Open Journal of Animal Sciences
Vol.3 No.3A(2013), Article ID:34782,9 pages DOI:10.4236/ojas.2013.33A004
Inhibition of Ehrlichia canis and Babesia canis transmission among ticks fed together on dogs vaccinated with Bm86 antigen
![]()
1Centro de Ingeniería Genética y Biotecnología, Habana, Cuba; *Corresponding Author: alina.rodriguez@cigb.edu.cu
2Universidade Estadual Paulista-UNESP, Jaboticabal, Brazil
3Facultad de Ciencias Agropecuarias, Universidad de La Salle, Bogotá DC, Colombia
4Facultad de Ciencias Agrarias, Universidad de Ciencias Comerciales, Altamira, Nicaragua
Copyright © 2013 Alina Rodríguez-Mallon et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 15 May 2013; revised 17 June 2013; accepted 27 June 2013
Keywords: Rhipicephalus sanguineus; Ehrlichia canis; Babesia canis; tick-borne diseases; dogs; Bm86
ABSTRACT
GAVAC (Heber Biotec S.A, Havana, Cuba) is a commercially available vaccine developed with the Rhipicephalus (Boophilus) microplus Bm86 recombinant antigen. Bm86 is a “concealed” antigen that is present in the plasmatic membrane of tick gut epithelial cells with unknown function so far. It is well known that after vaccination in the last fifteen years in Cuba, there was a significant decrease of babesiosis (Babesia bovis and Babesia bigemina) and anaplasmosis (Anaplasma marginale) in cattle. A reduced transmission capacity of ticks fed on tick-immune animals and humans has been reported for several tick-borne pathogens. Recent experiments have demonstrated that an anti-tick vaccine may contribute to the control of tick-borne pathogens not only by decreasing the exposure of susceptible hosts to ticks, but also by reducing the vector capacity of ticks. In this study, the potential of Bm86 vaccination to interfere with pathogen transmission among ticks was evaluated by using as experimental model the brown dog tick Rhipicephalus sanguineus and the tickborne Babesia canis and Ehrlichia canis pathogens. Dogs, vaccinated and not vaccinated, were infested with pathogen-infected ticks and noninfected nymphs of R. sanguineus. After feeding, the pathogen transmission to newly molted adults from co-feeding uninfected nymphs was studied by conventional PCR and qPCR. Results suggest that the anti-Bm86 antibodies could be able to block the transmission of B. canis and/or E. canis from infected to non-infected ticks.
1. INTRODUCTION
Ticks are obligate hematophagous ectoparasites of wild and domestic animals and humans. Perhaps the most serious impact comes through their capacity to vector the infectious agents that cause diseases in humans and other animals [1,2].
Babesiosis is a disease caused by intraerythrocytic protozoan parasites of the genus Babesia, transmitted by ixodid ticks. In Latin America there are two species, B. bovis and B. bigemina, which infect cattle, are both transmitted by the one-host tick R. (Boophilus) microplus [3]. R. sanguineus is the major vector of Babesia species, which infect dogs and it is invariably present in areas where canine babesiosis is endemic [4,5]. This tick species is a three-host tick that feeds primarily on dogs and occasionally on other hosts, including humans [6-8].
Ehrlichia species comprise a group of rickettsial agents that are obligate intracellular bacteria and reside within a cytoplasmic vacuole of infected eukaryotic cells [9]. Anaplasma marginale and Anaplasma phagocytophilum are closely related, but principally ruminants are cited as susceptible hosts [10]. Ehrlichia canis is a Gram-negative coccoid to ellipsoidal bacterium, occurring intracytoplasmically, either singly or in compact inclusions (morulae) in dog bone marrow derived cells. The first site of development of rickettsiae in ticks occurs in gut cells, but many other tick tissues subsequently become infected, including the salivary glands from where the rickettsiae are transmitted to the host during feeding [11].
The use of chemical pesticides constitutes the primary measure for tick control. Alternative strategies are required to control ticks as populations across the globe continue to evolve resistance to commercially available acaricides [12,13]. An understanding of the biological intricacies underlying vector-host-pathogen interactions is required to reach appropriate levels of innovation in sustainable tick management. GAVACTM (Heber Biotec S.A, Havana, Cuba) is a commercially available vaccine against ticks, which uses the R.B. microplus Bm86 antigen. Bm86 is a “concealed” antigen that is present in the plasma membrane of tick gut epithelial cells with unknown function so far [14,15]. It has been shown to be present in R.B. microplus strains from different regions of the world suggesting a certain degree of conservation of the gene [16-18]. Bm86 has demonstrated efficacy in the control of tick infestations in cattle under an integrated management system diminishing dramatically the frequency of acaricide applications [19]. It is a fact that after vaccination over the last fifteen years in Cuba, there is a significant decrease of babesiosis (B. bovis and B. bigemina) and anaplasmosis (A. marginale) in cattle [17, 19-21]. The efficacy of the Bm86 antigen on the biotic potential of R. sanguineus was investigated very recently. In this previous study, it was concluded that the Bm86 antigen used as a vaccine for dogs reduced the viability and biotic potential of this tick species [22].
A reduced transmission capacity of ticks fed on tickimmune animals and humans has been reported for several tick-borne pathogens [23-25]. For example, people who express an immune reaction against the vector tick Ixodes scapularis appear to acquire Lyme disease less frequently than those who experience no such immune response [26]. For insect vectors, seroconversion of humans against sand fly vectors correlates with development of protective immunity to leishmaniasis [27]. Recent experiments have demonstrated that an anti-tick vaccine may contribute to the control of tick-borne pathogens not only by decreasing the exposure of susceptible hosts to ticks but also by reducing the vector capacity of ticks [28,29].
Herein, we evaluate the potential of Bm86 dog vaccination to interfere with B. canis and E. canis transmission among ticks, by investigating if anti-Bm86 antibodies are able to block the dog-supported transmission of these two pathogens from infected R. sanguineous ticks to uninfected nymphs feeding together.
