © 2013 Yasuko Takanashi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 10 February 2013; revised 12 March 2013; accepted 21 March 2013
Keywords: Attention-Deficit/Hyperactivity Disorder (AD/HD); Asperger’s Disorder (AD); Wechsler Adult Intelligence Scale-Revised (WAIS-R); Adult; High-Functioning
ABSTRACT
Objective: The present study compared results on the Wechsler Adult Intelligence Scale-Revised (WAIS-R) among adult patients with attention-deficit/hyperactivity disorder (AD/HD) and those with Asperger’s disorder (AD). Method: WAIS-R results were compared between 16 adults with AD/HD (8 men and 8 women; mean age, 33.81 years; mean full-scale IQ, 101.5) and 15 adults with AD (12 men and 3 women; mean age, 30.93 years; mean full-scale IQ, 104.6). Results: Verbal IQ was significantly higher than performance IQ in the AD group. Among various subtests, scores were the highest for similarities in the AD/HD group and for block design in the AD group. Picture completion test scores were the lowest scores obtained in both groups. A comparison of subtest scores between the AD/HD and AD groups showed scores for information to be significantly higher in the AD group than in the AD/HD group. Conclusions: Our results suggest that there are no differences in verbal IQ, performance IQ, and full-scale IQ scores (except for scores on the information subtest) among adult patients with AD/HD compared with adult patients with AD.
1. INTRODUCTION
At the end of the twentieth century, a series of publications, feature programs, and articles on attention-deficit/ hyperactivity disorder (AD/HD) began to appear within the Japanese media. These programs sparked a rapid increase in the number of adults visiting medical facilities because they suspected they had AD/HD. An increase in adults seeking medical advice based on the suspicion that they have Asperger’s disorder (AD) has also been observed recently.
Actually, some individuals with AD/HD remain undiagnosed during childhood and adolescence because of high IQ, compliant behaviors, interpersonal charm, structured and tolerant home and school environments, and learned coping strategies [1,2]. Unlike children, in whom a high degree of hyperactivity or impulsivity at school is likely to annoy other people and result in visits to a medical doctor, adult patients often seek medical attention due to self-identified problems. These can include frequent errors/inability to fulfill work requirements or difficulties in interpersonal relationships.
Also early cognitive and language skills are not significantly delayed in AD, in contrast to autistic disorders, and mental retardation is not usually observed in AD [3]. Because of these characteristic features, AD, or ADrelated problems often go unnoticed during routine health check-ups of infants, in contrast to those with autistic disorders. Therefore, like a group of the AD/HD which mentioned above, it is not rare for AD patients to fail to attract medical attention during childhood, and to visit medical facilities only after recognizing their own poor social adjustment or as a result of developing secondary mental disorders in adolescence or adulthood.
Besides symptoms of over-activity and inattention are frequent in AD, and many individuals with these symptoms receive a diagnosis of AD/HD before a diagnosis of AD [3]. As with HFPDD, individuals with AD also experience symptoms common to AD/HD [4-7], including problems arising from hyperactivity, impulsivity, or inattention. It is actually reported that HFPDD might be confused with AD/HD [8], therefore it is required to establish effective clues for distinguishing PDD from AD/HD.
Based on the type of concerns that lead individuals to seek treatment as adults, it is likely that individuals given medical attention in childhood might differ from those who did not undergo medical evaluations until adolescence or adulthood. Therefore, in comparing AD/HD with PDD (especially HFPDD and AD), adults as well as children should be recruited.
For the purpose of making an adequate diagnosis of AD/HD and AD, it might be important to investigate the neuropsychological differences between AD/HD and AD. There are some reports that compared the difference of intelligence between AD/HD and AD in childhood [9- 11]. To our knowledge, however, no studies exist comparing the Wechsler Intelligence Scale (WAIS) profiles of adult patients with AD/HD and those with AD. The present study was designed to compare groups of adult patients with AD/HD and adult patients with AD in terms of WAIS-Revised (WAIS-R) scores. We expect the WAIS-R to delineate possible neuropsychological differences between the two groups.
