Open Journal of Animal Sciences
Vol.2 No.2(2012), Article ID:18528,21 pages DOI:10.4236/ojas.2012.22012
Effects of broccoli extract and various essential oils on intestinal and faecal microflora and on xenobiotic enzymes and the antioxidant system of piglets
![]()
1Institute of Agricultural and Nutritional Sciences, Preventive Nutrition Group, Martin Luther University of Halle-Wittenberg, Halle, Germany; *Corresponding Author: andreas.mueller@landw.uni-halle.de
2Institute of Veterinary Hygiene and Infectious Diseases of Animals, Justus Liebig University Giessen, Giessen, Germany
3Institute of Animal Nutrition, Free University of Berlin, Berlin, Germany
4Delacon Biotechnik Ges.m.b.H., Steyregg, Austria
Received 9 February 2012; revised 15 March 2012; accepted 26 March 2012
Keywords: Pigs; Phytogenic Feed Additives; Broccoli Extract; Essential Oils; Xenobiotic Enzymes; Antioxidant System
ABSTRACT
Objective: Since the ban of antibiotics as growth promoting feed additives in the EU in 2006 research in alternatives has gained importance. Phytogenic feed additives represent a heterogenous class of different plant derived substances that are discussed to improve the health of farm animals by direct and indirect antioxidant effects and by influencing microbial eubiosis in the gastrointestinal tract. Consequently our study aimed to investigate the influence of broccoli extract and the essential oils of turmeric, oregano, thyme and rosemary, as selected individual additives, on intestinal and faecal microflora, on xenobiotic enzymes, and on the antioxidant system of piglets. Methods: 48 four weeks old male weaned piglets were assigned to 6 groups of 8. The piglets were housed individually in stainless steel pens with slatted floor. The control group (Con) was fed a diet without an additive for 4 weeks. The diet of group BE contained 0.15 g/kg sulforaphane in form of a broccoli extract. 535, 282, 373 and 476 mg/kg of the essential oils of turmeric (Cuo), oregano (Oo), thyme (To) and rosemary (Ro) were added to the diets of the remaining 4 groups to standardise supplementation to 150 mg/kg of the oils’ key terpene compounds ar-turmerone, carvacrol, thymol and 1,8-cineole. The composition of bacterial microflora was examined by cultivating samples of jejeunal and colonic mucosa and of faeces under specific conditions. The mRNA expression of xenobiotic and antioxidant enzymes was determined by reversing transcrip- tase real time detection PCR (RT-PCR). Total antioxidant status was assayed using the Trolox Equivalent Antioxidant Capacity (TEAC), and lipid peroxidation was determined by measuring thiobarbioturic acid reactive substances (TBARS). Results: Compared to Con piglets all additives positively influenced weight gain and feed conversion in week 1. Over the whole trial period no significant differences in performance parameters existed between the experimental groups. Compared to group Con performance of Ro piglets was, however, slightly impaired. Compared to Con piglets Cuo, Oo and To increased the ratio of Lactobacilli:E. coli attached to the jejunal mucosa, whereas BE and Ro impaired this ratio slightly. In contrast in colonic mucosa Ro improved Lactobacilli:E. coli ratio. In faecal samples an improvement of Lactobacilli:E. coli ratio could be analysed for To and Ro. Ro was the only additive that reduced the incidence rate of piglets tested positive for enterotoxic E. coli (ETEC). All additives significantly increased jejunal TEAC and reduced TBA-RS. In the liver BE, Cuo, Oo and To increased TEAC in tendency and Ro significantly. Liver TBA-RS were slightly reduced by all additives compared to Con piglets. Whereas the influence of BE, To and Ro on jejunal TEAC mainly was derived from the induction of xenobiotic and antioxidant enzymes (indirect antioxidant effects), Cuo and Oo influenced TEAC by direct antioxidant effects. Discussion and Conclusions: Our results have shown: That within the labiatae oils Oo and To have the potential to improve performance slightly. That phytogenic substances have a small but not significant influence on intestinal microflora. That phytogenic feed additives up-regulate the antioxidant system of piglets either by direct or by indirect antioxidant effects and that they may thereby improve health status. That within the labiatae oils Oo has a high direct antioxidant potential whereas Ro potently induces xenobiotic and antioxidant enzymes. That broccoli extract is an attractive new phytogenic additive, improving antioxidant status by indirect antioxidant effects. That defined combinations of selected phytogenic substances may produce additive effects. That health promoting effects of phytogenic additives in the future should be studied systematically under the challenge with pathogenic microorganisms or food derived toxins.
1. INTRODUCTION
Weaned pigs and fast growing broilers are frequently affected by diarrhoea in the first weeks of their lives resulting in impaired performance or the loss of animals. Antibiotics as feed additives were therefore used for a long time in order to protect the animals from this harm. The complete ban of the last three antibiotic feed additives in the European Union (EU) in 2006 bore the necessity to intensify research in alternatives. Besides the supplementation of pig diets with preand/or probiotics or organic acids, phytogenic feed additives have turned out as promising substances for this purpose.
In this context some studies reported that in particular the essential oils (EO) of labiatae plants (Origanum vulgare, Thymus vulgaris and Rosmarinus officinalis) influenced growth and performance of piglets positively whereas other trials found no or even contrary effects [1-5]. These contradictory results with regard to performance are extensively summarised in a recent review [6].
Antibiotic-like bactericidal and/or bacteriostatic effects on several pathogenic intestinal microorganisms may represent another mechanism by which labiatae oils influence the health of farm animals positively. However, most of the studies regarding this topic have been carried out in vitro, frequently in the context of food safety of meat products [7-9]. The bactericidal activity of EOs in vivo is also discussed controversially. Oils or herbal preparations of labiatae plants rather seem to improve microbial eubiosis [10,11] than having direct bactericidal effects against pathogenic bacterial strains [12].
A further mechanism by which labiatae oils may contribute to animal health may consist in their direct antioxidant effects. Presumably the terpene compounds of labiatae oils largely contribute to both their antioxidant potential and to their influence on bacteria. The main terpene compounds of labiatae plants include the phenollic terpenes carvacrol (oregano), thymol (thyme and rosemary) and the epoxy-terpene 1,8-cineole (rosemary). The measurement of the Trolox Equivalent Antioxidative Capacity (TEAC, mmol TROLOX equivalent®/g test substance) represents a frequently applied method to test the antioxidant potential of labiatae oils. In this context it could be demonstrated that oregano had the highest antioxidant potential within labiatae plants (TEAC value: 100.7) followed by rosemary (38.8) and thyme (38.8) [13].
Similar mechanisms improving performance and health of farm animals as reported for labiatae extracts and oils are also discussed for turmeric preparations (Curcuma longa L.). Turmeric oil contains a highly active terpene compound, the aromatic sesquiterpene ar-turmerone, possessing both a strong bactericidal activity against several microorganisms [14] and direct antioxidant effects [15].
Broccoli extract, containing glucoraphanin (GRA), the glucosinolate precursor of the isothiocyanate sulforaphane (SFN), is a phytogenic substance that has been tested as a feed additive only in one broiler study which has been carried out in our group [16]. Due to the lack of studies with other farm animals broccoli extract has no current permission as a feed additive in the EU. In contrast in humans broccoli extract and SFN are generally accepted dietary supplements with a high potential in cancer prevention [17]. In vitro SFN exerted a strong bactericidal activity against various microorganisms such as Enterococcus faecalis, Staphylococcus aureus, Escherichia coli S1 and S2, and Salmonella typhi [18].
The cancer protective effects of turmeric and of SFN are believed to derive from the induction of enzymes with an “Antioxidant Response Element” (ARE) in their DNA. ARE containing enzymes include a large number of xenobiotic enzymes, such as glutathione-S-transferases (GST), epoxide hydrolases (EPHX), aflatoxin aldehyde reductases (AFAR) and of antioxidant enzymes, such as heme oxygenases (HMOX1) and cytosolic copper/zinc-superoxide dismutase 1 (SOD1) [19-24]. The increased transcription of genes with an ARE sequence is initiated by translocation of the transcription factor “Nuclear factor erythroid 2-related factor 2” (Nrf2) [25] to the nucleus and its subsequent association with the ARE sequence.
Both oxidative stress and electrophiles like sulforaphane or terpenes can increase Nrf2 translocation to the nucleus by modifying of redox sensitive cysteine-SHgroups of Kelch-like ECH-associated protein 1 (Keap1) to which Nrf2 is bound in the cytosol [25].
Consequently, in contrast to the direct antioxidant effect, the ability of phytogenic feed additives to induce ARE regulated genes is referred to as indirect antioxidant effect.
The systematical investigation of indirect antioxidant effects of phytogenic feed additives in different farm animal species, including pigs, has been carried out only in our broiler trial until today.
Another problem of a number of studies investigating the influence of phytogenic additives on performance parameters, microbial eubiosis, and on antioxidant effects consists in the use of blends of various phytogenic substances
• Consequently the first aim of our present study with piglets was to investigate systematically the impact of labiatae oils (oregano, thyme, rosemary), a zingiberaceae oil (turmeric) and of a broccoli extract on performance, microbial eubiosis and the antioxidant system.
• The second aim of our study was to standardise the addition of the different phytogenic substances to an equal dietary concentration of their key compounds.
• The third aim of our trial was to give suggestions for expedient combinations of the single additives in order to maximise the impact on performance, microbial eubiosis, and on direct and indirect antioxidant effects.
As the reference substances for the examination of indirect antioxidant effects and for the examination of xenobiotic enzyme induction we used a broccoli sprouts extract and turmeric oil, both having a proven impact on these parameters [15,26-28].
2. METHODS AND MATERIALS
2.1. Pigs Husbandry and Diets
The protocol of the piglet study was approved by the Regional Council of Halle and by the animal welfare committee of the Martin Luther University Halle Wittenberg (record token: 42502-3-559-MLU). Further a certificate of exemption for feeding the broccoli sprouts extract, which has no current permission as a feed additive in the EU was attested by the veterinary administrative office Saxony-Anhalt, Halle (record token: 203.2.1/ 22.10).
48 four weeks old male weaned pigs [(DL × DL) × Pietrain] were obtained from the Intitutes’ own piggery (NWZ Merbitz, Germany). After a 5 day acclimatisation period the piglets (mean body weight: 9.5 ± 0.11 kg) were assigned to 6 groups of 8 animals. Piglets were housed individually in stainless steel pens with slatted floor and fed the experimental diets for 4 weeks.
The control group (Con) was fed a piglet starter diet without any additive. 3694 mg/kg broccoli sprouts extract, providing 369.4 mg/kg GRA (150.0 mg/kg SFN), were added to the diet of group BE. 535 mg turmeric oil/kg diet (group Cuo), 282 mg oregano oil/kg diet (group Oo), 373 mg thyme oil/kg diet (group To) and 476 mg rosemary oil/kg diet (group Ro) were added to the diets of the other 4 groups in order to standardise total EO addition to the amount of 150.0 mg/kg diet of the individual key terpene compound of each essential oil. The standardised main terpenes were ar-turmeron for turmeric oil, carvacrol for oregano oil, thymol for thyme oil, and 1,8-cineole for rosemary oil. The active ingredient in the broccoli sprouts extract and the EOs, as analysed by Delacon Biotechnik Ges.m.b.H. (Steyregg, Austria), had the following concentrations (g/100g = %): broccoli sprouts extract (sulforaphane, 4.06), turmeric oil (ar-turmerone, 30.0), oregano oil (carvacrol, 65.0), thyme oil (thymol, 49.0), and rosemary oil (1,8-cineole, 45.0). Premixes of the essential oils using Sipernat® as the carrier matrix were prepared by Delacon Biotechnik Ges.m. b.H. and added to the basal diet (Table 1).
Piglets had free access to their respective diet and water. During the experiment temperature, humidity and lightening were controlled. The temperature was gradually reduced from 26˚C on day 1 to 21˚C on day 28. Lighting regime consisted of a 12 h light: 12 h dark circle. Minerals, vitamins and essential amino acids were added to all diets as recommended for pigs by the Society of Nutrition Physiology [29] and of the National Research Council [30].
Feed intake and individual live weight were recorded once a week. Feed conversion was calculated from the ratio of feed intake (g) and weight gain (g). On day 28 piglets were exsanguinated after stunning for organ sampling (blood, liver, jejunal mucosa, ileal mucosa and colon). Mucosa samples of small intestine were prepared from a 15 cm jejunal segment located 10 cm distal to the duodenum. Ileum mucosa was obtained from a 10 cm segment located proximal to the caecum. Colon tissue was taken from a 10 cm segment located distal to the caecum. At the beginning [first bacterial examination (BE)] and at the end (final BE) of the experiment faeces samples were collected directly from the piglets’ anus using rectal swabs.
2.2. Haemogram
Blood samples of each piglet were taken at the end of the experiment using ethylenediamine tetraacetic acid (EDTA) coated tubes. The haemogram was analysed at the “Klinik für kleine Klauentiere und Forensische Medizin” of the “Stiftung Tierärztliche Hochschule Hannover” using a high throughput haemoanalyzer (Nihon Kohden Cell Tac alpha, MEK-6450K).