2. MATERIALS AND METHODS
2.1. Hosts, Hemoparasites and Ticks
The trial was conducted under controlled conditions at the Department of Veterinary Pathology of the Faculty of Agronomic and Veterinary Sciences-FCAV, Jaboticabal campus, São Paulo State University-UNESP, Brazil. Dogs of different breeds and sexes, an average of 3 months old and weighting approximately 5 kg were used throughout the study. The animals were fed with a commercial pellet diet (Big BossTM) and water ad libitum. Dogs were maintained in separated boxes until the end of the experiment and were handled according to international guidelines for experimentation with animals. After infection with hemoparasites, dogs received medical attention. Once the experiments were over, dogs were included in an adoption program.
R. sanguineus ticks used in this study was obtained from a Brazilian strain maintained at FCAV-UNESPJaboticabal, Brazil [30]. Maintenance conditions comprised a biochemical oxygen-demand incubator-BOD with 80% relative humidity at 28˚C, and a 12-h photoperiod. B. canis and E. canis were obtained from stocks maintained at FCAV-UNESP-Jaboticabal, Brazil [31,32].
2.2. Infection of Ticks with E. canis and B. canis
Two healthy dogs without antibodies against E. canis and Babesia sp. were inoculated by intravenous injection with 1 mL of frozen dog blood infected either with E. canis or B. canis laboratory stocks, respectively. The dog infected with B. canis was splenectomized 24 hours before the pathogen inoculation. The body temperature and hematocrit of the animals were measured daily throughout the test. Confirmation of a dog’s infection with B. canis or E. canis was performed by thin blood smears stained with Giemsa and evaluated by standard microscopy methods. Whole-blood samples were collected from dogs, with EDTA used as anticoagulant. One hundred naive R. sanguineus nymphs were fed during the parasitemia of each infected dog. Engorged dropped-off ticks were kept in the incubator until molt. Genomic DNA from 5 whole individual adult ticks newly molted was used to test infection prevalence of naive ticks with each pathogen. DNA was extracted using a DNeasy Blood & Tissue Kit (Qiagen, USA, # 69506) according to the manufacturer’s instructions. The samples were eluted in a final volume of 100 μL. Five μL from each eluted individual DNA were used to perform specific Babesia and Ehrlichia PCR. The Babesia specific PCR amplified a 400-bp fragment of Babesia ssu-r DNA [33] using the primers, forward (5’ AATACCCAATCCTGAC ACAGGG 3’) and reverse (5’ TTAAATACGAATGCC CCCAAC 3’). In the case of Ehrlichia-specific PCR, a 350 bp fragment of the dsb gene [34] was amplified with primers forward (5’ ATGAATTGCAAAAAAATTCTTA TA 3’) and reverse (5’ TTAGAAGTTAAATCTTCCT CC 3’). PCR reactions were prepared in a final volume of 25 μL containing tick DNA prepared as described above plus 2.5 μL of 10× Taq polymerase buffer, 1.5 mM MgCl2, 10 pmol of each oligonucleotide, 200 μM dNTPs and 1.25 units of Taq polymerase.
The reaction mixtures were subjected to an initial denaturation of 5 min at 95˚C, followed by 30 cycles of denaturation-annealing-extension (for Ehrlichia PCR: 30 seconds at 95˚C, 1 minute at 52˚C and 2 minutes at 72˚C and for Babesia PCR: 1 minute at 95˚C, 1 minute at 55˚C and 1 minute at 72˚C) and a final extension of 5 minutes at 72˚C. Amplification products were visualized on an ethidium bromide-stained 1% agarose gel in TAE 1×.
2.3. Dog Immunizations with Gavac Tm
Six healthy dogs without previous antibodies to hemoparasites and negative PCR for Babesia and Ehrlichia were allocated at random into two experimental groups as follows:
Group 1: (n = 4) Dogs were immunized with 2 mL of GAVACTM (100 μg of Bm86 antigen) by subcutaneous injection on days 0, 14 and 28.
Group 2: (n = 2) Negative controls. Dogs were immunized by subcutaneous injection on days 0, 14 and 28 with 2 mL of PBS 1X with a similar composition to GAVAC, but lacking the active pharmaceutical principle, in a 60/40 proportion of water/oil using VG Montanide 888 adjuvant (prepared to 10% in mineral oil).
The general behavior and body temperature of the animals were monitored daily throughout the test. Animal serum samples were taken on days 0, 7, 14, 21, 28, 35, 42, 49 and 56. The antibody response against Bm86 was evaluated by indirect ELISA. Purified recombinant Bm86 (1 μg/well) was used to coat ELISA plates overnight at 4˚C. Sera were serially diluted in base 1:2 in PBS 1X. Each serum was assayed using three replicates. The plates were incubated with the diluted sera for 1 h at 37˚C and then incubated with 1:10,000 anti-dog IgGHRP conjugate (Sigma) for 1 h at 37˚C. The color reaction was developed with a substrate solution containing O-phenylenediamine 0.4 mg/mL in 0.1 M citric acid and 0.2 M Na2HPO4, pH 5.0 and 0.015% hydrogen peroxide. The reaction was stopped with 2.5 M H2SO4 and the OD490 nm was determined. The antibody titer was established as the reciprocal of the highest dilution at which the mean OD of the serum in question was three times the mean OD of the serum in negative controls. The mean antibody titer in the vaccinated group was determined from individual values in each dog.
2.4. Infestations of Vaccinated Dogs with Infected and Non Infected Ticks
Two feeding chambers were glued to shaved flanks of each vaccinated and control dog one day before the tick infestation, as described previously [30]. On day 36 of the experiment, (when anti Bm86 titers reached 1:3000), three dogs (two vaccinated and one control) were infested with 10 females and 5 males infected with B. canis in one chamber to guarantee infected ticks on each dog according to the infection prevalence determined before. The other three dogs were infested with E. canis infected ticks (20 females with 10 males) in one chamber in the same way described for the other pathogen. After 5 days for the B. canis infected group, and 10 days for the E. canis infected group, when the parasitemia was the highest and guaranteed the ingestion of a greater number of parasitized erythrocytes, which subsequently must result in a higher incidence of infection in the adult ticks [31,35], each dog was infested with 100 naive nymphs in the empty chamber. After ticks were released inside chambers, Elizabethan collars were placed on the dogs to avoid chamber removal.