2. METHODS
2.1. Subjects
Study subjects included 31 patients where objective data from childhood were available. None the subjects had been diagnosed with a developmental disorder during infancy or childhood. All had been diagnosed with AD/HD or AD after reaching adulthood. Subjects were chosen among patients 18 years of age or older, but younger than 60 years, who first visited the Department of Neuropsychiatry of Fukushima Medical University Hospital between January 2005 and August 2007. Because mental retardation is not generally observed in patients with AD, only patients with AD/HD with no evidence of mental retardation were included. All patients demonstrated WAIS-R full-scale IQs of 80 or higher, indicating that no patient suffered from mental retardation. At the time of the WAIS-R tests, 10 patients were already receiving psychotropic drug therapy and 21 were not. Written informed consent was obtained from all subjects.
The 31 patients included 16 with AD/HD (AD/HD group; 8 men and 8 women; mean age = 33.81, SD = 6.46 years; mean education = 13.12. SD = 1.46 years; 5 receiving psychotropic drug therapy) and 15 with AD (AD group; 12 men and 3 women; mean age = 30.93, SD = 7.43 years; mean education = 13.53, SD = 2.50 years; 5 receiving psychotropic drug therapy). There were no statistically significant differences in age, sex ratio, or mean education between the groups. The AD/HD subtypes in the AD/HD group were the combined type in 9 patients (56%), the predominantly inattentive type in 6
(38%), and the hyperactive-impulsive type in 1 (6%).
Seven patients (47%) met diagnostic criteria for AD/HD according to the DSM-IV-TR for all items excluding: symptoms do not occur exclusively during the course of a PDD, schizophrenia, or other psychotic disorder and are not better accounted for by another mental disorder. One patient (7%) met the criteria for combined type AD/HD, 4 patients (27%) met criteria for the predominantly inattentive type, and 2 (13%) met the criteria for the hyperactive-impulsive type. All subjects gave written consent to use their demographic and clinical data as well as examine their IQ results for the present study.
2.2. Diagnosis
Diagnoses of AD/HD and AD were made according to the DSM-IV-TR. As mentioned, the DSM-IV-TR [3] diagnostic criteria for AD/HD specify that symptoms do not occur exclusively during the course of a PDD, schizophrenia, or other psychotic disorder and are not better accounted for by another mental disorder. At the same time, many patients with PDD have clinical symptoms similar to those of AD/HD. Therefore, particular caution is necessary when a patient presents with a chief complaint of AD/HD-related symptoms. Various features including incomplete agreement with diagnostic criteria for PDD must be taken into consideration. The growth of the patient during childhood and, in AD/HD cases in particular, the presence/absence of difficulties due to AD/HD symptoms before the age of 7 should be reviewed using medical records and other sources to confirm a diagnosis. However, for adult patients seeking medical consultation because they suspect that they might have AD/HD or AD, it is often difficult to obtain objective information about developmental details during childhood. However, such information is indispensable for establishing a diagnosis. Therefore, only patients for whom relevant objective information was available were included in the present study. This was based on information provided by the parent(s) in the form of an interview, the mother-and-child health handbook, report cards, etc., as well as information obtained from patients during an interview.
2.3. Instruments
The Japanese WAIS-R was used in this study. This is a standardized Japanese version of the US WAIS-R and maintains the same basic framework as the original.
2.4. Statistical Analysis
Because IQ scores of each type and subtest score of the WAIS-R had a normal distribution in both the AD/HD and AD groups, the t-test was used for comparisons between groups. Verbal IQ and performance IQ scores were compared within each group using the paired t-test. Scores on subtests, classified as verbal or performance subtests, were subjected to multiple comparisons by Tukey’s method after conducting a one-way analysis of variance.
The subtest score profiles in each group were examined in terms of standard deviation (SD) ratios in line with Kaufman’s profile interpretation technique [12]. We determined SD ratios in the following manner: subtests were classified as verbal or performance subtests, and mean scores and SD values were obtained separately for each. Next, the SD ratio was calculated for each subtest item for individual patients in terms of the difference between each subtest score and the corresponding mean subtest score divided by the SD of the corresponding subtest group. Using the obtained SD ratio values for individual patients, mean SD ratio values for respective subtest items were calculated for the AD/HD group and the AD group.