2.3. Microbiological Determinations in Faeces, Jejunal and Colonic Segments of Piglets
In order to determine the counts of bacteria in faeces (first and final BE) rectal swabs were cut off, put in 5 ml cold sterile PBS-buffer and mixed for 10 s. To determine bacteria attached to the jejunal and the colonic mucosa immediately after slaughter the specific gut segments mentioned under “Pigs Husbandry and Diets” were thoroughly rinsed with sterile physiological sodium chloride solution (0.9%w/v). Subsequently the mucosa of these jenunal and colonic segments was scraped off with a sterile glass slide. Then 1 g of the mucosa preparations were suspended in 5 ml cold sterile PBS-buffer and mixed for 10 s. Solutions were transferred into 50 ml tubes containing 4 g sterile glass beads and stored at 4˚C. For microbiological determinations log10-serial dilutions of faeces and mucosa samples were prepared. 50 µl of each serial dilution were disseminated on agar plates. Specific culture media for the single bacterial strains and incubation conditions are shown in Table 2. Bacterial counts are presented as CFU (colony forming units) per g mucosa or per rectal swab. To determine the count of aerobic mesophilic bioburden mucosaand swab-preparations were plated on blood agar. For the determination of the fraction of facultative anaerobic bacteria (Enterobacteriaceae, e.g. Escherichia and Enterobacter) as a part of the aerobic mesophilic bioburden, Gassner agar was used for cultivation. In order to determine coliform bacteria (e.g. E. coli, Salmonella), representing lactose-metabolising Enterobacteriaceae, the samples were also incubated on Gassner agar with additional supplementation of the pH-indicator “soluble blue”. The analysis of the samples after cultivation on Violet Red Bile agar with 4
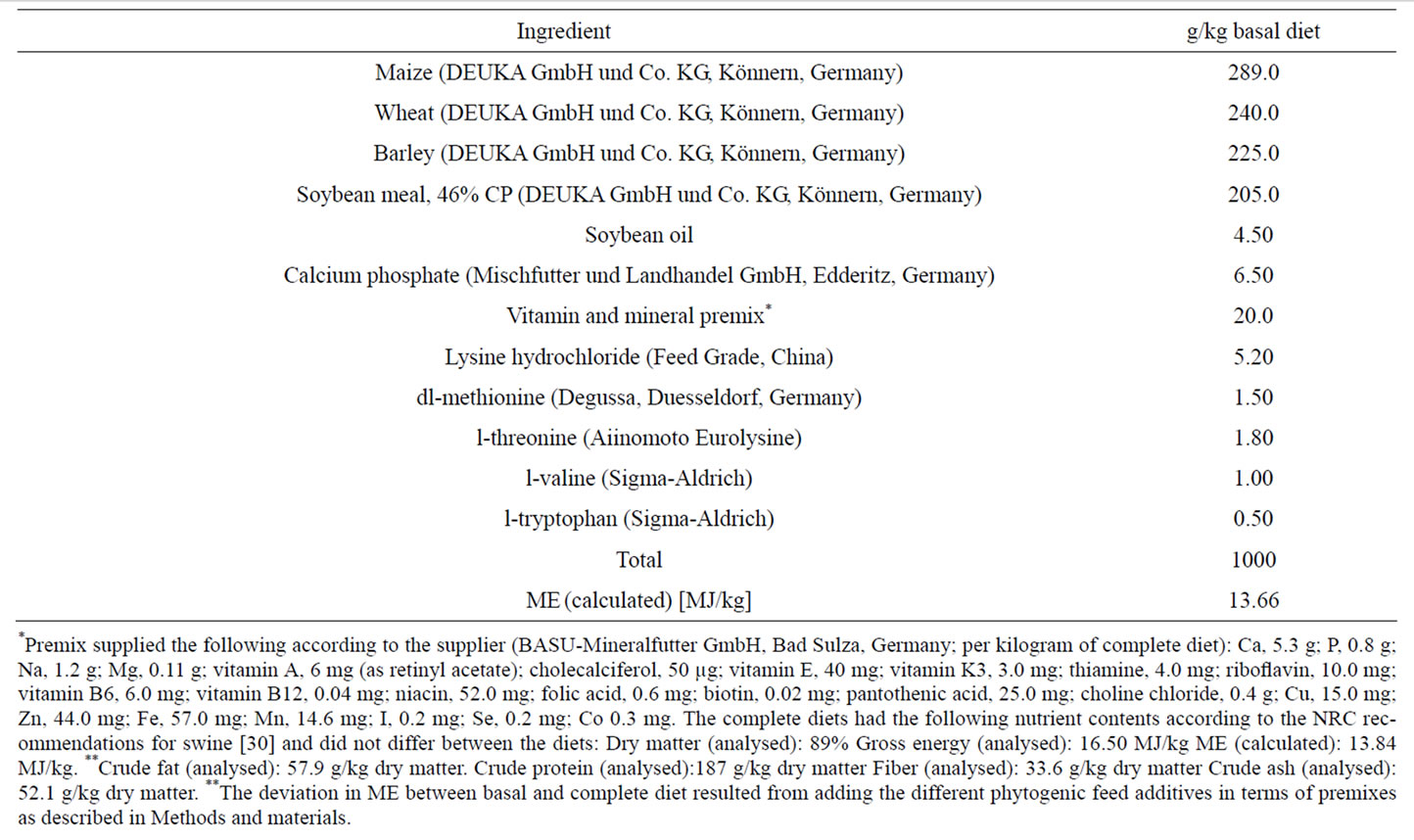
Table 1. Composition of the basal diet.
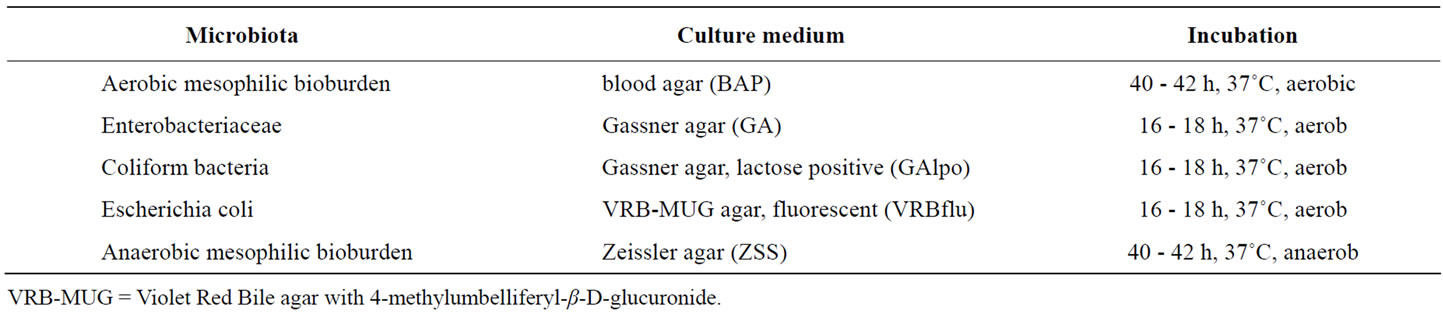
Table 2. Bacterial specific growing conditions.
methylumbelliferyl-β-D-glucuronide (VRB-MUG) under UV-exposure yielded the count of E. coli. Cultivation of the samples prepared from jejeunal and colonic mucosa and from rectal swabs on Zeissler agar under anaerobic conditions yielded the count of anaerobic mesophilic bioburden, including fastidious anaerobic (Lactobacilli) and facultative anaerobic bacteria.
The E. coli specific heat stable enterotoxin II (estb) was determined in faecal samples using semiquantitative PCR. Therefore bacterial colonies of selected agar plates were collected in a solution consisting of 1.5 ml sodium chloride (0.9%) and 0.5 ml glycerine. From this solution an aliquot of 3 µl was added to the PCR reaction mixture containing 1 × ammonium buffer, magnesium chloride (2 mM), estb-primer (0.5 µM each), nucleotides (133 µM each) and Taq Polymerase (0.03 U/µl). The nucleotide sequences of the estb primers were as follows: for: 5’ TGCCTATGCATC-TACACAAT 3’; rev: 5’ CTCCAGCAGTACCATCTCTA 3’. Annealing temperature of the primers was 55˚C. Following amplification for 30 cycles the resulting PCR products were run on 3% agarose gels and visualised under UV light in order to confirm the existence of enterotoxic estb producing E. coli in the sample.
2.4. RNA Preparation and Real Time RT-PCR Assay Including the Stability Analysis of Four Selected Reference Genes in Jejunal Mucosa, Ileal Mucosa, Colon and Liver
To determine mRNA expression levels of GPx1 (glutathione peroxidase 1), SOD1 (Cu/Zn-dependent superoxide dismutase), GSTa2 (glutathione-S-transferase alpha 2), HMOX1 (heme oxygenase 1), EPHX1 (microsomal epoxide hydrolase 1), AFAR (aflatoxin B1 aldehyde reductase) and KEAP1 (Kelch-like ECH-associated protein 1) total RNA was isolated from liver, jejunal mucosa, ileal mucosa and colon using Trizol® reagent (Invitrogen) according to the manufacturers’ protocol. Following the photometrical determination of RNA concentration and purity at 260 nm and 280 nm, RNA quality was checked by testing the integrity of the 18Sand 28Sribosomal RNA bands and by controlling the absence of genomic DNA in 1.2% agarose gels. Reverse transcripttion of total RNA (3 µg) was carried out using a commercial cDNA synthesis kit (RevertAidTM First Strand synthesis kit, Fermentas, Latvia). mRNA expression was analysed by real time detection polymerase chain reaction (RT-PCR) as described previously [16]. Subsequent to the identification of the correct length of the amplification products in 1.2% agarose gels, relative quantification of mRNA expression was performed using the ∆∆Ct method [31]. In accordance with the current guidelines for the proper determination of gene expression data in various tissues [32], a set of four reference genes was selected and their expression stability (M) was ranked according to the standard procedure [33-35]. Beta actin (b-Actin), glycerine aldehyde-3-phosphate dehydrogenase (GAPDH), ribosomal phosphoprotein large PO subunit (RPP0) and beta-2-microglobulin (B2M) were selected as capable reference genes reported in current literature [34,35], and their expression (Ct values) was measured in jejunum, ileum, colon and liver. The treatment independent expression stability (M) was determined by calculating the Ct ratios of one gene with all the other genes. Subsequently the standard deviation of the logarithmically transformed ratios was calculated and plotted [33]. According to their expression stability M, indicated by a decrease of standard deviation, a ranking of the most stable reference genes was compiled for each tissue investigated. The best set of housekeeping genes was used for normalisation of the expression data of the target genes. The expression values of target genes were normalised using the arithmetic mean of the Ct values of RPP0 and GAPDH in jejunal and ileal mucosa, of RPP0 and B2M in colon, and of GAPDH and b-Actin in the liver. Primer sequences used in PCR and their gene bank accession numbers are shown in Table 3.
2.5. TROLOX® Equivalent Antioxidant Capacity (TEAC) in Jejunal Mucosa, Colon and Liver Tissue
TEAC was measured in 1:5 (w/v) crude homogenates of jejunal mucosa, colon and liver using the method originally described by Miller et al. [36] with modifications of Wang et al. [37]. The method is based on monitoring the inhibition of ABTS radical formation spectrophotometrically at 600 nm and 20˚C for 15 min. The reaction mixture contained PBS buffer, ABTS reagent (0.15 mM), H2O2 (0.1 mM) and metmyoglobin (2.50 µM). Sample TEAC values were calculated by comparison to TEAC values of a TROLOX® standard curve (concentration range: 0 to 21.0 µM). The comparison was carried out in the linear range of the reaction (jejunal mucosa: 10 min, colon: 7 min, liver: 7 min). The TEAC values of the samples were expressed in µmol TROLOX® equivalent per g organ fresh matter and kg body weight. Samples were measured in duplicate.