DNA was extracted from blood samples of each dog with patent parasitemia (on day 5 or 11 after dogs were exposed to B. canis or E. canis infected ticks, respectively). The same specific PCR tests described above were performed to determine the presence of both pathogens. Sera were collected from experimental dogs after day 15 post-infection to be used in an Indirect Immunofluorescence Assay (IFA) described previously [32]. Briefly, antigen slides (sections of specific pathogen infected ticks) were flooded with serial dilutions in base two of test serum from 1:40 to 1:1280. Appropriate positive and negative control sera were included with each run. The slides were placed in a humidified chamber and incubated at 37˚C for 45 min; they were rinsed three times for 5 min each time in PBS, air-dried and then 10 μL of anti-dog IgG (KPL) labeled with fluorescein isothiocyanate at 1:30 dilution in PBS containing 0.01% Evans blue was applied. Incubation and rinse procedures were repeated as above. Mounting fluid containing buffered glycerin, pH 9.6 was placed on each slide, and covered with a cover slip. The slides were examined on a fluorescence microscope using a 40× objective (Olympus, BX-FLA).
2.5. Pathogen Infection Prevalence in Naive Ticks Feeding Together with Infected Ticks
Engorged dropped-off naive nymphs were collected and kept in a BOD incubator having a 12 h light: 12 h dark photoperiod, 28˚C and 80% relative humidity, until molt. Ten newly molted naive ticks from each group were analyzed by conventional PCR, as described above, for the presence of pathogens. The infection percentage of naive ticks was calculated for each experimental group.
Remaining newly molted naive ticks from each group were pooled into five groups with the same quantity of both sexes in each group and genomic DNA was analyzed by quantitative real time PCR using a Rotor-Gene 3000 Detection System (Corbett, Life Science). Briefly, 5 μL template DNA prepared from whole ticks in the same way as described above for individual ticks using a DNeasy Blood & Tissue Kit (Qiagen, USA, # 69506) was mixed with 6.5 μL of 2× SYBR Green PCR master mix (Quantitect SYBR Green PCR kit, Qiagen, USA, # 204143) and 0.3 μM of each primer (forward and reverse primers) in a final volume of 12.5 μL. The amplification program was 15 minutes at 95˚C and 45 cycles of 15 seconds at 94˚C, 30 seconds at 56˚C and 30 seconds at 72˚C. All reactions were run in triplicate.
Specific gene amplification efficiency in the real-time PCR was analyzed with a 2-fold dilution series of genomic tick DNA containing the genes of interest. Plots of log copy numbers of the tested gene at different dilutions versus the corresponding cycle threshold (CT) were generated. The slope of the linear plot is defined as −(1/log E), where E is the amplification efficiency. Thus, the quantity of target sequence relative to a reference gene can be calculated using the formula 2(−ΔCT), where ΔCT = (CT target − CT reference) [36]. Tick actin was used as reference gene. Oligonucleotides used are summarized in the Table 1.
Quantitative data obtained from ticks fed on vaccinated and non vaccinated groups infected with specific pathogens were compared using t tests (Prism, version 4.0 for Windows; GraphPad Software, USA).
3. RESULTS
3.1. Infection Prevalence of Ticks Fed on Dogs Intravenously Inoculated with Pathogens E. canis and B. canis
The dog inoculated with B. canis showed increased temperature after the fifth day post-infection (p.i.), when the haematocrit started to drop from 35% to 24% on day
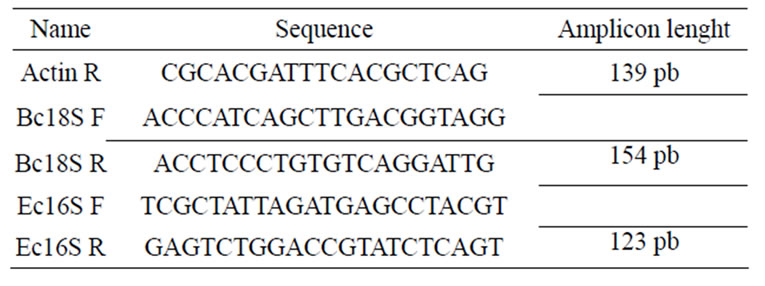
Table 1. Specific oligonucleotides used in quantitative real time PCR.