It was assumed that the range of subtest scores for individual patients in the AD/HD and AD groups (e.g. the difference between the highest and lowest scores in the subtest items for individual patients) would be homoscedastic in the classifications of verbal, performance, and overall subtests. Therefore, differences between these classifications were analyzed using the t-test. SPSS 11.5 J for Windows (SPSS Co., Ltd., Tokyo) was used for statistical analysis; the statistical significance level was set at p < 0.05.
3. RESULTS
3.1. IQ Values and Subtest Scores
The IQ values and results of subtests for both groups are shown in Table 1. In the AD/HD group, the mean verbal IQ was 102.31 (range, 92 - 117), the mean performance IQ was 99.81 (range, 78 - 119), and the mean overall full-scale IQ was 101.50 (range, 85 - 115). The corresponding IQ values in the AD group were 108.13 (range, 84 - 128), 99.13 (range, 78 - 118), and 104.60 (range, 80 - 124). All IQ values were at average levels in both the AD/HD and the AD groups.
There were no significant differences in any type of IQ between the AD/HD and AD groups. Comparisons of verbal IQ and performance IQ within each group showed that verbal IQ was significantly higher than performance IQ in the AD group (Table 1; t(14) = 3.98, p < 0.005). For the Japanese version of the WAIS-R, when determining discrepancies between verbal IQ and performance IQ, 11.31 points are required for significance at a 95% confidence level among those 16 - 74 years of age. Conversely, 8.31 points are required for significance at an 85% confidence level among those 16 - 74 years of age. When verbal IQ-performance discrepancies in our patients were examined according to these criteria, 9 patients (56%) in the AD/HD group and 9 (60%) in the AD group reached the 85% confidence level. Six patients (37.5%) in the AD/HD group and seven (46.7%) in the AD group met the 95% confidence level criteria.
Nine patients (56%) in the AD/HD group and 14 (93%) in the AD group had higher verbal IQ values than performance IQ values. Analysis using Fisher’s exact tests showed the percentage of patients with a verbal IQ superior to their performance IQ was significantly higher in the AD group than in the AD/HD group (p < 0.04, two-tailed).
3.2. Comparison of Subtest Scores
Among various subtests (as shown in Table 1), information scores were significantly higher in the AD group than in the AD/HD group, t(29) = 3.06, p < 0.01. Scores were highest for similarities in the AD/HD group and for block design in the AD group. Picture completion test scores were the lowest scores obtained in both groups.
Test scores for verbal subtests and performance subtests were examined by one-way analysis of variance separately for the AD/HD and AD groups. Results showed that verbal subtest parameters, F(5,90) = 3.14, p < 0.05, in the AD/HD group and performance subtest parameters in the AD group, F(4,70) = 2.90, p < 0.05, were significantly different. Multiple comparisons using Tukey’s method revealed scores to be significantly lower for information (p < 0.05) and arithmetic (p < 0.05) than for similarities in the AD/HD group. Picture completion was associated with lower scores than block design in the AD group (p < 0.05).
3.3. Discrepancies of Subtest Scores
The ranges of subtest scores (the discrepancies between the highest and lowest subtest scores) among individual patients in the AD/HD and AD groups were examined in relation to the classifications of verbal subtests, performance subtests, and overall subtests (Table 2). A comparison of the AD/HD and AD groups revealed that there were greater discrepancies in scores on performance subtests, t(29) = 2.91, p < 0.01, and overall subtests, t(29) = 2.57, p < 0.05, in the AD group than in the AD/HD group.
3.4. Subtest Score Profiles
The results of analyzing subtest score profiles for the AD/HD and AD groups are shown in Table 3. Deviations in performance IQ and verbal IQ subtest scores were examined using SD ratios. In this study, an SD ratio < −0.5 was defined as −−, −0.5 £ SD ratio < 0 as −, SD ratio = 0 as 0, 0 < SD ratio £ 0.5 as +, and 0.5 < SD
Table 1. IQ values and subtest scores in Adult AD/HD and Asperger’s disorder groups.
