Open Journal of Animal Sciences
Vol.06 No.01(2016), Article ID:62790,7 pages
10.4236/ojas.2016.61004
A Complex Interrelationship between Rectal Temperature and Dairy Cows’ Performance under Heat Stress Conditions
Meriem Rejeb, Raoudha Sadraoui, Taha Najar, Moncef Ben M’rad
National Agronomic Institute in Tunis, Animal Production Department University of Carthage Tunis, Tunis, Tunisia

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 29 October 2015; accepted 12 January 2016; published 15 January 2016
ABSTRACT
Upper limit of thermal stability and subsequent rise of thermoregulatory functions are affected by body temperature. This study was designed to determine the effects of rectal temperature (RT) on dairy cows’ performance (heart rates (HR), respiratory rates (RR), milk yield (MY), dry matter intake (DMI), digestibility, plasma concentration of vitamin C under hot climate. This study was carried out in 2009, in north-west of Tunisia using 30 Holstein cows in mid lactation. The experiment was performed in spring (15th of February-15th of March: P1) and summer (1st-30th of August: P2). On each test day, temperature-humidity index (THI), RT, HR, RR, MY, DMI, digestibility and plasma VC concentration were determined. All this parameters were affected (P < 0.001) when the THI increased from 65.62 (P1) to 83.27 (P2). Regression analyses were carried out between THI index and some parameters (HR, RR, MY, DMI, digestibility, plasma concentration of vitamin C) and between RT and same parameters (HR, RR, MY, DMI, digestibility, plasma concentration of vitamin C). Characteristics of regression analyses in the two modes were different as also were R2 and r (correlation coefficient) of the regressions. R2 in regressions on RT (R2 (RT, DMI) = 0.92 (P < 0.01); R2 (RT, MY) = 0.91 (P < 0.001)) was markedly higher relative to R2 in regressions on THI (R2 (THI, DMI) = 0.76 (P < 0.001); R2 (THI, MY) = 0.63 (P < 0.001)). The two regressions modes suggest that increasing R2 in regressions on RT confirms that rectal temperature constitutes a larger com- ponent of total variance of responses in dairy cows to hot environmental temperature.
Keywords:
Heat Stress, Rectal Temperature, Dairy Cows, Temperature-Humidity Index

1. Introduction
RT (rectal temperature) is a sensitive indicator of thermal balance and may be used to assess the negative effects of hot environments on growth, lactation and reproduction of dairy cows [1] . In [2] , Berman et al. noted during thermal equilibrium, RT was independent of air temperature but related to energy metabolism in man, dog, and rat. In [3] , Heusner found that metabolic rate and RT were interrelated during their circadian changes at thermo-neutral ambient temperatures in the rat and in the domestic.
However, it has been shown that a rise of 1˚C or less in RT is enough to reduce intake and production in dairy cows [4] . Furthermore it has been reported that body temperature is usually maintained by the thermoregulatory system within 1˚C of its normal under ambient conditions that do not impose severe heat stress [2] . Various studies under field conditions have reported increases in RT when lactating cows are subject to temperatures above their thermo-neutral zone [5] . Increased RT signifies lack of thermal balance and increased water intake to replace increased evaporative losses [6] .The amplitude of the body temperature rhythm increases as ambient temperature rises [7] . Evaluation of these lag times, changes in amplitude and other features of the body temperature rhythm in response to different environmental stressors may provide an insight into how dairy cows change their thermoregulatory mechanisms during periods of heat stress [8] . Heat stress causes changes in the homeostasis status of the animals and has been quantified through measurements of RT [9] [10] . In [2] , Berman reported that a relationship between RT and metabolic rate can be expected under thermal comfort conditions. The present study was part of a large study aiming to detect the relationship between RT and several parameters, such as heart rates (HR), respiratory rates (RR), milk yield (MY), dry matter intake (DMI), digestibility, and plasma concentration of vitamin C under high environmental temperature.