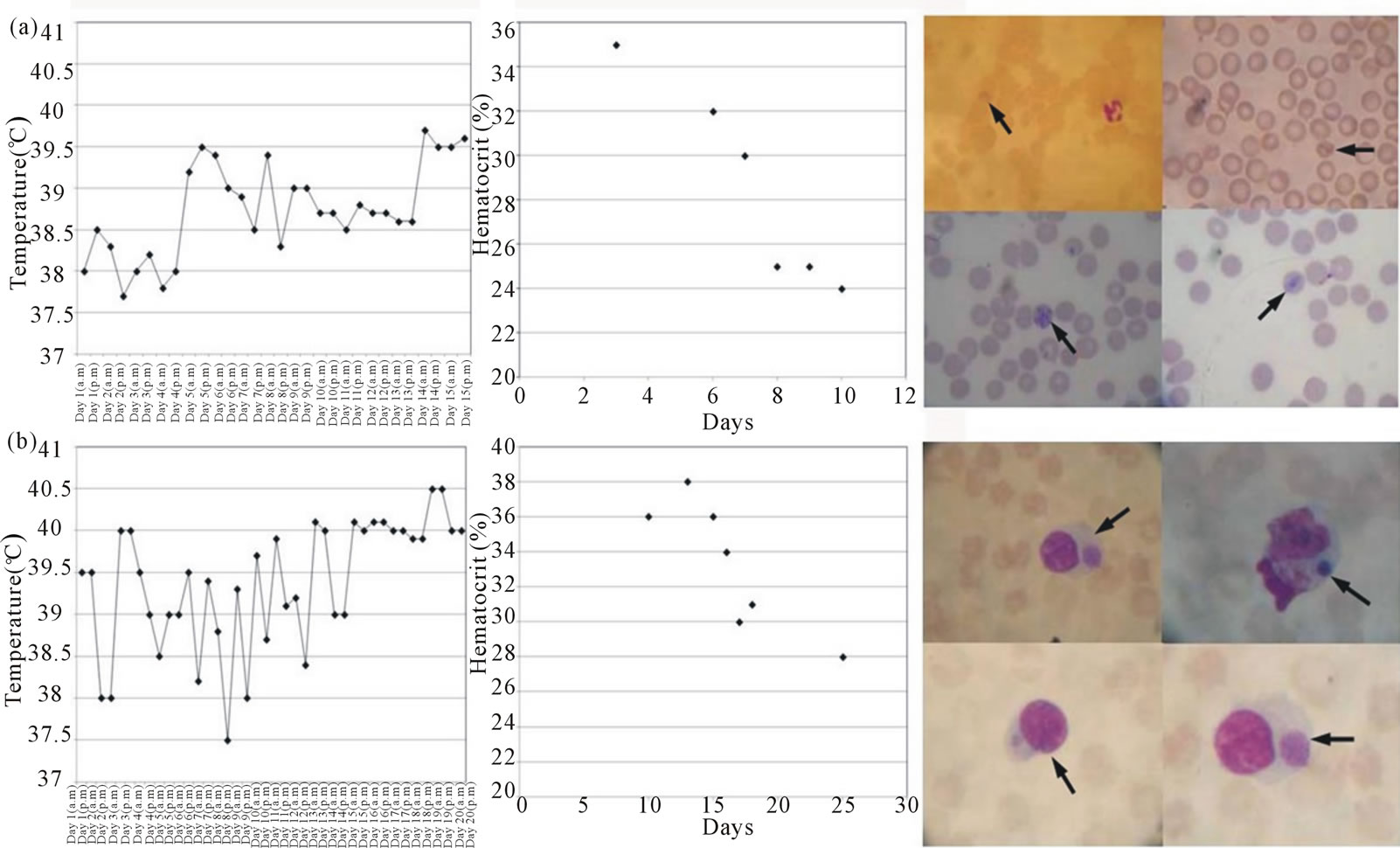
Figure 1. Temperature, hematocrit and blood smears stained with Giemsa in the splenectomized dog inoculated with B. canis (panel (a)) and in the dog inoculated with E. canis (panel (b)). Arrows indicate typical Babesia trophozoites in (a) and characteristic Herlichia morulae in (b).
10 p.i. Typical Babesia trophozoites including piriform, ameboid and Maltese cross forms were observed in peripheral blood since day 6 p.i. (Figure 1(a)), but parasi taemia became very low after three days.
In the case of the dog inoculated with E. canis, the highest temperatures were observed after day 11. The haematocrit started to drop from 38% to 28% on day 13 p.i. Ehrlichial morulae were observed in peripheral blood of this dog after day 15 p.i. (Figure 1(b)).
Two of five (2/5) and 1/5 nymphs become infected when they are fed on B. canis and E. canis experimentally inoculated dogs, respectively, under the laboratory conditions. Representative images of ethidium bromidestained gel electrophoresis of the specific amplicons of tick infection are presented in Figure 2. The remained adult ticks were used to infest vaccinated dogs.
3.2. Antibodies against Bm86 Seem Interfere with E. canis and B. canis Transmission among Ticks
There was no change in normal behavior, nor any fever or clinical signs of disease in any of the animals during the immunization experiment (data not shown). Local inflammatory responses down the vaccine application in one side, were evident in two out of four vaccinated dogs, but after two or three days of treatment, disappeared. This issue should be taken in consideration to address changes in the vaccine composition when recommended for pet dogs. Specific titers against Bm86 were obtained only in animals immunized with GAVACTM (Figure 3). On day 35, the anti-Bm86 titer average was
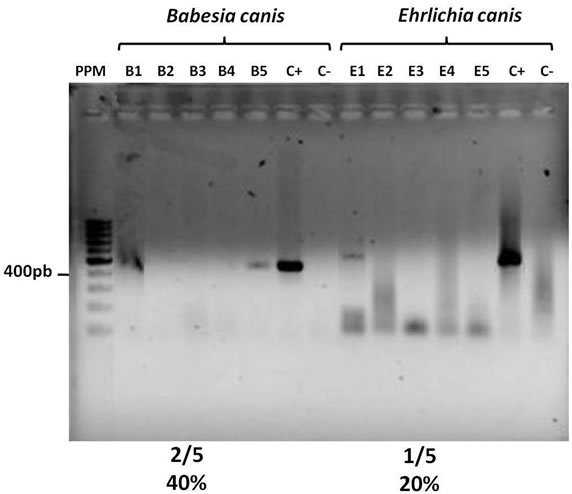
Figure 2. Infection frequency in ticks fed on dogs intravenously inoculated with E. canis and B. canis. Electrophoresis in a 1% agarose gel in 1X TAE. PPM, Molecular Weigth Marker. B1-5 Amplification products in the B. canis specifc PCR from individual tick genomic DNA. E1-5 Amplification products in the E. canis specifc PCR from individual tick genomic DNA, C+ and C-Positive and negative controls in each specific PCR.