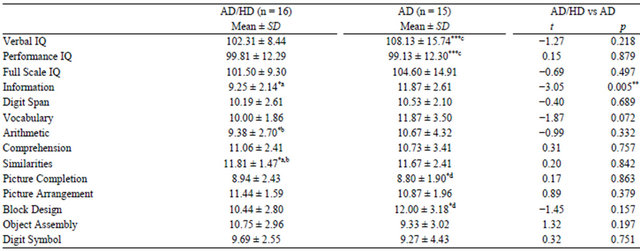
Comoarisons were made between verbal and performance IQs and among subtest scores for each subject group. Comparisons were also made between AD/HD and Asperger’s disorder groups for verbal, performance and full scale IQs as well as all subtest scores. (*p < 0.05; **p < 0.01; ***p < 0.005; a, b, c and d represent compared pairs, respectively). IQ, intelligent quotient; AD/HD, attention-deficit/ hyperactivity disorder; SD, standard deviation; AD, Asperger’s disorder.
Table 2. Range of subtest scores for all subtests, performance subtests, and verbal subtests of WAIS-R.

Subtest scores are expressed as average ± SD. Comparisons were made between AD/HD and Asperger’s disorders groups. (**p < 0.01; *p < 0.05; a and b represent compared pairs, respectively). WAIS-R, Wechsler adult intelligent scale-revised; AD/HD, attention-deficit/hyperactivity disorder; AD, Asperger’s disorder; SD, standard deviation.
Table 3. Deviations in verbal and performance IQ subtest scores of WAIS-R as expressed by SD ratios.
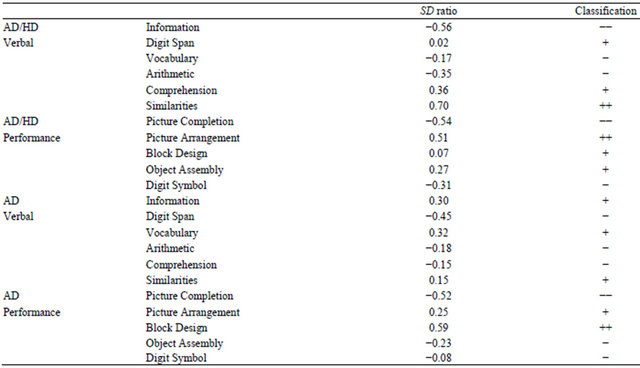
Refer to statistical analysis section for details of SD ratios. Deviations in performance IQ and verbal IQ subtest scores were examined using SD ratios. In this study, an SD ratio < −0.5 was defined as −−, −0.5 £ SD ratio < 0 as −, SD ratio = 0 as 0, 0 < SD ratio £ 0.5 as +, and 0.5 < SD ratio as ++. IQ, intelligent quotient; WAIS-R, Wechsler adult intelligent scale; SD, standard deviation; AD/HD, attention-deficit/hyperactivity disorder; AD, Asperger’s disorder.
ratio as ++. Using these classifications, variations in the scores of subtests among individual patients were examined separately for the AD/HD and AD groups. High scores for similarities and picture arrangement and low scores for information and picture completion were evident in the AD/HD group. Conversely, high scores for block design and low scores for picture completion characterized the AD group.
4. DISCUSSION
To our knowledge, this is the first study to clarify similarities and differences between adult patients with AD/HD and those with AD using the WAIS-R profile. The lack of differences in IQ values (performance, verbal, and full scale) between these two groups indicates that there is no marked difference in overall intelligence between adult patients with AD/HD and those with AD. However, these two groups showed different intellectual trends. Patients with AD/HD demonstrated no significant differences between verbal IQ and performance IQ, whereas verbal IQ was significantly higher than performance IQ in patients with AD. A distinct verbal IQperformance IQ discrepancy at the 95% confidence level was observed in 6 patients (37.5%) in the AD/HD group and 7 patients (46.7%) in the AD group. Conversely, the present study demonstrated that the percentage of patients with verbal IQ scores higher than their performance IQ scores was significantly larger in the AD group than in the AD/HD group.