2.6. 2-Thiobarbituric Acid-Reactive Substances (TBA-RS) in Jejunal Mucosa and the Liver
To analyse lipid peroxidation in intestine and liver samples 2-Thiobarbituric acid-Reactive Substances (TBARS) were measured in 25 µl of 1:5 (w/v) crude homogenates of jejunal mucosa and liver according to a modified protocol from Wong et al. [38]. After adding 375 µl
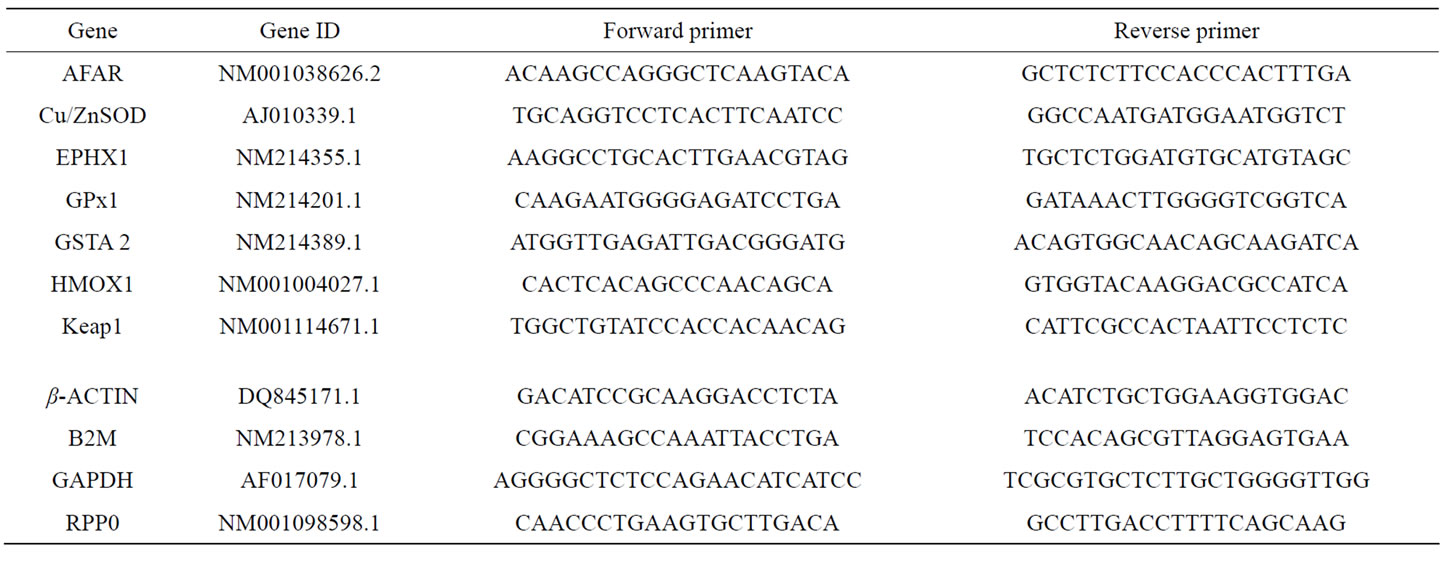
Table 3. Primer sequences of the porcine primers for PCR analyses.
H3PO4 (0.44 M), 225 µl aqua bidest. and 125 µl 0.6% 2- thiobarbituric acid, the samples were incubated in a thermo block at 100˚C for 60 min. Blanks were determined using 25 µl of potassium phosphate buffer (0.1 M) instead of the different crude homogenates. Subsequent to heating the samples were chilled on ice and mixed with 750 µl of methanolic NaOH (10 ml of 1 M NaOH, 90 ml methanol). After vortexing and centrifugating the samples at 4000 g at 4˚C for 10 min, the extinction was measured spectrophotometrically at 532 nm. To calculate the samples’ TBA-RS concentrations a calibration curve with 1,1,3,3,-tetraethoxypropane in a concentration range of 0.60 - 1.20 mM was prepared. The TBA-RS values were expressed in nmol per g organ fresh matter and kg body weight. Each sample (n = 8) was measured in duplicate.
2.7. Protein Concentration of Samples
Protein concentration in homogenates of jejunal mucosa, colon and liver was determined using the standard method of Bradford (1976) [39], adapted to the needs for measurement in a 96 well plate reader.
2.8. Statistical Analysis
Data are presented as means ± SEM or as medians ± min. and max. values for bacterial counts. Following assurance the normality of distribution (Shapiro Wilk test and Kolmogorov Smirnov test) and the homogeneity of variances (Levene’s test), data were analysed with SPSS 19.0 for Windows using the one-way ANOVA procedure. If variances were homogenous, significant differences between means (P < 0.05) were evaluated with the Least Significant Difference (LSD) test, if not the Games Howell test was used. All tables were prepared with Microsoft Excel (Version 2003).
3. RESULTS
3.1. Performance Parameters (Feed Intake, Body Weight, Weight Gain, Feed Conversion Ratio)
With the exception of two piglets (1 from group BE and 1 from group Ro) which needed a single antibiotic treatment, all other piglets showed no clinical abnormalities during the whole experiment. The haemogram of piglets in all groups (Table 4) indicated a good health status, since no parameter laid outside the normal range.
During the whole experimental period no significant differences in feed intake could be registered between the experimental groups (Table 5). Piglets of groups Cuo, Oo and To had somewhat higher final body weights compared to Con piglets. However, these differences were not significant. Although piglets of group To had the best feed conversion (1.35:1) compared to all the other experimental groups, this parameter did not differ significantly from any group.
3.2. Microflora in Jejunal and Colonic Mucosa
Due to a high individual variation, no significant differences for aerobic mesophilic bioburden, Enterobacteriaceae, coliform bacteria, E. coli and anaerobic mesophilic bioburden in jejunal mucosa existed between the experimental groups (Table 6). However, it is obvious from the jejunal data, that Con pigs had the highest maximum counts for all bacterial classes investigated (50% of the animals above the median value), including coliform bacteria and E. coli. In contrast the number of Lactobacilli, calculated from the difference between anaerobic mesophilic bioburden and Enterobacteriaceae was the lowest in the jejunal mucosa of Con pigs. Nevertheless, comparison of E. coli median values revealed
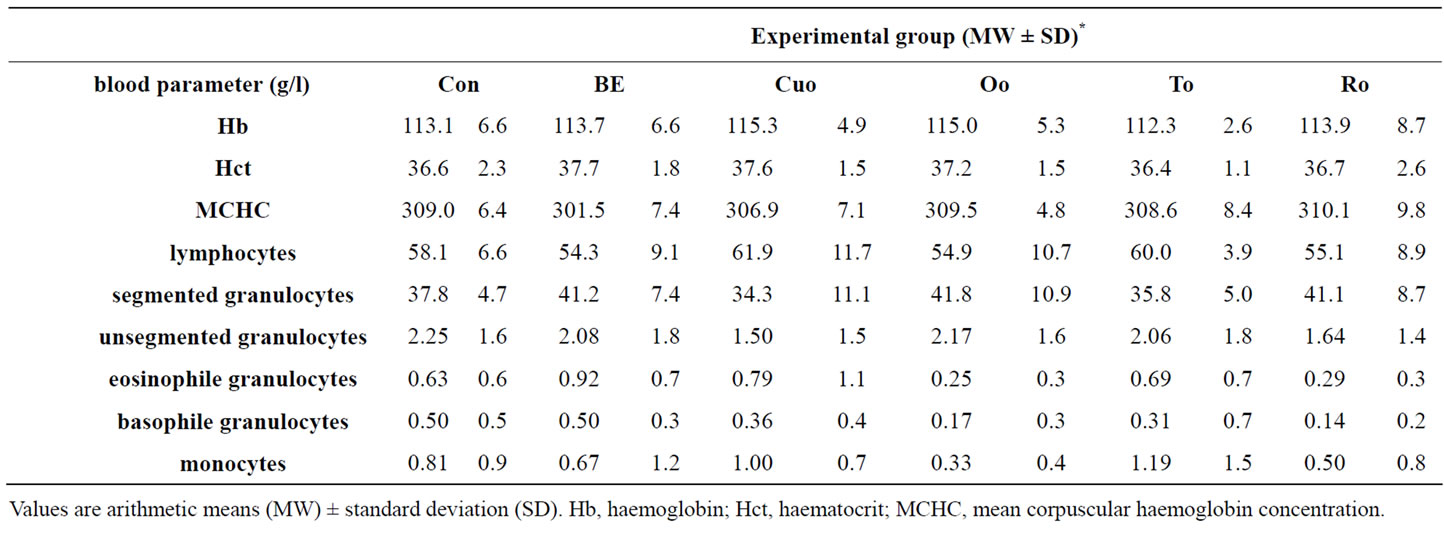
Table 4. Blood parameters of piglets feeding diets containing different phytogenic feed additives for 28 days.
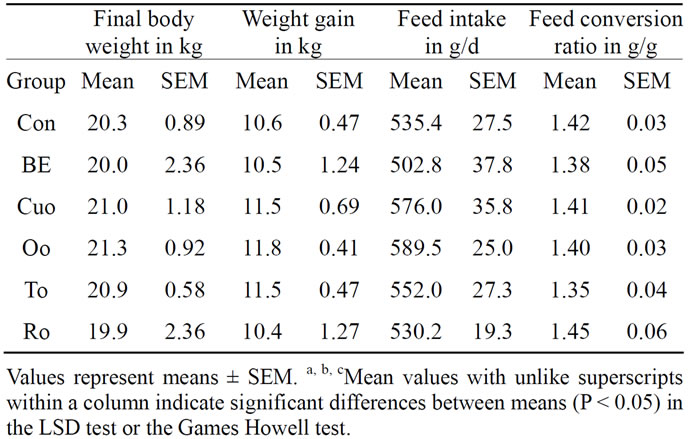
Table 5. The effects of feeding diets containing different phytogenic feed additives for 28 days on final body weight (kg), body weight gain (kg), daily feed intake (g) and feed conversion ratio (g/g) in piglets.
that Cuo, Oo, and To reduced the number of E. coli bacteria compared to Con piglets, whereas BE and Ro increased their number.
As similarly observed in jejunal mucosa also in colonic mucosa no statistically significant differences regarding all bacterial classes investigated existed between the experimental groups (Table 7). In contrast to the results for jejunal microflora in particular Ro piglets showed a reduced E. coli number attached to colonic mucosa compared to Con piglets. Moreover in colonic mucosa Lactobacilli:E. coli ratio was better in Ro piglets than in Con piglets.
3.3. Microflora in Faecal Samples Collected with Rectal Swabs
Additionally to the determination of bacteria attached to jejunal and colonic mucosa we have determined bacterial counts in the faeces of the piglets at the beginning and at the end of the trial (Table 8). Although the treatment of the piglets with the different phytogenic additives caused no significant changes in faecal E. coli and Lactobacilli counts as well as in the Lactobacilli:E. coli ratio, To and Ro influenced these parameters in tendency. Whereas there existed no differences in the initial E. coliand Lactobacilli counts between the experimental groups (E. coli: 3.73 ± 0.18; Lactobacilli: 6.54 ± 0.12; Ratio Lactobacilli:E. coli: 1.50 ± 0.04), both To and Ro reduced final E. coli count distinctly and improved Lactobacilli:E. coli ratio compared to Con piglets and to piglets of all the other treatments.
In this context our data regarding the heat stable enterotoxin estb produced by enterotoxic E. coli strains of the serotypes O149:K91 with F4 or F6 fimbriae and O138:K81 with F18 fimbriae are of interest. At the beginning of the experiment estb was detectable with a varying incidence rate [12.5% (1 of 8) to 25.0% (2 of 8)] in faecal samples of all experimental groups. The incidence of estb detection rate remained unchanged in groups BE, Oo and To. One more piglet of the Cuo group was estb positive (+12.5%) at the end of the experiment. As the only additive Ro reduced estb incidence rate by 12.5%, whereas in Con piglets the highest increase in estb incidence rate (+25%) was analysed at the end of study (Figure 1).