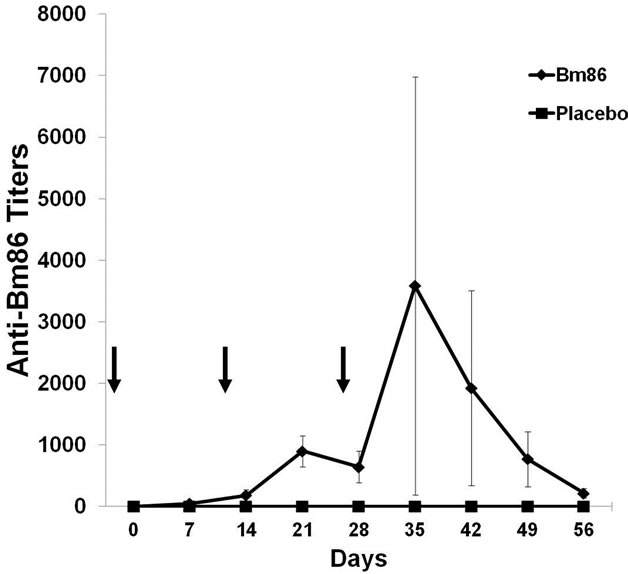
Figure 3. Antibody titers against Bm86 antigen from immunized dogs measured by indirect ELISA. Data are expressed as the reciprocal of the antibody titer average in each experimental group (Vaccinated with GAVAC, n = 4 and negative controls, n = 2). The antibody titer in each vaccinated dog was determined as the last serum dilution with an average OD greater than three times the average OD of the sera in the negative control group. Standard deviations are represented. Arrows indicate immunization days.
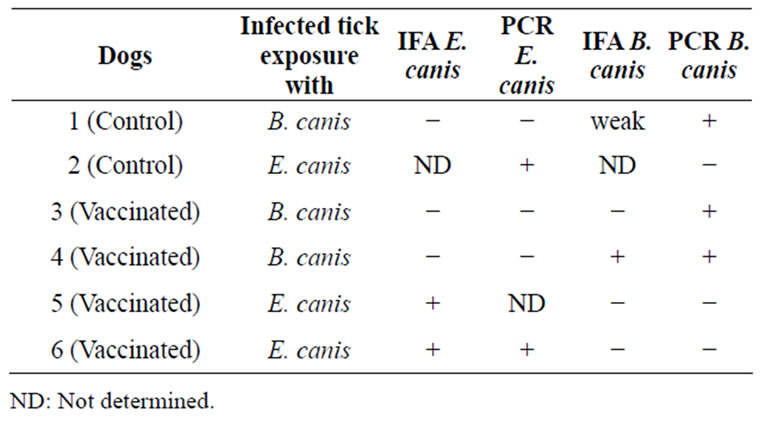
Table 2. Dog infection after infected tick exposure as determined by IFA and conventional PCR.
higher than 1:3000.
Table 2 shows the dog infections with pathogens after exposure to infected ticks as determined by IFA and conventional PCR. The low numbers of dogs preclude any statistical analysis of the effect of vaccination with GAVACTM on infection of the dogs with E. canis or B. canis, but these results suggest that the anti-Bm86 anti body titers didn’t interfere with the pathogens’ transmission from ticks to host during tick feeding.
The infection percentage of naive ticks fed together with infected ticks on vaccinated dogs was lower than on control dogs for both pathogens as determined by conventional specific PCRs from genomic DNA of individual ticks (Figure 4(a)). For B. canis, 67% of the uninfected nymphs feeding on the control dog became in-
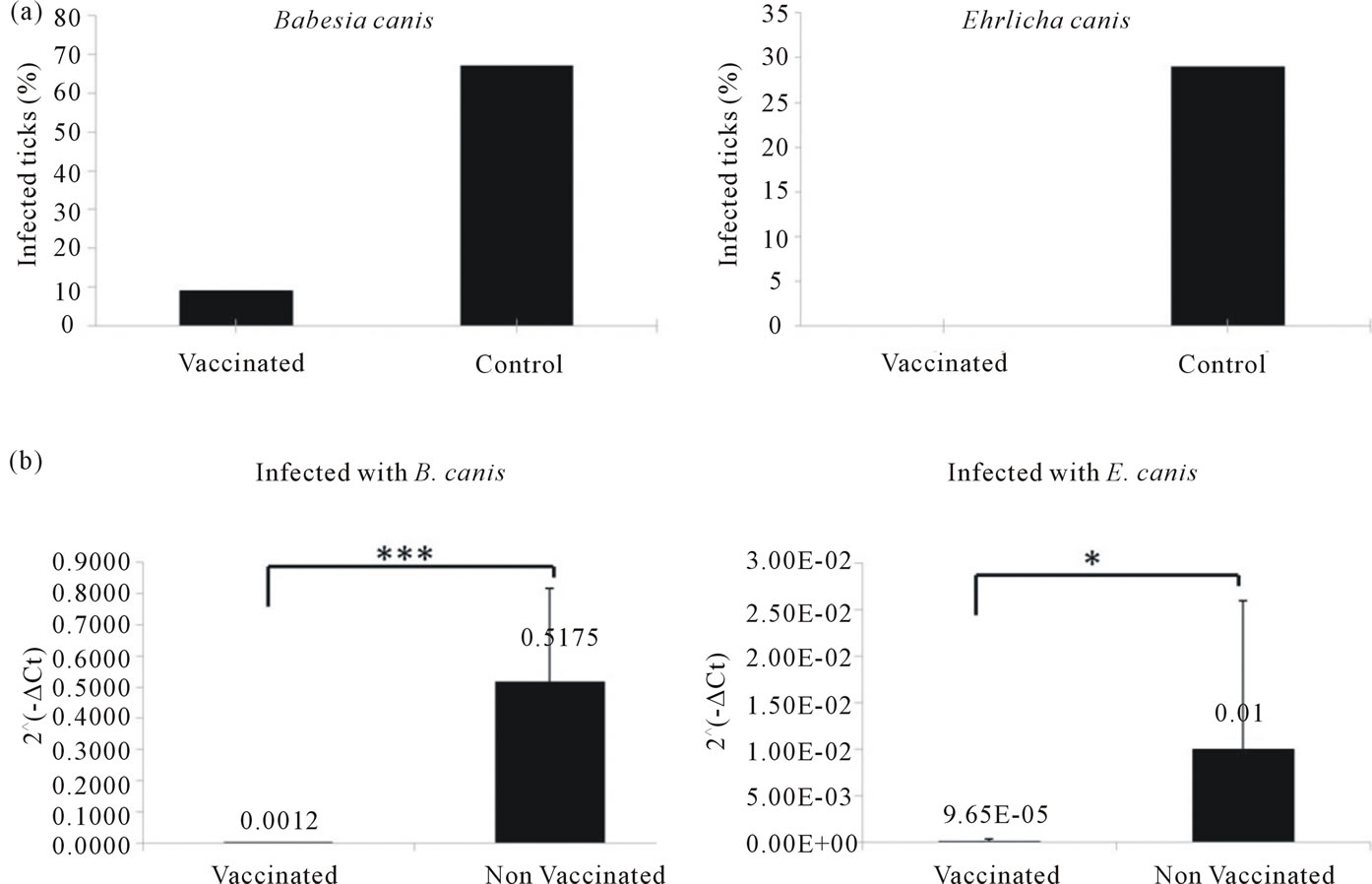
Figure 4. Incidence of infection of naive nymphs fed together with E. canis and B. canis infected ticks determined by conventional pathogen specific PCRs from individual tick genomic DNA (a) and by pathogen specific Real Time PCRs from five different pools of tick genomic DNA from each experimental group (b). Actin gene was used to normalize. Standard deviations are represented. Statistical significances are represented by asterisks (*p < 0.05; ***p < 0.001).