2. Materials and Methods
2.1. Cows, Measurements and Sampling
The study was carried out in 2009 at the OTD Badrouna dairy farm Bousalem (north-west of Tunisia), which is located 36˚6' latitude north and 8˚9' longitude west. Thirteen multiparous and primiparous lactating Holstein Friesian dairy cows (512 ± 16 kg BW, 171 ± 17 DIM and 27 ± 1.5 kg/d milk yield) were used. The experiment was conducted in two different periods: Spring (15th of February-15th of March: P1) and summer (1st-30th of August: P2). Cows were housed in free stalls with concrete surfaces and bedded with hay. The cows were fed individually in mangers (0.70m wide and 1.2m long) in front of the stalls. The diets were typical of those in the region and consisted of about 61% forage and 39% concentrate mix on a dry matter (DM) basis. Corn, Barley grain, soybean meal, Wheat bran and a mineral and vitamin supplement were the feed ingredients in the concentrate mixture. The diets fed to animals during the experiment contained, on average, 32.3% DM, 13.9% crude protein (CP), and 41.8% Neutral Detergent Fiber (NDF) on a DM basis in spring. The percentages of DM, CP and NDF in summer were 28.7, 16 and 39.6%. The amounts were calculated according to the milk production level. Forage and Concentrate were separately provided to the cows to allow for the measurement of individual refusals. Drinking water was made available at all times.
At each test day Ta, RH, RT, HR and RR were recorded. Measures started at 12 p.m. and finished around 3 p.m. Ta and RH were measured using a thermo hygrometer (HI 91610C, Hanna instrument, Portugal). Estimation of THI (Temperature Humidity Index) was performed for each test day using the equation described by Kibler in [11] . RT was measured by inserting a veterinary digital thermometer approximately 60 mm into the rectum for 60 s (precision ± 0.01˚C). The HR was determined using a medical stethoscope for one minute (breaths/minute). RR was measured by counting the flank movements of the individual cows for one minute period of uninterrupted breathing and reported as the number of inspirations per minute (inspirations/minute).
To determine daily DMI, the amounts of the feed offered and refused were recorded daily throughout the experiments. Refused feed was removed and weighed daily just prior to the morning feeding. All cows consumed all of the concentrate; therefore, weigh-back consisted of only forage. The samples of feed and refusal were taken daily and one fraction was used for DM determination by drying at 105˚C in a forced air oven for 24 h. Digestibility of rations was evaluated at each test day using Acid-Insoluble Ash (AIA) as a natural marker. The precision of the AIA marker was performed according to the method reported by Van Keuleun and Young in [12] . Sampling started at 12 p.m. and finished around 3 p.m.
Cows were machine-milked two times on all test days (2×) at 6:00 a.m. and 5:00 p.m. in a herringbone parlor (Alpha Laval, The Netherlands). Routine milking included udder and teat cleaning as well as teat dipping in an iodine solution (Iodine, Veto Lab, Tunisia). MY of the individual cows was recorded at each milking on all test days.
Blood samples were collected at each test day at approximately 1 p.m. from the caudal vein puncture of each cow into vacuum tubes (10 ml) in P1 (15 February-15 March) and again in P2 (1st-30th of August). In each tube EDTA solution (anticoagulant substance) was placed before sterilization. The samples were kept in an ice bath for a few hours until centrifugation (3000 tours/minute at 4˚C) to recover plasma. Plasma vitamin C concentration (VC) was performed according to the method reported by Roe and Kuether in [13] .
2.2. Data Analysis
To estimate the effect of period (P1, P2) on RT, RR, HR, DMI, Digestibility, MY and VC concentration a mixed model was used:
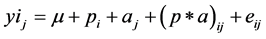 (1)
(1)
where yij is the measured values of RT, RR, HR, DMI, Digestibility, MY and VC concentration. μ is the model mean value, pi fixed effect of period, aj fixed effect of animal, (p × a)ij interaction period-animal, eij is the residual error. Data was conducted using SPSS (version 17.0) (Aug 23, 2008). Differences were considered significant at P < 0.05.