3.4. Expression of ARE Regulated Xenobiotic and Antioxidant Enzymes in Jejunal and Ileal Mucosa and in Colon and Liver Tissue
The expression patterns of selected ARE regulated xenibiotic and antioxidant enzymes depended on the phytogenic additive and on the tissue investigated. In the jejunum BE, used as one reference substance, potently up-regulated the expression of all xenobiotic and antioxidant enzymes investigated compared to Con piglets, with significant changes for AFAR (factor: 2.14), GSTa2
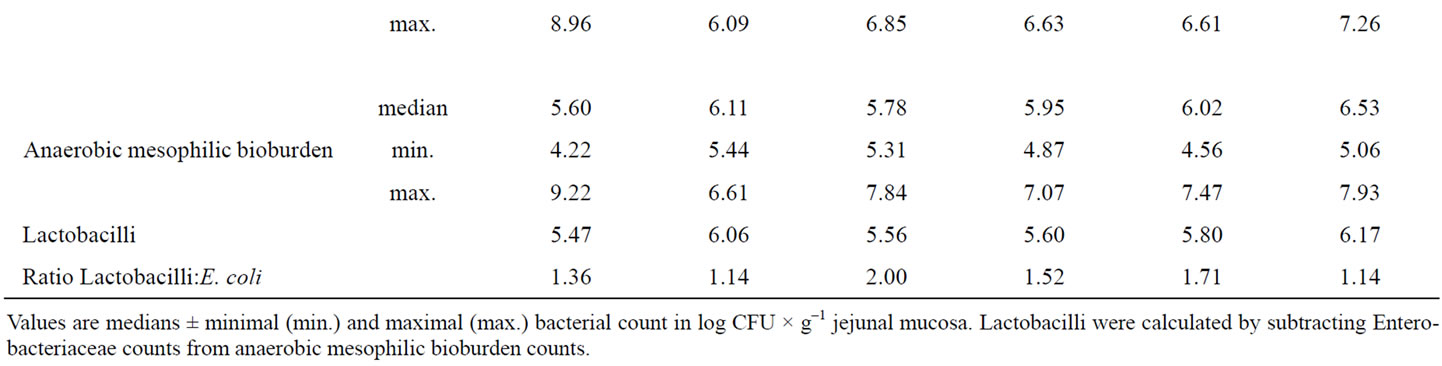
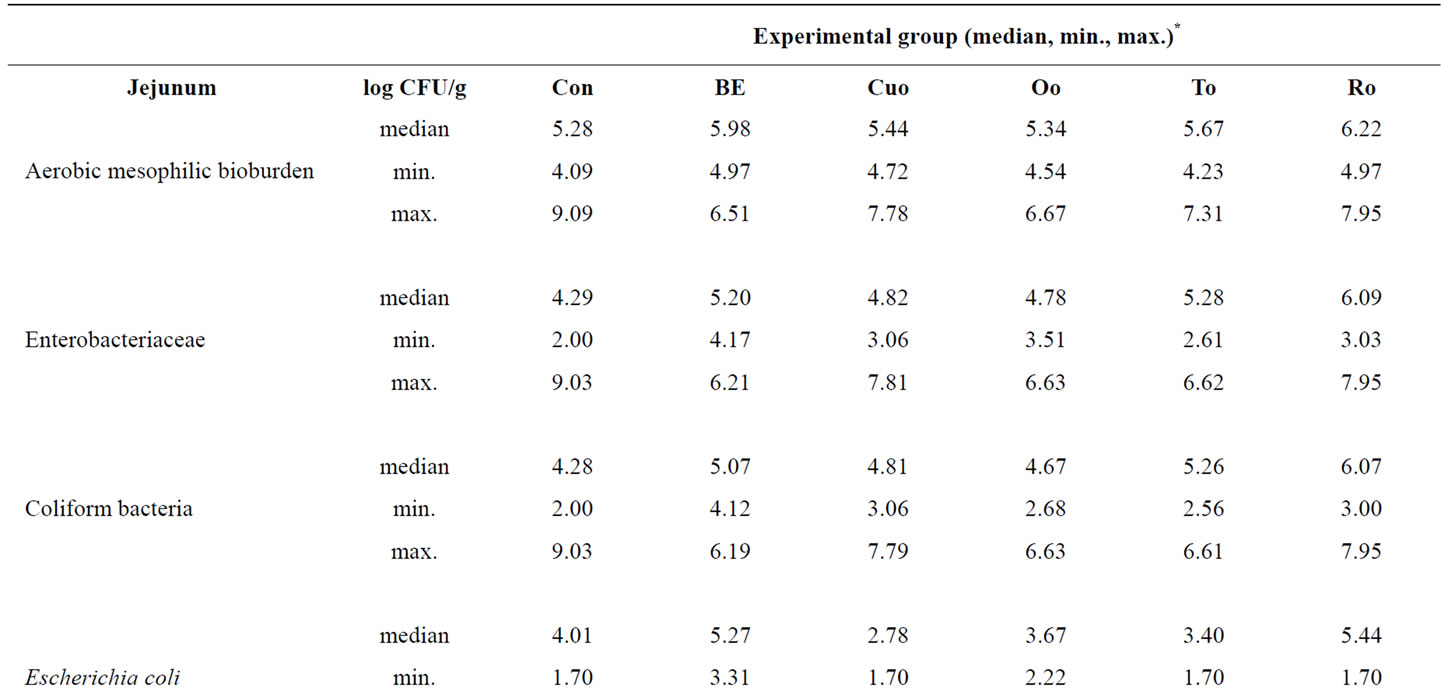
Table 6. Influence of different phytogenic feed additives on bacterial microflora in jejunal mucosa of piglets.
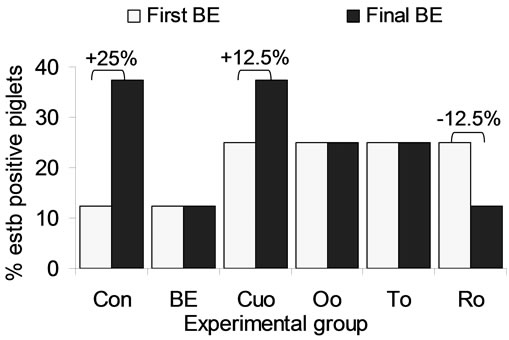
Figure 1. Influence of different phytogenic feed additives on the detection of the heat stable enterotoxin estb produced by enterotoxic E. coli strains of the serotypes O149:K91 with F4 or F6 fimbriae and O138:K81 with F18 fimbriae in faeceal samples.
(factor 2.15), GPx1 (factor 1.79) and SOD1 (factor 2.14) (Table 9(a)). Within the labiatae oils to and in particular Ro were most effective in the up-regulation of xenobiotic and antioxidant enzymes. Both oils increased the mRNA expression of jejunal GPx1 and EPHX1 significantly compared to Con piglets. Whereas To additionally increased SOD1 mRNA level significantly, Ro strongly influenced AFAR expression compared with Con piglets. Jejunal mRNA expression of some of the genes investigated was more potently increased by To and in particular by Ro than by BE. Jejunal HMOX1 mRNA was upregulated by feeding the rosemary and BE diets. However, due to a high standard deviation for this enzyme the effect was not statistically significant. In contrast to our expectations, the second reference additive Cuo caused no increase in the mRNA levels of the jejunal xenobiotic and antioxidant enzymes as well as Oo, coming along with a high direct antioxidant capacity (Table 9(a)). A completely different induction pattern for the xenobiotic and antioxidant enzymes was analysed in the ileum. No effects of the most potent additives BE, To and Ro could be measured with regard to the induction of the antioxidant enzymes GPx1, SOD1 and HMOX1 (Table 9(b)). In contrast the mentioned additives positively affected the expression of the xenobiotic enzymes AFAR, EPHX1 and GSTa2, with significant effects of BE on AFAR (factor 3.80) and EPHX1 (factor 2.78), of To on GSTa2 (factor 1.93), and of Ro on AFAR (factor 3.40). More
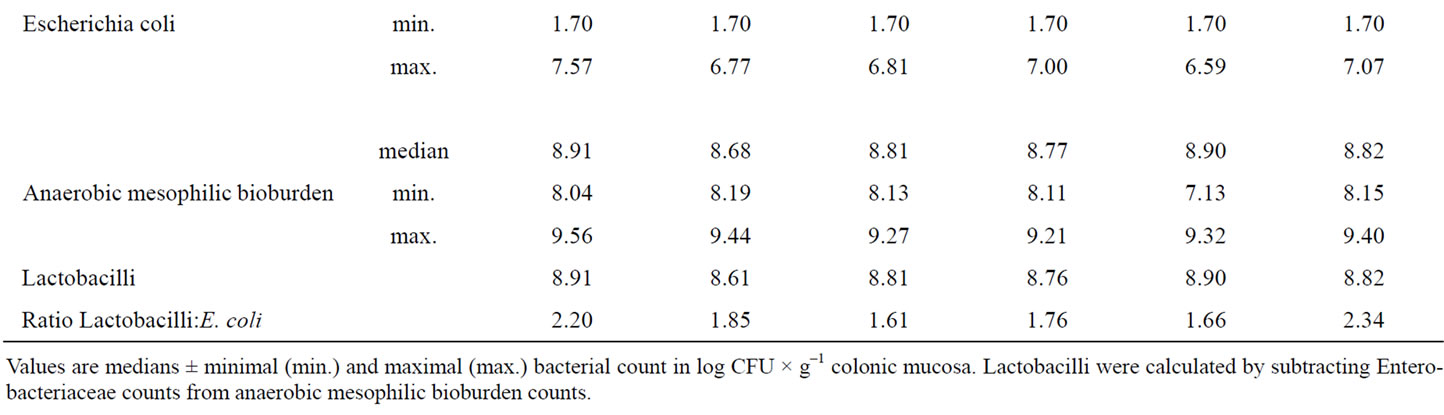
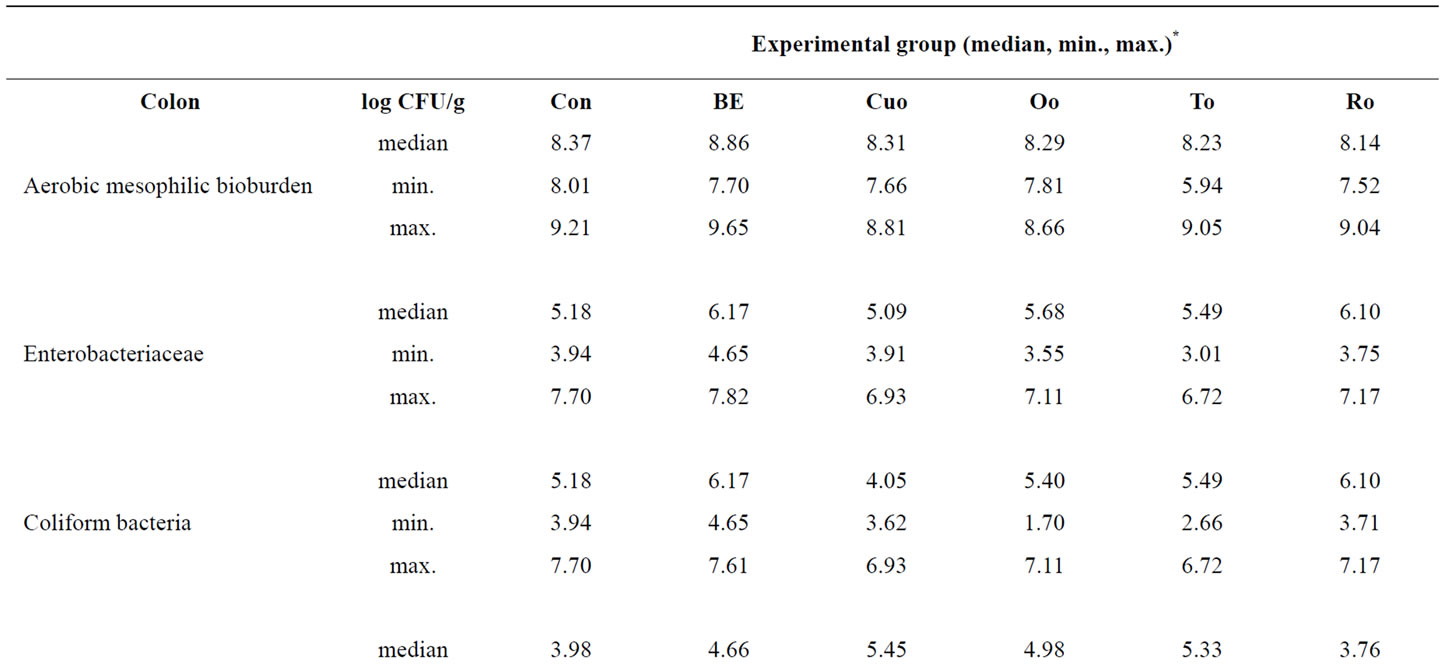
Table 7. Influence of different phytogenic feed additives on bacterial microflora in colonic mucosa of piglets.
over the impact of BE and Ro on ileal AFAR expression (average factor: 3.60) compared to Con piglets was distinctly higher than in the jejunum.
As observed in the jejunum, the addition of Cuo and Oo caused no significant changes in the expression of xenobiotic and antioxidant genes also in the ileum.
Most interestingly in the colon a completely different regulation profile of antioxidant and xenobiotic enzymes was measured due to feeding the pyhtogenic substances. In colon rosemary was the only additive that powerfully induced both antioxidant enzymes (GPx1, factor 3.20; SOD1, factor 1.62) and xenobiotic enzymes (AFAR, factor 2.17; GSTa2, factor 1.71). The other feed additives only increased GPx1 mRNA expression to small and not significant extent, whereas nearly no changes or even a marked down-regulation could be observed for the xenobiotic enzymes (AFAR, EPHX1, GSTa2).
In the liver the reference additives BE and Cuo significantly increased the mRNA concentrations of AFAR (average factor 1.46) and EPHX1 (average factor 2.12) compared to the Con group (Table 9(c)). EPHX1 expression was also significantly increased in piglets of the Oo group (factor 1.97) and in the Ro group (factor 1.55). In groups BE and Ro the expression of GSTa2 was downregulated by 39% to 43% of the Con level (Table 9(d)). While BE significantly increased hepatic GPx1 mRNA expression (factor 1.66), the addition of Cuo increased SOD1 mRNA levels (factor 1.47) in liver tissue.
3.5. TROLOX® Equivalent Antioxidant Capacity (TEAC) in Jejunum Mucosa, Colon and Liver Tissue
TEAC values in jejunum, colon and liver are shown in Figure 2. Jejunal and hepatic TEAC values showed a similar height, whereas colonic TEAC reached only about 30% to 50% of those in jejeunum and liver (Figure 2). All phytogenic feed additives increased jejunal TEAC by 26% to 64% compared with Con piglets. In the colon no effects of the phytogenic additives on TEAC could be measured. Although BE (20.7%), Cuo (13.5%), Oo (10.3%), To (26.1%) increased liver TEAC distinctly compared to Con piglets, only the rise produced by feeding Ro (46.4%) was significant.