fected as compared to only 9% on the vaccinated dogs. In the case of E. canis also, fewer nymphs became infected on the vaccinated dogs compared with the controls (0% compared to 29%). The qPCR results confirmed the results shown by conventional PCR. Statistically significant differences were found in the average quantity of pathogen DNA between naive tick DNA pools co-feeding with infected ticks on vaccinated dogs compared to the control group for both pathogens (Figure 4(b)).
4. DISCUSSION
For tick-borne transmission of E. canis and B. canis, pathogens are transmitted from an infected tick to the uninfected dog on which it feeds. These pathogens infect host cells and then are transmitted from the infected dog to uninfected ticks cofeeding on the animal. Conventional PCR and qPCR results suggest that immunization with the commercial GAVACTM vaccine provided transmission blocking activity because fewer nymphs became infected compared with the controls as demonstrated by the significantly lowered presence of pathogen DNA in ticks fed on the vaccinated dogs.
The anti-tick vaccine, Gavac, induces an antibody-mediated response that results in rupture of the midgut, tick mortality, and reduced reproductive output [16,19]. This experiment was not designed to assess the effect of Bm86 immunization on R. sanguineus tick feeding and tick survival, as demonstrated previously [22], but rather the transmission-blocking effects of GAVACTM. Results indicate that it could affect the likelihood of nymphs acquiring the infection. It is a challenge to speculate on the possible functions of Bm86 in the gut of ticks and its role in the vector capacity of the ticks.
It is known that Bm86 is a membrane-bound glycoprotein expressed mainly on the surface of the digestive tract of R.B. microplus ticks [37,38]. Its expression is restricted to a few sites on the digestive cell membrane, in the microvilli exposed to the gut lumen [16,39]. The function of Bm86 has not been elucidated; however, it has been speculated that Bm86 is involved in endocytosis of the tick’s blood meal [38,40]. Lysis of midgut digestive cells mediated by anti-Bm86 antibodies during tick feeding occurs in ticks that feed on Bm86 vaccinated cattle, resulting in leakage of blood meal into the tick hemocoel [41]. In addition, it has been shown that this protein contains several epidermal growth factor (EGF)-like domains that may be involved in blood coagulation and cell growth [38,42]. Recent studies silencing of the Bm86 gene via RNA interference (RNAi) are contradictory [43, 44]. Understanding the role of the Bm86 protein in tick biology is critical for understanding the effect of antibodies against Bm86 on ticks and how that could interfere with pathogen transmission.
Both B. canis and E. canis are pathogens infective to the tick. Certain aspects of the Babesia life cycle inside ticks, especially the initial phase in the tick gut lumen are still not well known. However, it is known that after a tick has ingested infected blood, there is a rapid destruction of red blood cells (hemolysis), releasing intracellular forms of Babesia in the lumen of the tick gut. The fact that only a small proportion of blood forms survive intestinal digestion to continue its cycle within the tick has led to the acknowledge that there is a sexual stage of Babesia [45]. Ingested piroplasms develop into male and female gametes within a cell of the intestinal epithelium in the tick. The microgametes fuse with macrogametes to form motile zygotes [43]. The zygotes then multiply and the ‘‘vermicules’’ that result break through the intestinal epithelium towards the hemolymph and invade numerous organs of the tick, including ovaries. In the salivary glands cells, “vermicules” will develop into infectious forms [45]. When the tick attaches to a new host, maturation of the sporozoites takes place and the host is infected with saliva from the tick. Probably, all of the described process in the tick gut requires an intact intestinal epithelium, which is destroyed by antibodies against Bm86 or these antibodies block, in some way, the entry of Babesia piroplasms into cells of the tick intestinal epithelium.
E. canis has a complex and largely unknown life cycle inside the R. sanguineus tick. Ehrlichiae attach to a cell receptor through an adhesin, to enter the host cell [31]. Probably, the mechanism for entering the tick gut cells, its first site of development inside ticks, is the same. After this initial development, many other tick tissues subsequently become infected, including the salivary glands from where the rickettsiae are transmitted during feeding [11]. Defining the binding specificities of Ehrlichiae in a given tick species may lead to the development of a novel type of disease control whose mode of action would be based on competing for the ligands that bind to the pathogen receptors or preventing adhesion to host tissues (transmission-blocking), thereby preventing infection. These data show that antibodies against Bm86 could be blocking, in some way, the E. canis transmission to the R. sanguineus tick. Whether this blocking effect is due to destruction of midgut cells or is due to a more specific effect remains to be clarified. Further studies are suggested to corroborate this finding.
In conclusion, it appears that high antibody titers against Bm86 could help reducing the infection incidence of naive ticks fed together with B. canis and E. canis infected ticks, which suggests the potential of Bm86 vaccination for controlling tick-borne diseases by reducing the vector capacity of the ectoparasite.
5. ACKNOWLEDGEMENTS
The The authors thank Paulo Henrique Sampaio and Patricia Martínez Evora for their help in the execution of the experimental procedures involving animal manipulations and Lídice Méndez Pérez and Yamilet Cárdenas Cuellar for their collaboration in the experimental procedures involving qPCRs. The authors thank Dr. Carlos Borroto for his administrative management and his review of this article. The authors also thank Dr. John van der Meer for his valuable English review of this article.