3. Results and Discussion
3.1. Environmental Conditions during the Experimental Periods
Mean Ta, RH and calculated THI by the experimental period are shown in Table 1. Average values of environmental variables (Ta, RH and THI) were higher in P1 than in P2. Average THI was 65.62 ± 2.43 in the spring, and therefore was characterized by a lack of heat stress conditions. In contrast to the spring period, the summer period was characterized by heat stress conditions and THI exceeded 72. A threshold THI value of 72 repre- sented the point at which heat stress begins for dairy cattle [14] .
3.2. Heat Stress Effects
In this study, heat stress altered (P < 0.001) RT, RR and HR (Table 1). Rectal temperature increased from P1
Table 1. Least squares means of rectal temperature, respiratory rates, heart rates, dry matter intake, Digestibility, milk yield and vitamin C concentration of dairy cows in summer and spring period.
(THI = 65.62) with 38.15˚C to P2 (THI = 83.27) with 39.2˚C. Respiratory rates increased from P1with 61.95 Insp./min to P2 with 78.05 Insp./min. heart rates increased from P1with 43.75 Beat/min to P2 with 79.4 Beat./ min. Such response changes are adaptive mechanisms initiated by the cow in an attempt to restore its thermal balance. In summer conditions (P2) RT, RR and HR raised by 0.32˚C, 3.64 Insp./min and 4.6 Beat/min, respectively per increase of THI unit. Similar results were observed by Coppock et al. in [15] , by Bouraoui et al. in [5] and by Ben youness in [10] .
The results in Table 1 showed a significant decreases (P < 0.001) in DMI and MY and significant increases (P < 0.001) in digestibility under summer heat stress. As the THI values increased from 65.62 to 83.27, DMI decreased by 2.31 kg, and milk production decreased by 5.59 kg. The regression equation obtained under high environmental temperatures of the present work indicates that DMI and milk yield drops by 0.73 kg /DM/day and 1.1kg /day for each point increase in the value of THI above 81 in P2. However, the adverse effect on milk yield was most likely mediated through a reduction in DMI in order to reduce metabolic heat production. This suggested that an adaptive mechanism must have occurred in cows under heat stress conditions, resulting in lower efficiency of energy use for milk production. This, combined with the decrease in DMI, would explain the decreased milk yield for these cows [12] . Evaluation of various studies under field conditions shows that there is an increase in digestibility in response to high temperature that may be explained by reduced DMI [16] [17] and a prolonged retention of feed in the gastrointestinal tract [18] .
Our data indicated that VC concentration decreased significantly (P < 0.001) from the spring (2.96 mg/l) to the summer (1.79 mg/l). Other authors have reported similar results in [19] -[22] . The biosynthetic pathway for VC starts with the production of UDP glucose from glucose-1-phosphate in the liver. Generally, cows that are heat stressed have decreased DMI and therefore glucose who presented the sole precursor of VC in animal body.
3.3. Rectal Temperature a Determinant of Heat Stress
Define abbreviations and acronyms the first time they are used in the text, even after they have been defined in the abstract. Abbreviations such as IEEE, SI, MKS, CGS, sc, dc, and rms do not have to be defined. Do not use abbreviations in the title or heads unless they are unavoidable.
Rectal temperature was of utmost importance in heat stress and adaptation process. Heat stressed cows evoke thermal regulatory reactions in order to try to maintain heat balance [23] . These reactions include increased RR and HR, reduced DMI increased MY and VC concentration via increased body temperature. Research has indicated that RT may be used as indicators of climatic stress in order to assess the negative effects of hot environments on growth, lactation and reproduction of dairy cows [1] [5] . The regression estimates according RT and THI during summer 2009 are given in Table 2, the regressions indicate that, the value of the relationship according RT for predictive purposes is relatively higher than according THI, as depicted by the R2 value. However, a large part of the variation in daily several parameters determined in this study could therefore be attributed to RT.