4.1. Comparison of Subtest Results between the AD/HD and AD Groups
Comparisons of the two groups on subtest scores revealed higher scores for information and higher scores for vocabulary in the AD as compared to the AD/HD group. We used Kaufman and colleagues’ interpretations of WAIS-R profiles as the basis for our discussion given that they are noted experts on cognitive functioning [12]. When examining the high scores for information and vocabulary subtests together, it is possible that memory and information performances are better in the AD group than in the AD/HD group. Given that “intellectual curiosity and striving” and “richness of early environment” are factors that influence subtest scores, these results may reflect the differences between patients with AD/HD and those with AD in terms of endurance and how to express interest.
Discrepancies among subtest scores required for reaching the 85% confidence level on the Japanese WAIS-R range from 1.17 to 4.24, with a mean of 2.39. In the present study, the mean maximum discrepancies among all subtest scores were high in both the AD/HD (M = 7.00, SD = 1.63) and AD groups (M = 8.53, SD =
1.68). This suggests that there may be a greater imbalance among subtest scores in the AD/HD and AD groups than in the general population. In addition, when the AD/HD and AD groups were compared with each other, greater discrepancies in scores for both the performance subtests and overall subtests were observed in the AD as compared to the AD/HD group. This finding suggests that such an imbalance is more distinct in the AD group than in the AD/HD group.
4.2. Comparisons with Previous Studies
We are not aware of any published reports of comparisons between adults with AD/HD and AD according to the WAIS-R. A study that compared AD and AD/HD using the Wechsler Intelligence Scale for Children-III (WISC-III) demonstrated that block design scores were generally higher in patients with AD than in those with AD/HD; however, these differences were not significant [10]. Another study compared patients with AD to those with patients with deficits in attention, motor control, and perception (DAMP), a population more or less overlapping with AD/HD, using the Wechsler Intelligence Scale for Children-Revised (WISC-R) norms. AD patients performed significantly better on all items except for performance IQ, comprehension, picture arrangement, object assembly, and, with the most significant differences obtained for information [13]. Our results regarding the information subtest appear to support these findings. Patients with AD showed better performance than those with AD/HD on the information subtest. Sztmari et al. also compared AD, high-functioning autism (HFA), and outpatient controls (control group of socially impaired child psychiatric outpatients), including those with AH/HD. However, patient age and IQ levels were not controlled in their study [11]. Another study focused on changes in WISC-III scores in three groups of patients: patients with AD, AD/HD, and patients with a reading and writing disorder over a 2-year period. However, No inter-group comparisons were carried out in this study [14].
Several investigators have observed that verbal IQ is lower than performance IQ in patients with autistic disorder, whereas verbal IQ is higher than performance IQ in patients with AD [9,15-17]. Comparisons of patients with AD and those with HFA have also revealed that verbal IQ is significantly higher in patients with AD [13,17,18]. Cederlund and Gillberg [15] examined 100 boys with AD and reported that more than half had a considerably higher verbal IQ than performance IQ, whereas only 6% showed the reverse. The trend for a higher verbal IQ than performance IQ found in previous investigations as well as the present study and the higher percentage showing this tendency among patients with AD may reflect the characteristic features of AD. In contrast to autistic disorder, early cognitive and language skills are not significantly delayed in AD, and mental retardation usually is not observed. Although high scores for block design have been reported in both patients with HFA and those with AD [13], patients with AD have displayed significantly higher vocabulary scores than HFA patients [9,17,18]. The possibility of overlapping features, common to both HFA and AD, is a controversial issue. However, we restricted the subjects of this study to patients with AD. This approach facilitated the accurate extraction of characteristic features of an AD sample in comparison to an AD/HD sample using the WAIS-R.
4.3. Influences of Sex Ratios and Copresence of AD with AD/HD
Although the difference was not statistically significant, the sex ratio (male:female) was 1.1 in the AD/HD group, but 4.1 in the AD group. This yielded a rather unmatched feature for comparison. Classic autism and AD are the two clearest subgroups of autistic spectrum disorder, and both affect males more often than females. Baron-Cohen et al. [19] reviewed evidence suggesting that specific aspects of autistic neuroanatomy may also be the extremes of typical male neuroanatomy. The presence of gender differences has also been identified by WAIS-R tests [12]. In patients 20 - 34 years of age, which corresponds to the mean age of patients examined in the present study, the mean IQ values for males minus the mean IQ for females was +2.6 for verbal IQ, +2.2 for performance IQ, and +2.8 for full IQ; thus, IQ values were greater in males than in females [12]. Comparisons of subtests in patients 20 - 34 years of age, scores were greater in males than in females (p < 0.001) for the information, arithmetic, and block design items [20]. Conversely, digit symbol scores were greater in females than in males (p < 0.001) [12]. Koyama et al. [21] investigated sex differences in children with HFPDD using the WISC-III and found that girls scored significantly higher than boys on coding and symbol search and significantly lower than boys on block design. Boys scored significantly higher on block design than compared to any other performance subtests. No significant differences among the six subtests were observed in girls.