This work was supported by the ICGEB-TWAS-UNESCO/IBSP Joint Project on Capacity Building in Basic Molecular Biology, Center for Genetic Engineering and Biotechnology, Havana, Cuba and Universidade Estadual Paulista-UNESP, Jaboticabal-SP, Brazil.
REFERENCES
- Parola, P. and Raoult, D. (2001) Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clinical Infectious Diseases, 32, 897-928. doi:10.1086/319347
- Suarez, C.E. and Noh, S. (2010) Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Veterinary Parasitology, 180, 109-125. doi:10.1016/j.vetpar.2011.05.032
- Alonso, M., et al. (1992) Epidemiology of bovine anaplasmosis and babesiosis in Latin America and the Caribbean. Revue Scientifique et Technique (International Office of Epizootics), 11, 713-733.
- Dantas-Torres, F. and Figueredo, L.A. (2006) Canine babesiosis: A Brazilian perspective. Veterinary Parasitology, 141, 197-203. doi:10.1016/j.vetpar.2006.07.030
- Dantas-Torres, F. (2008) Canine vector-borne diseases in Brazil. Parasites & Vectors, 1, 25. doi:10.1186/1756-3305-1-25
- Bourdoiseau, G. (2006) Canine babesiosis in France. Veterinary Parasitology, 138, 118-125. doi:10.1016/j.vetpar.2006.01.046
- Garcia, S., Chinikar, S., Coudrier, D., Billecocq, A., Hooshmand, B., Crance, J.M., Garin, D. and Bouloy, M. (2006) Evaluation of a Crimean-Congo hemorrhagic fever virus recombinant antigen expressed by Semliki Forest suicide virus for IgM and IgG antibody detection in human and animal sera collected in Iran. Journal of Clinical Virology, 35, 154-159. doi:10.1016/j.jcv.2005.02.016
- Kjemtrup, A.M., Wainwright, K., Miller, M., Penzhorn, B.L. and Carreno, R.A. (2006) Babesia conradae, sp. Nov., a small canine Babesia identified in California. Veterinary Parasitology, 138, 103-111. doi:10.1016/j.vetpar.2006.01.044
- Otranto, D., Dantas-Torres, F. and Breitschwerdt, E.B. (2009) Managing canine vector-borne diseases of zoonotic concern: Part one. Trends in Parasitology, 25, 157- 163. doi:10.1016/j.pt.2009.01.003
- Derdakova, M., et al. (2011) Emergence and genetic variability of Anaplasma species in small ruminants and ticks from Central Europe. Veterinary Microbiology, 153, 293- 298. doi:10.1016/j.vetmic.2011.05.044
- Chapman, A.S., et al. (2006) Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: A practical guide for physicians and other health-care and public health professionals. MMWR—Recommendations and Reports, 55, 1-27.
- Fernandez-Salas, A., Rodriguez-Vivas, R.I. and AlonsoDiaz, M. (2011) First report of a Rhipicephalus microplus tick population multi-resistant to acaricides and ivermectin in the Mexican tropics. Veterinary Parasitology, 172, 109-113.
- Miller, R.J., Davey, R.B. and George, J.E. (2007) First report of permethrin-resistant Boophilus microplus (Acari: Ixodidae) collected within the United States. Journal of Medical Entomology, 44, 308-315. doi:10.1603/0022-2585(2007)44[308:FROPBM]2.0.CO;2
- de la Fuente, J., Almazan, C., Canales, M., Perez de la Lastra, J.M., Kocan, K.M. and Willadsen, P. (2007) A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Animal Health Research Reviews, 8, 23-28. doi:10.1017/S1466252307001193
- Willadsen, P., McKenna, R.V. and Riding, G.A. (1988) Isolation from the cattle tick, Boophilus microplus, of antigenic material capable of eliciting a protective immunological response in the bovine host. International Journal for Parasitology, 18, 183-189. doi:10.1016/0020-7519(88)90059-8
- Penichet, M., et al. (1994) Detection of Bm86 antigen in different strains of Boophilus microplus and effectiveness of immunization with recombinant Bm86. Parasite Immunology, 16, 493-500. doi:10.1111/j.1365-3024.1994.tb00377.x
- de la Fuente, J., et al. (1999) Vaccination against ticks (Boophilus spp.): The experience with the Bm86-based vaccine Gavac. Genetic Analysis, 15, 143-148. doi:10.1016/S1050-3862(99)00018-2
- Sossai, S., Peconick, A.P., Sales-Junior, P.A., Marcelino, F.C., Vargas, M.I., Neves, E.S. and Patarroyo, J.H. (2005) Polymorphism of the bm86 gene in South American strains of the cattle tick Boophilus microplus. Experimental and Applied Acarology, 37, 199-214. doi:10.1007/s10493-005-3262-7
- Rodriguez, M., et al. (1995) Control of Boophilus microplus populations in grazing cattle vaccinated with a recombinant Bm86 antigen preparation. Veterinary Parasitology, 57, 339-349. doi:10.1016/0304-4017(94)00678-6
- Valle, M.R., et al. (2004) Integrated control of Boophilus microplus ticks in Cuba based on vaccination with the anti-tick vaccine Gavac. Experimental and Applied Acarology, 34, 375-382.
- de la Fuente, J., et al. (1998) Field studies and cost-effectiveness analysis of vaccination with Gavac against the cattle tick Boophilus microplus. Vaccine, 16, 366-373. doi:10.1016/S0264-410X(97)00208-9
- Perez-Perez, D., Bechara, G.H., Machado, R.Z., Andrade, G.M., Del Vecchio, R.E., Pedroso, M.S., Hernandez, M.V. and Farnos, O. (2010) Efficacy of the Bm86 antigen against immature instars and adults of the dog tick Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). Veterinary Parasitology, 167, 321-326. doi:10.1016/j.vetpar.2009.09.034
- Jones, L.D. and Nuttall, P.A. (1990) The effect of host resistance to tick infestation on the transmission of Thogoto virus by ticks. Journal of General Virology, 71, 1039-1043. doi:10.1099/0022-1317-71-5-1039
- Mishaeva, N.P. (1990) The protection of vertebrate animals from experimental tick-borne encephalitis with active and passive immunization against tick antigens. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii, 93-98.