The correlations with RT and THI were calculated. THI index has been widely used to evaluate environments for dairy cows in Mediterranean climate [5] . THI showed a low correlation with animals ‘responses to thermal environment compared with RT (Table 2). The values of these correlations were assumed as indications of the efficiency RT as indicators of the animals ‘response to the environment. RT usually is maintained by the thermoregulatory system within 1˚C of its normal under ambient conditions that do not impose severe heat stress [2] . A rise of 1˚C or less in rectal temperature is associated with gradual deterioration of feed intake and productivity [4] . RR was positively correlated to RT (r = 0.93; P < 0.01). Similar results were reported by Gaughan et al. in [24] who found lower correlation. The results of present study showed that cows increased RR (>40 Insp./min) when RT exceeded 38.7˚C. Thatcher et al. in [25] reported that cows may be considered stressed by hot environmental temperature, when RT was above 39.2˚C and RR exceeds 60 Insp./min.
Table 2 shows that milk production is a function of RT and THI. The negative slope of the regressions line indicates that milk production decreases as RT and THI increases. The regressions indicates that, in general, for each point increase in the THI value above 81.4, there was a decrease in milk yield of 1.10 kg/day, and for each point increase in RT value above 38.2˚C, there was a decrease in milk yield of 1.61 kg/day. Johnson et al. in [4] suggested that milk yield declines when body temperature exceeds 38.6˚C, and, for each 0.56˚C increase in RT, milk yield and intake of total digestible nutriment decline by1.8 and 1.4 kg, respectively. [1] indicated that Fans
Table 2. Regressions of RT, RR, HR, DMI, digestibility, MY and VC concentration deviations from means of individual cows on rectal temperature and THI in summer (P2).
and sprinklers reduced the diurnal rise in body temperature in cows by 0.4˚C to 0.9˚C and increased milk yield by 2 to 2.6 kg/d in cows exposed to high temperatures. However, the value of relationship between MY and RT (R² = 0.92; P < 0.01) for predictive purposes is relatively higher than Relationship between MY and THI (R² = 0.63; P < 0.001). A large part of the variation in daily milk yield in heat stress conditions could be attributed to the RT increases. This agreed with results reported by Igono in [26] , which indicate that higher body temperatures were associated with increasing milk yield.
Decreasing Feed intake is always accompanied with an increase in rectal temperature [18] . The reduction in appetite under heat stress is a result of elevated body temperature and may be related to gut fill, and consequently decreases in DMI may help to maintain homeothermy through reduced metabolic heat production. Regression equation (DMI, RT) reported in Table 2 indicates that, for each point increase in RT value above 38.5˚C, there was a decrease in DMI of 1.31 kg/day. The value of R² = 0.91 (P < 0.001) confirms that a relation between RT and DMI is relatively higher than those between DMI and THI (R² = 0.76; P < 0.001). The calculated correlations indicated that DMI was negatively correlated to RT (r = −0.85; P < 0.001) and to THI (r = −0.81; P < 0.001).
Under heat stress conditions, reduction of DMI is generally associated with an increase in diet digestibility. During summer, correlation between digestibility and RT (r = 0.64; P < 0.01) was higher relative to correlation between digestibility and THI (r = 0.32; P < 0.01). The results also showed that R2 was high in regression of digestibility on RT, and indicates that RT explains 63% of total variance of digestibility. The present results (Table 2) indicated that THI and RT explain 72% and 82%, respectively of plasma VC variation in hot environmental conditions. However, RT appears to be a better predictor of changes in plasma vitamin C concentration than THI index. These results are in accordance with Tanaka et al. in [27] , who reported that plasma VC level depends on the body temperature in heat stress conditions.
4. Conclusion
Our results suggest that RT is an indicator of thermal balance and may be used to assess the adversity of the thermal environment which can affect the growth, lactation, and reproduction of dairy cows. The interaction of rectal temperature and cattle performance form a complex interrelationship affects the dissipation of body heat. Daily milk yield and feed intake are associated with metabolic heat production. At higher ambient temperature, increases of RT could act as a factor limiting milk production. However, in an attempt to reduce body temperature, dairy cows reduce heat production from fermentation, digestion and other metabolic processes.