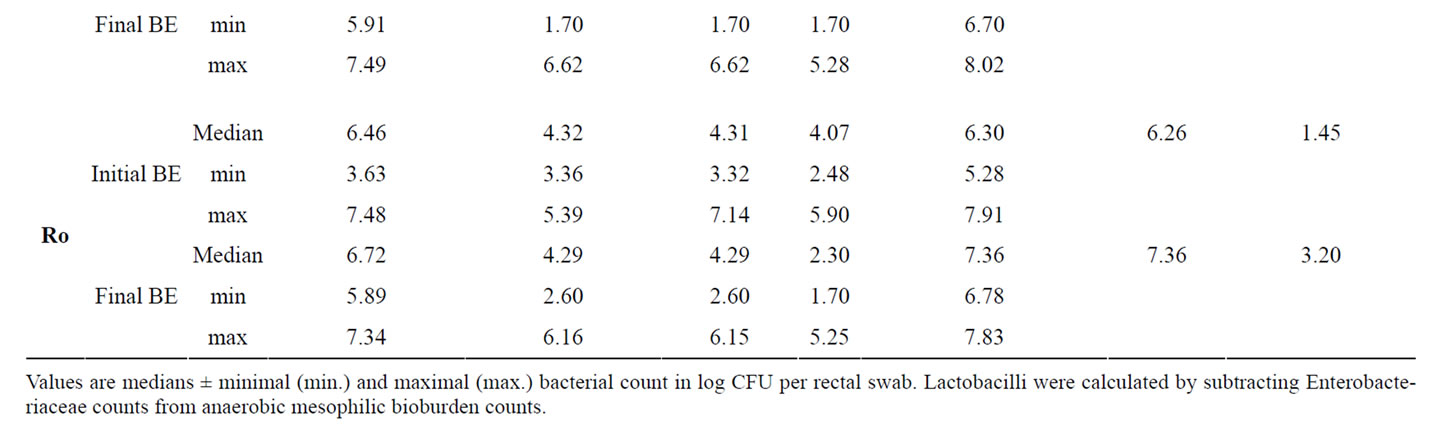
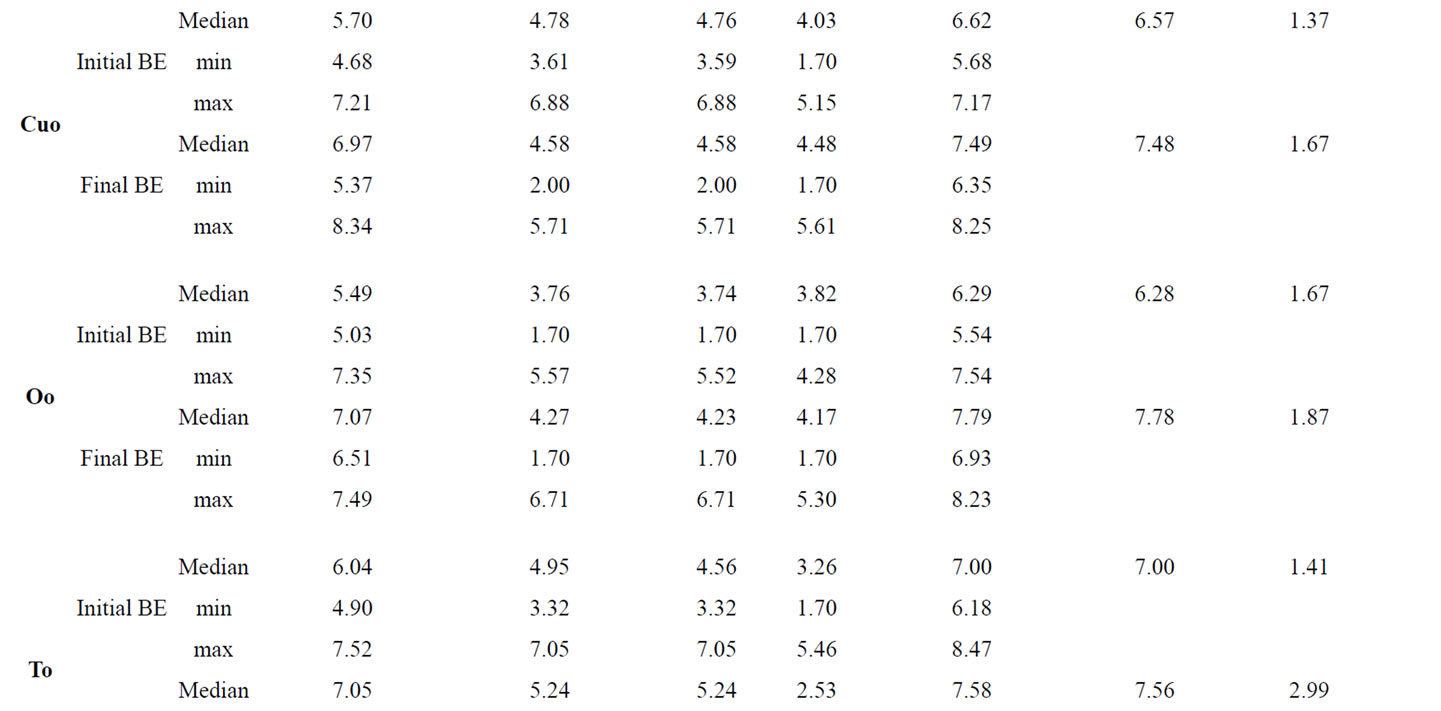
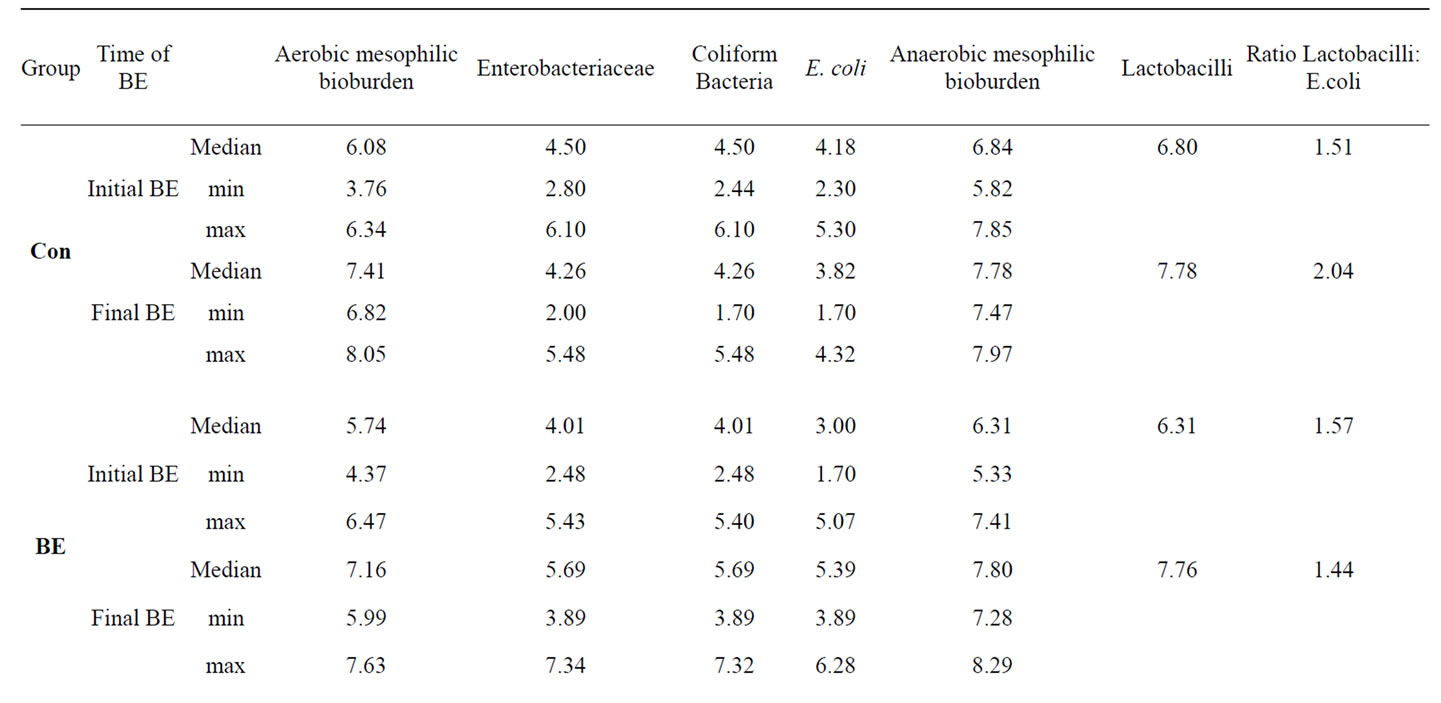
Table 8. Influence of different phytogenic feed additives on bacterial microflora in faecal samples of piglets.
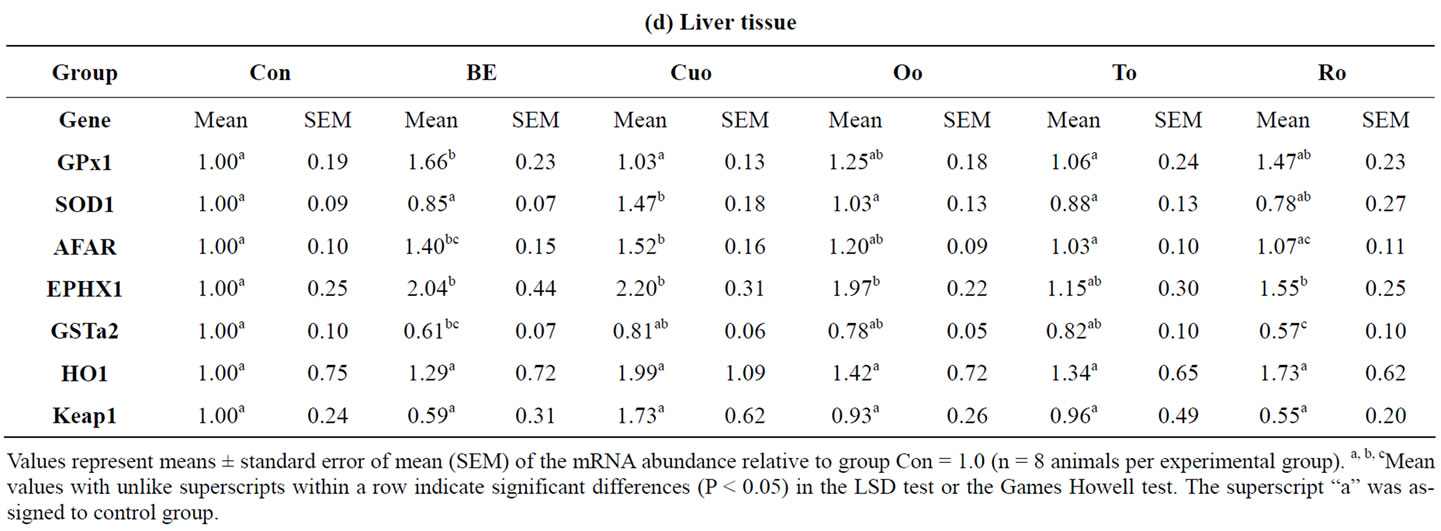
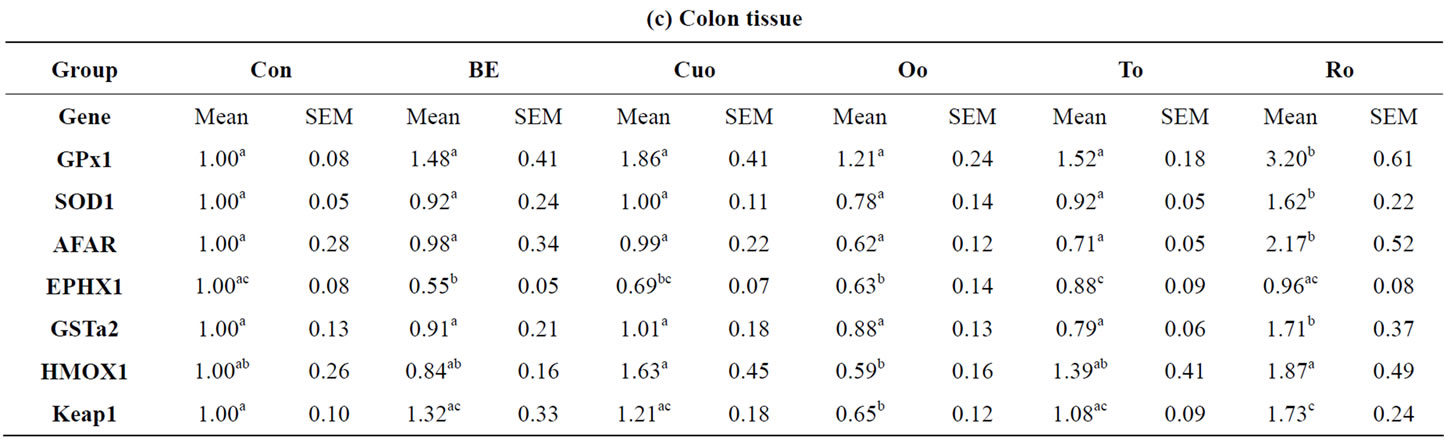
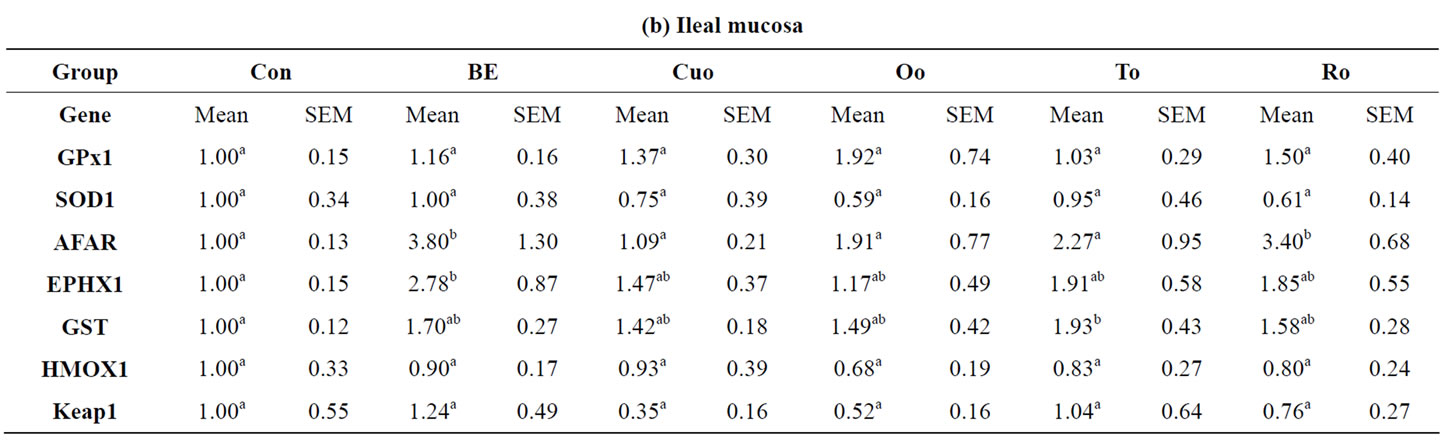
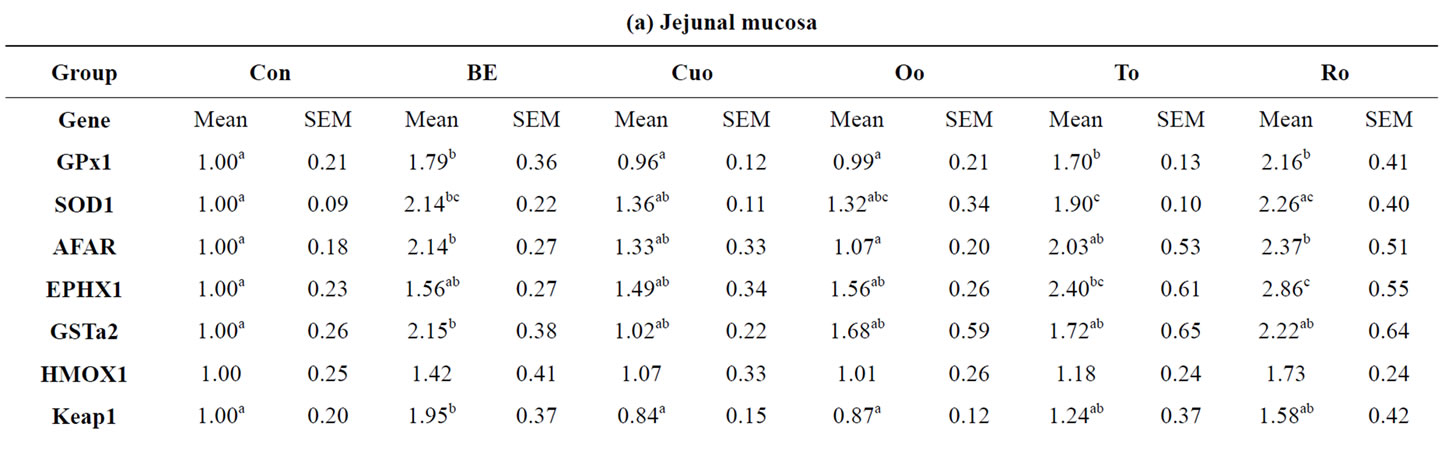
Table 9. Relative mRNA concentration of xenobiotic (GSTa2, EPHX1, AFAR) and antioxidant enzymes (GPx1, SOD1, HMOX1) and KEAP1 in jejunal and ileal mucosa, colon and liver tissue of piglets.
3.6. 2-Thiobarbituric Acid-Reactive Substances (TBA-RS) in Jejunal Mucosa and the Liver
In the jejunum TBA-RS concentration was distinctly (P < 0.150) but not significantly reduced by BE (16.1%), Cuo (14.2%), To (25.9%), and Ro (16.0%). Oo (25.7%) and To (25.9%) decreased jejunal lipid peroxidation to a significant extent (Figure 3). Liver TBA-RS were reduced by feeding all phytogenic additives to a greater or lesser extent (BE: 21.3%, Cuo: 8.22%, Oo: 23.8%, To: 11.5%, Ro: 23.2%). However, in the liver no significant differences resulted from these data, due to a high variation within the groups.
4. DISCUSSION
4.1. Performance Parameters (Feed Intake, Body Weight, Weight Gain, Feed Conversion Ratio)
As stated in the introduction data from the literature,
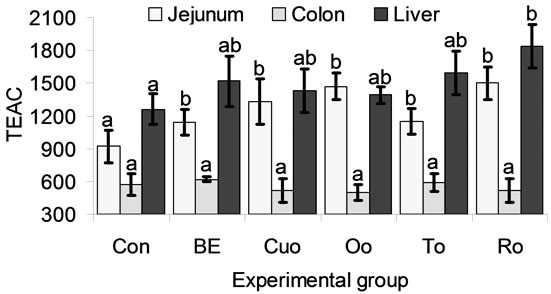
Values represent means ± SEM (n = 8 animals per experimental group). a,bMean values with unlike superscripts indicate significant differences between means (P < 0.05) in the LSD test. The superscript “a” was assigned to the control group.
Figure 2. TROLOX® equivalent antioxidant capacity (TEAC in µmol per g organ fresh matter and kg body weight) in jejunal mucosa, colon and the liver of piglets.
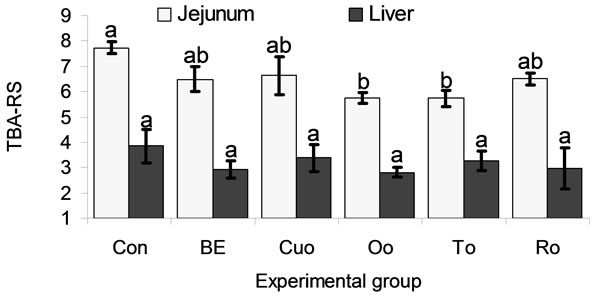
Values represent means ± SEM (n = 8 animals per experimental group). a,bMean values with unlike superscripts indicate significant differences between means (P < 0.05) in the LSD test. The superscript “a” was assigned to the control group.
Figure 3. Thiobarbituric acid reactive substances (TBARS in nmol per g organ fresh matter and kg body weight) in jejunal mucosa and the liver of piglets.
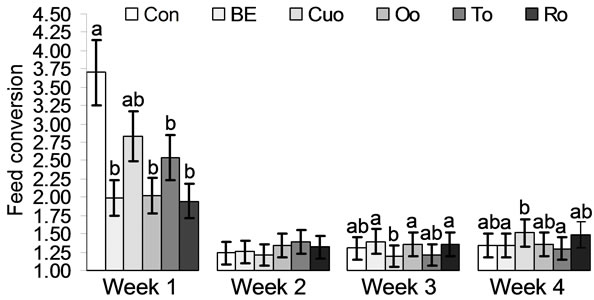
justifying the declaration of phytogenic feed additives and in particular of labiatae oils as appetite stimulating substances and as growth promoters are inconsistent. Whereas some studies reported on beneficial effects of even low dietary concentrations of oregano oil (0.0125 g/kg to 0.025 g/kg diet) [1] and of blends of essential oils from oregano, anise, citrus peels, and chicory [3] on weight gain and feed efficiency ratio, other studies showed no effects [4] or even opposite effects on these parameters [5,40]. Due to the standardisation of the essential oils’ concentration to that of their main terpenes (150 mg main terpene/kg diet) in our study the added oil concentrations were rather high. Thus it can be remarked as a positive result of our study that the high phytogenic concentrations in the diets caused no mentionable adverse effects on performance parameters at all. With the exception of Ro in our study all essential oils improved weight gain or feed conversion ratio in comparison to Con piglets to a greater or lesser extent (Table 5). However, these differences were not significant over the whole experimental period.