Previous studies concerning PDD and autism spectrum disorders (ASD) focused mainly on males; the percentage of male patients was also high in our AD group. Therefore, the results may reflect not only the characteristic features of the disease itself but also the influences of the sex differences on these measures. Further investigation is needed that considers the influence of gender differences on IQ scores.
It should be considered that slightly less than 7 of the 15 AD group patients met the diagnostic criteria for AD/HD, excluding criterion E. To investigate the influence of AD/HD features, it is necessary to compare patients with both AD and AD/HD with those who have AD without AD/HD within a larger population. Conversely, some differences in trends between AD/HD and AD, including AD with AD/HD were extracted. Thus, differences between AD/HD and AD may have been extracted regardless of whether AD occurred with AD/HD.
The results of the present study showed trends in both similarities and differences in cognitive function between adult patients with AD/HD and those with AD. However, it should be highlighted that this trend is restricted to adult AD/HD and AD groups. Profiles of individual patients are variable and are not necessarily consistent with the overall trend of the patient group. We should be aware that the WAIS-R results provide information only about some aspects of cognitive function in these patients. To use the results of the WAIS-R in actual clinical practice, it is important to consider the individual patient in a comprehensive manner. This includes taking into account the patient’s method for dealing with subtests, his or her attitude toward the tests, the relation of subtest scores to difficulties in actual daily living, and IQ levels and subtest profiles.
The present study is the first to compare adult patients with AD/HD and those with AD with regard to intelligence. Our results revealed certain characteristic features of intelligence in each group of patients. However, the two-group design of this study had drawbacks due to the sex ratio difference, and the AD group included a number of patients who had AD/HD-related difficulties. Shared characteristics between AD/HD and AD groups, such as picture completion, reflect a property of AD/HD in both groups. Future research needs to consider not only WAIS-R scores but also the individual differences in effort and attitudes toward the tests.
4.4. Conclusions
Our results revealed no differences in verbal IQ, performance IQ, or full-scale IQ scores in adult patients with AD/HD compared with adult patients with AD. Additionally, our results suggest that there are no major differences among verbal IQ, performance IQ, or fullscale IQ scores in the AD/HD group. However, among the AD group, verbal IQ was significantly higher than performance IQ. Differences among subtest scores were observed for the AD group. Thus, imbalance in abilities may be greater in the AD than that in the AD/HD group.
Finally, differences were observed between AD/HD and AD for the information subtest, with the AD/HD group displaying a lower score than AD group. This might suggest a particular deficit for AD/HD that distinguishes this disorder from AD.
REFERENCES
- Dulcan, M. (1997) Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 85S-121S. doi:10.1097/00004583-199710001-00007
- Ratey, J.J., Greenberg, M.S., Bemporad, J.R. and Lindem, K.J. (1992) Unrecognized attention-deficit hyperactivity disorder in adults presenting for outpatient psychotherapy. Journal of Child and Adolescent Psychopharmacology, 2, 267-275. doi:10.1089/cap.1992.2.267
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th Edition, American Psychiatric Press, Arlington.
- Ghaziuddin, M., Weidmer-Mikhail, E. and Ghaziuddin, N. (1998) Comorbidity of Asperger syndrome: A preliminary report. Journal of Intellectual Disability Research, 42, 279-283.