Acknowledgements
The authors would like to acknowledge the financial support of this work by grants from General Direction of Scientific Research (DGRST), Tunisia.
Cite this paper
MeriemRejeb,RaoudhaSadraoui,TahaNajar,Moncef BenM’rad, (2016) A Complex Interrelationship between Rectal Temperature and Dairy Cows’ Performance under Heat Stress Conditions. Open Journal of Animal Sciences,06,24-30. doi: 10.4236/ojas.2016.61004
References
- 1. West, J.W. (1999) Nutritional Strategies for Managing the Heat—Stressed Dairy Cow. Journal of Animal Science, 77, 21-35.
- 2. Berman, A., Folman, Y., Kaim, M., Mamen, M., Herz, Z., Wolfenson, D., Arieli, A. and Graber, Y. (1985) Upper Critical Temperatures and Forced Ventilation Effects for High-Yielding Dairy Cows in a Subtropical Climate. Journal of Dairy Science, 68, 1488-1495
- 3. Heusner, A.A. (1972) Criteria for Standard Metabolism. In: Smith, R.E., Ed., Proceeding of the International Symposium on Environmental Physiology: Bioenergetics, Federation of the American Societies of Experimental Biology, Baltimore, 15-21.
- 4. Johnson, H.D., Ragsdale, A.C., Berry, I.L. and Shanklin, M.D. (1963) Temperature-Humidity Effects Including Influence of Acclimation in Fed and Water Consumption of Holstein Cattle, University of Missouri, Research Bulletin, No. 846.
- 5. Bouraoui, R., Lahmar, M., Majdoub, A., Djemali, M. and Belyea, R. (2002) The Relationship of Temperature-Humidity Index with Milk Production of Dairy Cows in a Mediterranean Climate. Animal Research, 51, 479-491.
http://dx.doi.org/10.1051/animres:2002036 - 6. Mohammed, M.E. and Johnson, H.D. (1984) Effect of Growth Hormone on Milk Yields and Related Physiological Functions of Holstein Cows Exposed to Heat Stress. Journal of Dairy Science, 68, 1123-1133.
http://dx.doi.org/10.3168/jds.S0022-0302(85)80938-3 - 7. Brown-Brandl, T.M., Nienaber, J.A., Eigenberg, R.A., Hahn, G.L. and Freetly, H. (2003) Thermoregulatory Responses of Feeder Cattle. Journal of Thermal Biology, 28, 149-157.
http://dx.doi.org/10.1016/S0306-4565(02)00052-9 - 8. Hahn, G.L. (1999) Dynamic Responses of Cattle to Thermal Heat Loads. Journal of Animal Science, 77, 10-20.
- 9. Ferreira, F., Pires, M.F.A. and Martinez, M.L. (2006) Physiologic Parameters of Cross Are Cattle Subjected to Heat Stress.
- 10. Ben Younes, R., Ayadi, M., Najar, T., Caccamo, M., Schadt, I. and Ben M’Rad, M. (2011) Hormonal (Thyroxin, Cortisol) and Immunological (Leucocytes) Responses to Cistern Size and Heat Stress in Tunisia. Journal of Life Sciences, 5, 257-265.
- 11. Kibler, H.H. (1964) Thermal Effects of Various Temperature-Humidity Combinations on Holstein Cattle as Measured by Eight Physiological Responses. University of Missouri Agricultural Experiment Station, Research Bulletin Missouri Agricultural Experiment Station, 862, 1-42.
- 12. Van Keulen, J.V. and B.A. Young. (1977) Evaluation of Acid Insoluble Ash as a Natural Marker in Ruminant Digestibility Studies. Journal of Animal Science, 44, 282.
- 13. Roe, J.M. and Kuether, C.A. (1942) Detection of Ascorbic Acid in Whole Blood and Urine through the 2,4-DNPH Derivative of Dehydroascorbic Acid. The Journal of Biological Chemistry, 147, 399-407.
- 14. Johnson, H.D. (1987) Bioclimates and Livestock. In: Johnson, H.D., Ed., Bioclimatology and the Adaptation of Livestock, Chap. 1, Elsevier Science Publishers, Amsterdam, 3-16.