- Wikel, S.K., Ramachandra, R.N., Bergman, D.K., Burkot, T.R. and Piesman, J. (1997) Infestation with pathogenfree nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infection and Immunity, 65, 335-338.
- Burke, G., Wikel, S.K., Spielman, A., Telford, S.R., McKay, K. and Krause, P.J. (2005) Hypersensitivity to ticks and Lyme disease risk. Emerging Infectious Diseases, 11, 36-41.
- Andreotti, R., Gomes, A., Malavazi-Piza, K.C., Sasaki, S.D., Sampaio, C.A. and Tanaka, A.S. (2002) BmTI antigens induce a bovine protective immune response against Boophilus microplus tick. International Immunopharmacology, 2, 557-563. doi:10.1016/S1567-5769(01)00203-X
- Titus, R.G., Bishop, J.V. and Mejia, J.S. (2006) The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunology, 28, 131-141.
- Labuda, M., Trimnell, A.R., Lickova, M., Kazimirova, M., Davies, G.M., Lissina, O., Hails, R.S. and Nuttall, P.A. (2006) An antivector vaccine protects against a lethal vector-borne pathogen. PLOS Pathogens, 2, e27. doi:10.1371/journal.ppat.0020027
- Bechara, G.H., Szabó, M.P.J. and Ferreira, B.R. (1995) Rhipicephalus sanguineus tick in Brazil: feeding and reproductive aspects under laboratorial conditions. Brazilian Journal of Veterinary Parasitology, 4, 61-66.
- Botelho de Castro, M., Zacarias Machado, R., Cury Tomaz de Aquino, L.P., Alessi, A.C. and Tinucci Costa, M. (2004) Experimental acute canine monocytic ehrlichiosis: Clinicopathological and immunopathological findings. Veterinary Parasitology, 119, 73-86. doi:10.1016/j.vetpar.2003.10.012
- Furuta, P.I., Ferreira de Sousa Oliveira, T.M., Alves Teixeira, M.C., Gouveia Rocha, A., Zacarias Machado, R. and Tinucci-Costa, M. (2009) Comparison between a soluble antigen-based ELISA and IFAT in detecting antibodies against Babesia canis in dogs. Revista Brasileira de Parasitologia Veterinária, 18, 41-45. doi:10.4322/rbpv.01803007
- Olmeda, A.S., et al. (1997) A subtropical case of human babesiosis. Acta Tropica, 37, 229-234. doi:10.1016/S0001-706X(97)00045-4
- Doyle, V., Beugnet, F. and Carithers, D. (2005) Comparative efficacy of the combination fipronil-(S)-methoprene and the combination permethrin-imidacloprid against Dermacentor reticulatus, the European dog tick, applied topically to dogs. Veterinary Therapeutics, 6, 303-310.
- Kumar, S., Malhotra, D.V., Sangwan, A.K., Goel, P., Kumar, A. and Kumar, S. (2007) Infectivity rate and transmission potential of Hyalomma anatolicum ticks for Babesia equi infection. Veterinary Parasitology, 144, 338- 343. doi:10.1016/j.vetpar.2006.10.009
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using Real-Time quantitative PCR and the 2(-ΔΔCT) method. Methods, 25, 402-408.
- Rand, K.N., Moore, T., Sriskantha, A., Spring, K., Tellam, R., Willadsen, P. and Cobon, G.S. (1989) Cloning and expression of a protective antigen from the cattle tick Boophilus microplus. Proceedings of the National Academy of Sciences of the United States of America, 86, 9657- 9661. doi:10.1073/pnas.86.24.9657
- Willadsen, P., et al. (1989) Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. Journal of Immunology, 143, 1346-1351.
- Bastos, R.G., Ueti, M.W., Knowles, D.P. and Scoles, G.A. (2010) The Rhipicephalus (Boophilus) microplus Bm86 gene plays a critical role in the fitness of ticks fed on cattle during acute Babesia bovis infection. Parasites & Vectors, 3, 111. doi:10.1186/1756-3305-3-111
- Gough, J.M. and Kemp, D.H. (1993) Localization of a low abundance membrane protein (Bm86) on the gut cells of the cattle tick Boophilus microplus by immunogold labeling. Journal of Parasitology, 79, 900-907. doi:10.2307/3283728
- Agbede, R.I. and Kemp, D.H. (1987) Ultrastructure of secretory cells in the gut of the cattle-tick Boophilus microplus. International Journal for Parasitology, 17, 1089- 1098. doi:10.1016/0020-7519(87)90161-5
- Kemp, D.H., Pearson, R.D., Gough, J.M. and Willadsen, P. (1989) Vaccination against Boophilus microplus: Localization of antigens on tick gut cells and their interacttion with the host immune system. Experimental and Applied Acarology, 7, 43-58. doi:10.1007/BF01200452
- Nijhof, A.M., Taoufik, A., de la Fuente, J., Kocan, K.M., de Vries, E. and Jongejan, F. (2007) Gene silencing of the tick protective antigens, Bm86, Bm91 and subolesin, in the one-host tick Boophilus microplus by RNA interference. International Journal for Parasitology, 37, 653- 662. doi:10.1016/j.ijpara.2006.11.005
- Liao, M., Zhou, J., Hatta, T., Umemiya, R., Miyoshi, T., Tsuji, N., Xuan, X. and Fujisaki, K. (2007) Molecular characterization of Rhipicephalus (Boophilus) microplus Bm86 homologue from Haemaphysalis longicornis ticks. Veterinary Parasitology, 146, 148-157. doi:10.1016/j.vetpar.2007.01.015
- Mehlhorn, H. and Shein, E. (1984) The piroplasms: Life cycle and sexual stages. Advances in Parasitology, 23, 37-103. doi:10.1016/S0065-308X(08)60285-7