According to results of a prior study [1] piglets of groups Oo and To had the best feed conversion compared to the essential oil groups Cuo and Ro and to group Con. Within the labiatae oils Oo and in particular To improved weight gain during the whole experiment compared to Ro as another labiatae oil. That within the labiatae oils thyme seems to unfold the most positive effects on performance parameters has been demonstrated in a choice experiment with piglets [40]. In a study investigating the growth promoting effects of curcumin (supplementation of 200 mg curcumin/kg to the diets of Large White × Landrace × Duroc piglets), body weight gain and feed intake of supplemented piglets remained unaffected compared to the control group [41]. Our results for turmeric oil, containing mainly turmeron derivatives and only traces of curcumin, deviated from these results (Table 5 and Figures 4(a), (b)). Over the whole experimental period weight gain of Cuo piglets was comparable to that of Oo and To piglets. In week 3 of the experiment Cuo piglets even had the highest weight gain and the best feed conversion compared to all other experimental groups (Table 5 and Figures 4(a), (b)). Thus our results for Cuo are rather in coincidence with data of Wenk (2005), reporting on a dose-dependent positive effect of turmeric on performance of pigs and broilers [42]. A very interesting result with regard to the performance parameters could be observed for broccoli extract (BE), not permitted as a feed additive in the EU until today. While BE piglets had the second best feed conversion after To piglets, weight gain of BE piglets was the second lowest. Only Ro piglets had a somewhat lower total weight gain. Thus BE seems to maximise feed conversion, but to slow weight gain slightly (Table 5 and Figures 4(a), (b)). This
 (a)
(a) 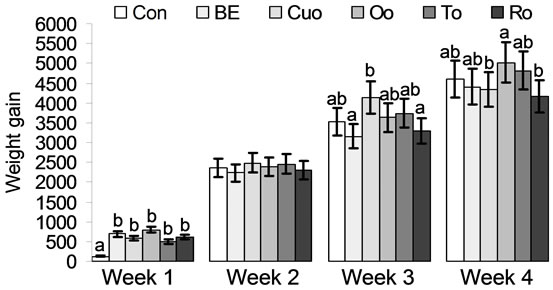 (b)
(b)
Values represent means ± SEM (n = 8 animals per experimental group). a,bMean values with unlike superscripts indicate significant differences between means (P < 0.05) in the LSD test.
Figure 4. (a) Feed conversion (g/g) and (b) Weight gain (g) of piglets fed different phytogenic feed additives for 28 days.
most interesting aspect of BE may have derived from potential goitrogenic effects of the isothiocyanate sulforaphane contained in a high concentration in the extract (4.06% w/w) and of other goitrogenic isothiocyanates contained in the extract in very low concentrations (<0.1% w/w). However, studies in humans have proven that sulforaphane, even in higher concentrations, has no or only a negligible influence on thyroid metabolism [43, 44].
Nevertheless, an important fact which should be remarked at the end of the discussion concerning performance parameters is that all phytogenic feed additives had a distinct positive effect on the performance parameters feed conversion and weight gain in the first week of the experiment (Figures 4(a), (b)). While about half of the Con piglets gained no weight in the first week or even lost weight, all phytogenic feed additives counteracted this undesired aspect. In particular in the first week after weaning it is of importance that the piglets have a good performance in order to prevent the loss of animals and to protect them from infections. This is in accordance with other reports [45,46].