- 15. Coppock, C.E., Grant, P.A., Portzer, S.J., Charles, D.A. and Escobosa, A. (1982) Lactating Dairy Cow Responses to Dietary Sodium, Chloride, and Bicarbonate during Hot Weather. Journal of Dairy Science, 65, 566-576.
http://dx.doi.org/10.3168/jds.S0022-0302(82)82234-0 - 16. Doreau, M., Grimaud, P. and Michalet-Doreau, B. (2000) La sous-alimentation chez les ruminants : ses effets sur la digestion. INRA Productions Animales, 13, 247-255.
- 17. Morand-Fehr, P. and Doreau, M. (2001) Ingestion et digestion chez les ruminants soumis au stress de chaleur. INRA Productions Animales, 14, 15-27.
- 18. Kadzere, C.T., Murphy, M.R., Silanikove, N. and Maltz, E. (2002) Heat Stress in Lactating Dairy Cows: A Review. Livestock Production Science, 77, 59-91.
http://dx.doi.org/10.1016/S0301-6226(01)00330-X - 19. Padilla, L., Matsui, T., Ikeda, S., Kitagawa, M. and Yano, H. (2007) The Effect of Vitamin C Supplementation on Plasma Concentration and Urinary Excretion of Vitamin C in Cattle. Journal of Animal Science.
http://dx.doi.org/10.2527/jas.2007-0060 - 20. Coates, M.E. (1984) Metabolic Role of the Vitamins. In: Freeman, B.M., Ed., Physiology and Biochemistry of the Domestic Fowl, Vol. 5, Academic Press, New York, 27-36.
- 21. Tanaka, M., Kamiya, Y., Suzuki, T., Kamiya, M. and Nakai, Y. (2008) Relationship between Milk Production and Plasma Concentrations of Oxidative Stress Markers during Hot Season in Primiparous Cows. Animal Science Journal, 79, 481-486.
http://dx.doi.org/10.1111/j.1740-0929.2008.00553.x - 22. Padilla, L., Matsui, T., Kamiya, Y., Kamiya, M., Tanaka, M. and Yano, H. (2006) Heat Stress Decreases Plasma Vitamin C Concentration in Lactating Cows. Livestock Science, 101, 300-304.
http://dx.doi.org/10.1016/j.livprodsci.2005.12.002 - 23. Ben Younes, R., Ayadi, M., Najar, T., Caccamo, M., Iris, S. and Ben M’Rad, M. (2011) Effect of Thermal Stress, Cistern Size and Milking Frequency on Plasma Mineral Concentrations in Holstein Dairy Cows. Journal of Life Sciences, 5, 739-746.
- 24. Gaughan, J.B., Holt, S.M., Hahn, G.L., Mader, T.L. and Eigenberg, R. (2000) Respiration Rate—Is It a Good Measure of Heat Stress in Cattle? Asian-Australasian Journal of Animal Sciences, 13, 329-332.
- 25. Thatcher, W.W., Flamenbaum, I., Block, J. and Bilby, T.R. (2010) Interrelationships of Heat Stress and Reproduction in Lactating Dairy Cows. High Plains Dairy Conference, Amarillo, Texas, 2010, 45-60.
- 26. Igono, M.O., Steevens, B. J., Shanklin, M.D. and Johnson, H.D. (1985) Spray Cooling Effects on Milk Production, Milk, and Rectal Temperatures during a Moderate Temperate Summer Season. Journal of Dairy Science, 68, 979.
http://dx.doi.org/10.3168/jds.S0022-0302(85)80918-8 - 27. Tanaka, M., Kamiya, Y., Kamiya, M. and Nakai, Y. (2007) Effect of High Environmental Temperatures on Ascorbic Acid Sulfhydryl Residue End Oxidized Lipid Concentrations in Plasma of Dairy Cows. Animal Science Journal, 78, 301-306.
http://dx.doi.org/10.1111/j.1740-0929.2007.00439.x