- Lee, D.O. and Ousley, O.Y. (2006) Attention-deficit hyperactivity disorder symptoms in a clinic sample of children and adolescents with pervasive developmental disorders. Journal of Child and Adolescent Psychopharmacology, 16, 737-746. doi:10.1089/cap.2006.16.737
- Tani, P., Lindberg, N., Appelberg, B., Nieminen-von Wendt, T., von Wendt, L. and Porkka-Heiskanen, T. (2006) Childhood inattention and hyperactivity symptoms self-reported by adults with Asperger syndrome. Psychopathology, 39, 49-54. doi:10.1159/000089910
- Yoshida, Y. and Uchiyama, T. (2004) The clinical necessity for assessing attention-deficit/hyperactivity disorder (ADHD) symptoms in children with high-functioning pervasive developmental disorder (PDD). European Child & Adolescent Psychiatry, 13, 307-314. doi:10.1007/s00787-004-0391-1
- Perry, R. (1998) Misdiagnosed ADD/AD/HD; rediagnosed PDD. Journal of the American Academy of Child and Adolescent Psychiatry, 37, 113-114. doi:10.1097/00004583-199801000-00024
- Ehlers, S., Nydén A., Gillberg, C., et al. (1997) Asperger syndrome, autism and attention disorders: A comparative study on the cognitive profiles of 120 children. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 38, 207-217. doi:10.1111/j.1469-7610.1997.tb01855.x
- Koyama, T., Tachimori, H., Osada, H., Kanai, C., Shimizu, K. and Kurita, H. (2004) A comparison of cognitive profiles on WISC-III between Asperger. Seishin Igaku Clinical Psychiatry, 46, 645-647.
- Szatmari, P., Tuff, L., Finlayson, M.A. and Bartolucci, G. (1990) Asperger’s syndrome and autism: Neurocognitive aspects. Journal of the American Academy of Child and Adolescent Psychiatry, 29, 130-136. doi:10.1097/00004583-199001000-00021
- Kaufman, A.S. (1990) Assessing adolescent and adult intelligence. Allyn and Bacon, Inc., Boston.
- Gilchrist, A., Green, J., Cox, A., Burton, D., Rutter, M. and Le Couteur, A. (2001) Development and current functioning in adolescents with Asperger syndrome: A comparative study. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 42, 227-240. doi:10.1111/1469-7610.00714
- Nydén, A., Billstedt, E., Hjelmquist, E. and Gillberg, C. (2001) Neurocognitive stability in Asperger syndrome, AD/HD, and reading and writing disorder: A pilot study. Developmental Medicine and Child Neurology, 43, 165- 171.
- Cederlund, M. and Gillberg, C. (2004) One hundred males with Asperger syndrome: A clinical study of background and associated factors. Developmental Medicine and Child Neurology, 46, 652-660. doi:10.1111/j.1469-8749.2004.tb00977.x
- de Bruin, E.I., Verheij, F. and Ferdinand, R.F. (2006) WISC-R subtest but no overall VIQ-PIQ difference in Dutch children with PDD-NOS. Journal of Abnormal Child Psychology, 34, 263-271. doi:10.1007/s10802-005-9018-3
- Ghaziuddin, M. and Mountain-Kimchi, K. (2004) Defining the intellectual profile of Asperger syndrome: Comparison with high-functioning autism. Journal of Autism and Developmental Disorders, 34, 279-284. doi:10.1023/B:JADD.0000029550.19098.77
- Koyama, T., Tachimori, H., Osada, H., Takeda, T. and Kurita, H. (2007) Cognitive and symptom profiles in Asperger’s syndrome and high-functioning autism. Psychiatry and Clinical Neurosciences, 61, 99-104. doi:10.1111/j.1440-1819.2007.01617.x
- Baron-Cohen, S., Knickmeyer, R.C. and Belmonte, M.K. (2005) Sex differences in the brain: Implications for explaining autism. Science, 310, 819-823. doi:10.1126/science.1115455
- Shinagawa, F., Kobayashi, S., Fujita, K. and Maekawa, H. (1990) Nihonban WAIS-R seijin chinou kensahou (Japanese Wechsler Adult Intelligence Scale Revised). Nihon Bunka Kagakusha, Tokyo.
- Koyama, T., Kamio, Y., Inada, N. and Kurita, H. (2009) Sex differences in WISC-III profiles of children with high-functioning pervasive developmental disorders. Journal of Autism and Developmental Disorders, 39, 135-141. doi:10.1007/s10803-008-0610-6
NOTES
*Corresponding author.