Weight gain and feed conversion are the two most important parameters in today’s animal nutrition, and the feed industry frequently advertises their phytogenic additives as growth promoters. In addition, both weight gain and feed conversion are frequently associated with general animal health. In contrast the results of the entire experimental period in our present trial and the outcome of a number of other studies mentioned above [4,5,40] suggest that phytogenic feed additives frequently do not fulfil the criteria acting as mere growth promoters. To complicate matters: One explanation for the lacking or sometimes slightly negative effect of phytogenic additives on performance may surprisingly be the consequence of their beneficial antioxidant effects. In this context an in vitro study has shown the potent inhibition of porcine pancreatic amylase by phenolic oregano compounds [47]. However, as discussed above, in particular in the critical first week, all additives tested, exerted their potential to acting as growth promoters [45,46]. Moreover, in the majority of these studies [4,5,40], including our present experiment, the piglets were not challenged with pathogenic microorganisms or toxic substances. It can be assumed that the threat of infections and the challenge with feed contaminants increase under practical feeding conditions with a high stocking rate [48]. In this context experiments in which broilers were infected with Eimeria tenella or fed aflatoxin containing diets, the essential oil of oregano or turmeric powder counteracted the depressed feed intake and growth [49-51]. Accordingly some other studies with rats as model animals and tissue cultures have demonstrated the beneficial effects of thyme, rosemary and of sulforaphane on mycotoxin detoxification [28,52-55].
However, until today there is a lack of broadly based studies in farm animals regarding this aspect. To our opinion the verification of beneficial effects of phytogenic additives on animal performance under challenged conditions represents an important field in future research and a useful instrument to evaluate commercial feed additives.
4.2. Microflora in Jejunal Mucosa, Colonic Mucosa, Faecal Samples and Detection Rate of Estb
The analysed counts of bacteria attached to the jejunal mucosa showed differential patterns depending on the phytogenic feed additive. With regard to E. coli the maximum counts (50% of the piglets of a group) were reduced by all phytogenic feed additives. The median values for jejunal E. coli were higher in BE and Ro piglets compared to the control, whereas Cuo, Oo and To treatment produced lower counts. However, due to the high individual variation E. coli counts did not differ significantly. In contrast all phytogenic additives slightly but not significantly increased jejunal Lactobacilli compared to Con piglets. A study analysing jejunal chymus of piglets found tendentially reduced E. coli and increased Lactobacilli counts due to feeding diets containing a blend of 300 mg/kg carvacrol (oregano), cinnamaldehyde and capsicum oleoresin. In this trial the ratio of Lactobacilli:E. coli was even significantly increased [46]. Thus our results for Oo, containing noteworthy amounts of carvacrol, are in accordance with the outcome of the above mentioned trial. Moreover in our trial Oo also increased the Lactobacilli:E. coli ratio (Oo, 1.52:1) compared to Con piglets (1.37:1). Moreover in our study To also distinctly increased the Lactobacilli:E. coli ratio (1.71:1). In the literature both for To and for the essential oil of Curcuma longa (Cuo) no reports on their effects on jejunal E. coli and Lactobacilli exist. Only one study reported on the lacking effect of an essential labiatae oil mixture on microbial counts in the stomach and the ileum of pigs [10]. However, in our trial Cuo was most effective in improving the Lactobacilli:E. coli ratio (2.00:1). For sulforaphane currently only in vitro data on a potent bactericidal activity against several E. coli strains exist [18], which could not be confirmed in vivo in our present study. Similarly, for Ro the majority of studies, investigating its bactericidal effects, including those against E. coli, was carried out in vitro in the context of investigating the storage stability of meat [7-9]. However, due to an higher increase in jejunal E. coli compared to jejunal Lactobacilli BE and Ro had the lowest Lactobacilli:E. coli ratios of 1.14:1 and 1.13:1, respectively.
In contrast to the moderate positive effects of Cuo, Oo and To on microorganisms attached to jejunal mucosa we could not find any differences in the bacterial populations of colonic mucosa. To the best of our knowledge no studies reporting on effects of phytogenic additives on bacterial populations in the colonic mucosa exist so far. Only few studies reported on the effects of phytogenic substances on caecal and faecal microbial counts. Feeding a blend of the essential oils of gingermint (labiatae plant), anis and sage (300 mg/kg diet) had no influence on colonic digesta E. coli counts whereas Lactobacilli were increased [10]. In contrast in our trial no effects of the labiatae oils Oo and To could be analysed for both bacterial classes attached to the colonic mucosa. Only Ro slightly increased Lactobacilli:E. coli ratio compared to Con piglets. This result for Ro was further confirmed by the distinct improvement of Lactobacilli to E. coli ratio in faecal samples collected at the end of the experiment.
A further interesting and important result of our study consisted in the influence of the phytogenic additives on the incidence of estb as a marker for the existence of enterotoxic E. coli strains of the serotypes O149:K91 with F4 or F6 fimbriae and O138:K81 with F18 fimbriae in the gut of the piglets (Figure 1). Whereas the incidence rate of estb existence in Con piglets distinctly increased till the end of the experiment, Ro was the only additive which even reduced estb incidence. In the other labiatae oil groups (Oo and To) and in the BE group estb incidence did not increase. Only in the Cuo group a slight increase in estb incidence could be observed which however remained below the Con group. Since enterotoxic E. coli strains (ETEC) are an important risk factor for the development of diarrhoea, the reduction of these strains is of particular importance. In a trial in which piglets were inoculated with a mixture of ETEC with F4-, F5-, F6-, F18- and F41 fimbriae ETEC with F4 fimbriae caused diarrhoea preferentially in the first 2 weeks after weaning, strains with F18 fimbriae were responsible for diarrhoea in 3 to 5 week old piglets [56]. Since estb is marker for strains with both F4 and F18 fimbriae future studies should investigate the selective reduction of ETEC by phytogenic feed additives. Moreover our results demonstrate the need of future studies in which piglets are challenged with ETEC in order to evaluate the suppressive effect of phytogenic additives on these strains in vivo. As stated with regard to the performance parameters this topic would represent a useful tool to evaluate commercial feed additives and to optimize mixtures of phytogenic additives.
4.3. ARE Regulated Xenobiotic and Antioxidant Enzymes, TROLOX® Equivalent Antioxidant Capacity (TEAC), and Thiobarbituric Acid Reactive Substances
To the best of our knowledge our data have shown for the first time that labiatae oils (Oo, To and Ro), broccoli extract (BE), and turmeric oil (Cuo) have promising effects on a panel of ARE regulated xenobiotic enzymes and the antioxidant system in piglets. Only a trial of our group has previously demonstrated similar results for growing chicken. However, the data of the broiler trial and those of the current piglet study exert some fundamental differences with regard to the organ specific influence of the phytogenic additives. Whereas in the broiler trial ARE-regulated xenobiotic and antioxidant enzymes were mainly up-regulated in the small intestine and partially down-regulated in the large intestine and in the liver, the data of our present piglet study showed a potent up-regulation of these enzymes by Ro in the large intestine and by BE, Cuo and Ro also in the liver. This particular result may be the consequence of the higher dietary concentration of the phytogenic additives in the piglet trial due to the standardisation of the active compounds. Because of their known impact on the induction of ARE-regulated genes we have applied broccoli extract and turmeric oil [15,57] as reference additives in our study. Most interestingly, the reference substances showed a completely different induction profile of ARE-regulated genes. Whereas sulforaphane, derived by bacterial cleavage from its glucosinolate precursor glucoraphanin (GRA) contained in BE [58], had a strong effect on the up-regulation of xenobiotic and antioxidant enzymes in the small intestine, Cuo unrolled its maximum effect on this process in the liver (Tables 9(a), (b), (d)). This results differs from our chicken trial in which we have observed a distinct induction of ARE regulated genes by Cuo already in the small intestine. That broccoli extract can influence the gene expression of xenobiotic and antioxidant enzymes already in the small intestine with a lower bacterial colonisation than in the large intestine is supported by results of most recent studies. In these trials the incubation of precision slices of different tissues with GRA effected a strong induction of ARE-regulated antioxidant and xenobiotic enzymes, suggesting that GRA per se can up-regulate the mentioned enzymes [26,27]. While in most other studies, investigating the effects of various turmeric extracts on the up-regulation of antioxidant and xenobiotic enzymes, dried curcumin-rich extracts were used [59,60] we have fed turmeric oil containing ar-turmerone as the primary active compound. Both curcumin and ar-turmerone have been shown to induce antioxidant and xenobiotic enzymes potently. In our study we have deliberately chosen the ar-turmerone rich turmeric oil [15], since the oil is easier to add to the diets and its price is more attractive for animal nutrition. In former studies with laboratory animals and with humans the main intention to use GRA, SFN and turmeric extracts was to study their preventive effects against intestinal cancers. Their safety has been verified [43,44,61]. In farm animals, having a short live span and needed for food production, the emphasis on application of these inducers of ARE-regulated genes rather aims on their efficiency to strengthen the intestinal barrier against oxidative stress in the organism and to activate their defence mechanisms against bacterial toxin and food borne toxins.
For the labiatae oils the results of our present study have confirmed the data of the broiler trial with regard to their differentiated effects on antioxidant processes (Tables 9(a), (b), (d)). Within the labiatae oils Ro and To induced jejunal ARE regulated enzymes to a much higher extent compared to Oo. In this context it is important that Ro in contrast to the broiler trial had an even higher influence on the induction of antioxidant and xenobiotic enzymes in the small intestine than the reference substance BE (Tables 9(a), (b)). In colon nearly all AREregulated enzymes showed a lowered response to feeding the phytogenic feed additives. As mentioned above only Ro maintained its strong inductive effect also in the colon.
Differences in the antioxidant mechanisms of labiatae oils can be assumed to deriving from their main terpene compounds. Whereas oregano and thyme mainly contain the phenolic terpenes carvacrol and thymol, Ro contains the epoxy-terpene 1,8-cineole. Phenolic groups again possess direct antioxidant effects and therefore have a rather low influence on the induction of ARE-regulated xenobiotic and antioxidant enzymes accounting for indirect antioxidant properties. In contrast the reactive epoxide group of 1,8 cineole (rosemary) contributes to a stronger induction of the indirect antioxidant system via activating the ARE of xenobiotic and antioxidant enzymes [62,63]. In addition to the modification of sensor - SH-groups of KEAP1 or by triggering Nrf2 phosphorylation [25,64] there may exist another mechanism for ARE-gene induction. This particular mechanism involves the metabolisation of the terpenes by phase I cytochrome P450 enzymes followed by the induction of antioxidant and xenobiotic enzymes through the activated phase I metabolites [65].
Since Keap1 per se has been shown to be induced via Nrf2, another important point to discuss is the impact of phytogenic feed additives on Keap1 de novo synthesis [64,66]. Whereas BE caused a significant and Ro a tendential increase in jejunal Keap1 expression, Cuo and Oo rather led to a decreased Keap1 mRNA concentration. Moreover the increased hepatic Keap1 mRNA level by feeding Cuo corresponded to the strongest induction of antioxidant and xenobiotic enzymes in the liver [64].
Our results for the differentiated response of the direct and the indirect antioxidant systems due to feeding phytogenic feed additives demonstrates the need of future studies with tissue cultures and model animals investigating the detailed mechanisms by which phytogenic feed additives and their key compounds influence these systems (e.g. by Keap1 modification, by Nrf 2 phosphorylation or subsequent to phase I up-regulation).
In this context our results for the TEAC values and for lipid peroxidation in jejunal mucosa, colon and the liver are of interest (Figures 2, 3). The TEAC value of a tissue comprises direct and indirect antioxidant effects by secondary mechanisms like ARE induction. In the jejunum the addition of all phygenic feed additives affected a significant increase in TEAC compared to Con piglets. These data are in accordance with our broiler trial. Thus it can be assumed that the rise in jejunal TEAC by BE, containing the isothiocyanate SFN, without any direct antioxidant mainly was basing on the up-regulation of antioxidant enzymes and therefore on an indirect antioxidant effect [57]. This result is in accordance with our results for the gene expression data (Table 9(a)). Within the oils tested in our trial Ro had the highest TEAC value of 156 mmol/100 mL, followed by Oo (120), To (116) and Cuo (90). In accordance with these data Ro increased jejunal TEAC most powerful which may be the consequence of both its high direct antioxidant potential and of the strong induction of xenobiotic and antioxidant enzymes. A similar mechanism may exist for To [63]. In contrast Oo which had only a weak influence on the upregulation of xenobiotic and antioxidant enzymes may have increased jejunal TEAC predominantly by its direct antioxidant effects as explained above [13]. Similarly the increase in jejunal TEAC by feeding Cuo may have derived from its main aromatic terpene ar-turmerone as a potent radical scavenger [67] (Figure 2). Most interestingly ar-turmerone has been shown to possess both direct antioxidant effects and strong indirect antioxidant effects [15]. Due to the contrary results for Cuo on jejunal AREinduction in our broiler trial it can be assumed that there exists a dose-dependent effect due to the predominance of direct or indirect antioxidant effects. This particular mechanism needs intensive investigation in the future. In accordance with the decreased influence of the phytogenic additives on antioxidant and xenobiotic enzymes’ expression colonic TEAC remained uninfluenced. This particular result may derive from the fact that distinctly lower concentrations of the active compounds of the phytogenic substances reach the large intestine than they are present in the small intestine. Despite an overall lower influence of the phytogenic substances on the upregulation of liver xenobiotic and antioxidant enzymes all additives influenced liver TEAC positive in tendency or even significantly. Higher liver TEAC values in piglets fed phytogenic additives may derive from the increased antioxidant potential in the small intestine. This particular barriere hypothesis has been suggested in detail for the selenoprotein glutathione peroxidase 2 [68, 69].
In the jejunum all phytogenic feed additives reduced iron provoked lipid peroxidation to a larger or smaller extent compared to Con piglets with significant effects for Oo and To. This result confirms the important function of the small intestine as an effective barrier against oxidative stress [69].
However, in the liver the influence of the phytogenic additives on TEAC and TBA-RS was less pronounced than in the small intestine. In contrast in our broiler trial we could measure a significant increase in liver TEAC and a significant decrease in liver TBA-RS. This phenomenon may be explained by differences in feed intake between pigs and broilers. Due to the fact that feed consumption of pigs increases with body weight and the feed conversion drops it can be assumed that a longer feeding period would have increased liver TEAC significantly and concomitantly reduced TBA-RS. However, in two other pig trials feeding diets supplemented with different amounts of Oo (0.25, 0.50 and 1.00 mL/kg diet) or the essential oils of rosemary, oregano and ginger (500 mg/kg diet) for a comparable short period of 35 days also remained without an influence on meat characteristics and lipid peroxidation parameters [70,71]. In contrast, feeding a rather high amount of an oregano extract (60 mL) to finisher pigs for 2 weeks significantly improved the antioxidant potential and the storage stability of the meat [72].
A final overview of our current results regarding the influence of broccoli extract and various essential oils on xenobiotic enzymes, on the antioxidant system, and on intestinal and faecal microflora of piglets is given in Table 10. These summarised data may give a good basis to discuss the development of rational and expedient combinations of the single additives in order to maximise the impact on performance, microbial eubiosis, and on direct and indirect antioxidant effects.
Compared to the control group in our study To followed by Oo and Cuo showed a positive influence on the performance parameters of the piglets. In the small intestine To exerted both, direct and indirect antioxidant effects. However, its influence on the xenobiotic enzymes in the liver was rather weak. Thus the combination of To with BE or Cuo, both acting as potent inducers of xenobiotic enzymes in the liver, may contribute to the optimisation of detoxification processes in the whole organism. Similarly a combination of Oo, having a strong direct antioxidant effect, with BE may represent a further rational combination of two additives. Because of its superior direct antioxidant potential and its weak effects on xenobiotic enzymes Oo may further be a very good additive for finisher pigs in order to improve the antioxidant potential of the meat prior to slaughter. Moreover feeding Oo to finisher pigs may increase storage stability of the meat, as suggested by the above mentioned study. In our trial Ro turned out as the all-rounder additive due to its strong effects on the induction of xenobiotic enzymes in all organs investigated. Moreover Ro as the only additive even reduced the incidence rate of enterotoxic E. coli existence in the faeces. Only the performance parameters were influenced slightly negative by Ro.
Maybe replacement of a small percentage of Ro addition by To or Oo could contribute to the improvement of performance. With regard to the microbial parameters BE, Oo and To had a rather neutral influence since in the mentioned groups no increase in the number of piglets infected with enterotoxic E. coli could be detected. Only in the Cuo group the incidence rate of estb positive piglets increased. This rather negative effect should be intensively investigated in future studies and lowers the very positive impact of Cuo on performance.
In summary we have shown that the different phytogenic feed additives tested significantly and differentially influence the direct and the indirect antioxidant system (xenobiotic system) in various organs of piglets. In particular the induction of the xenobiotic system is important to improve the defense against microbial and feed derived toxic substances. Future studies are needed to
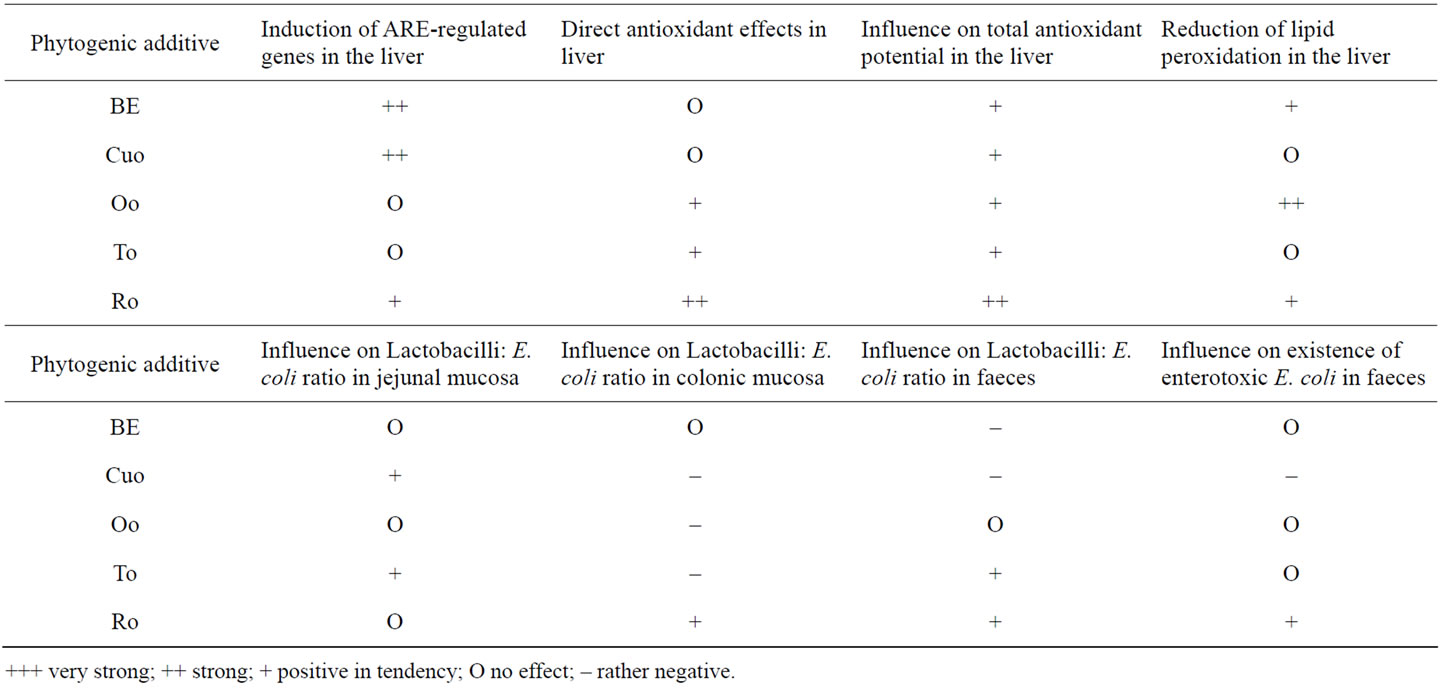

Table 10. Summary of the effects of different phytogenic feed additives on performance, the antioxidant system and on microflora in jejunal mucosa, colonic mucosa and faeces.
investigate the impact of the different feed additives on detoxification processes even under conditions with environmental challenges. The results of these studies could further contribute to the development of optimised combinations of phytogenic feed additives.
5. ACKNOWLEDGEMENTS
This study was supported by Delacon Biotechnik Ges.m.b.H, Steyregg (Austria) to study fundamental mechanisms of phytogenic feed additives and by the H. Wilhelm Schaumann foundation, Hamburg by funding Dipl. troph. Kristin Mueller with a doctoral scholarship. We thank Devakumari Hiller (Jarrow Deutschland GmbH, Berlin, Germany) arranging the contact with Mr. Jarrow L. Rogovin from Jarrow Formulas, Los Angeles, USA, who provided us with the broccoli seed extract. Moreover, we thank our stockmen Olaf Hoedel and Detlev Barth for their help with diet preparation, animal care, and organ sampling. Thank is also addressed to Mr. Frank Schmidt for slaughtering the pigs. Further thank is addressed to our master students Jette Rahn, Annett Schulz, Marianna von Jagemann, and Bettina Hommel for their help with analyses within the scope of their master theses.
REFERENCES
- Kyriakis, S.C., Sarris, K., Lekkas, S., Tsinas, C., Giannakopoulos, C., Alexopoulos, C. and Saoulidism, K. (1998) Control of post weaning diarroea syndrome of piglets by in-feed application of origanum essential oils. Proceedings of the 15th IPVS Congress 1998, Birmingham, England, 218.
- Tsinas, A.C., Giannakopoulos, C.G., Papasteriades, A., Alexopoulos, C., Mavromatis, J. and Kyriakis, S.C. (1998) Use of origanum essential oils as growth promoter in pigs. Proceeding 15th IPVS Congress 1998, Birmingham, England, 221.
- Zitterl-Eglseer, K., Wetscherek, W., Stoni, A., Kroismayr, A. and Windisch, W. (2008) Bioverfügbarkeit der ätherischen öle eines phytobiotischen futterzusatzes und der einfluss auf die leistung bzw. Nährstoffverdaulichkeit bei absetzferkeln. Die Bodenkultur, 59, 121-129.
- Mares, P., Novák, L., Zeman, L., Kratochvílová, P. and Vecerek, M. (2007) The evaluation of phytogenic additives using in pig nutrition. 6th BOKU-Symposium Tierernährung, 149-151.
- Oswald, J. and Wetscherek, W. (2007) Wirkung von oregano auf die aufzuchtleistung von ferkeln. 6th BOKUSymposium Tierernährung, 174-181.
- Hashemi, S.R. and Davoodi, H. (2011) Herbal plants and their derivatives as growth and health promoters in animal nutrition. Veterinary Research Communications, 35, 169-180. doi:10.1007/s11259-010-9458-2
- Schirmer, B.C. and Langsrud, S. (2010) Evaluation of natural antimicrobials on typical meat spoilage bacteria in vitro and in vacuum-packed pork meat. Journal of Food Science, 75, 98-102. doi:10.1111/j.1750-3841.2009.01485.x
- Kong, B., Wang, J. and Xiong, Y.L. (2007) Antimicrobial activity of several herb and spice extracts in culture medium and in vacuum-packaged pork. Journal of Food Protection, 70, 641-647.
- Busatta, C., Vidal, R.S., Popiolski, A.S., Mossi, A.J., Dariva, C., Rodrigues, M.R., Corazza, F.C., Corazza, M.L., Vladimir Oliveira, J. and Cansian, R.L. (2008) Application of Origanum majorana L. essential oil as an antimicrobial agent in sausage. Food Microbiology, 25, 207- 211. doi:10.1016/j.fm.2007.07.003
- Maenner, K., Vahjen, W. and Simon, O. (2011) Studies on the effects of essential-oil-based feed additives on performance, ileal nutrient digestibility, and selected bacterial groups in the gastrointestinal tract of piglets. Journal of Animal Science, 89, 2106-2112. doi:10.2527/jas.2010-2950
- Castillo, M., Martín-Orúe, S.M., Roca, M., Manzanilla, E.G., Badiola, I., Perez, J.F. and Gasa, J. (2006) The response of gastrointestinal microbiota to avilamycin, butyrate, and plant extracts in early-weaned pigs. Journal of Animal Science, 84, 2725-2734. doi:10.2527/jas.2004-556
- Hagemüller, W., Jugl-Chizzola, M., Zitterl-Eglseer, K., Gabler, C., Spergser, J., Chizzola, R. and Franz, C. (2006) The use of Thymi Herba as feed additive (0.1%, 0.5%, 1.0%) in weanling piglets with assessment of the shedding of haemolysing E.coli and the detection of thymol in the blood plasma. Berliner und Munchener Tierarztliche Wochenschrift, 119, 50-54.
- Shan, B., Cai, Y.Z., Sun, M. and Corke, H. (2005) Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of Agricultural and Food Chemistry, 53, 7749-7759. doi:10.1021/jf051513y
- Negi, P.S., Jayaprakasha, G.K., Rao, J.M. and Sakariah, K.K. (1999) Antibacterial activity of tumeric oil: A byproduct from curcumin manufacture. Journal of Agricultural and Food Chemistry, 47, 4297-4300. doi:10.1021/jf990308d
- Anonymous (2012) Evonik Industries product information (no authors listed). TEGO® Turmerone: The distilled fraction of tumeric oil extracted from the roots of Curcuma longa by supercritical carbon dioxide. http://www.cenerchem.com/PDFs/TEGO%20Turmerone%20Tech%20Lit%200208.pdf
- Mueller, K., Blum, N.M., Kluge, H. and Mueller, A.S. (2011) Influence of broccoli extract and various essential oils on performance and expression of xenobioticand antioxidant enzymes in broiler chickens. British Journal of Nutrition, 1-15. [Epub ahead of print] doi:10.1017/S0007114511005873
- Clarke, J.D., Dashwood, R.H. and Ho, E. (2008) Multitargeted prevention of cancer by sulforaphane. Cancer Letters, 269, 291-304. doi:10.1016/j.canlet.2008.04.018
- Aires, A., Mota, V.R., Saavedra, M.J., Rosa, E.A.S. and Bennett, R.N. (2009) The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from human intestinal tract. Journal of Applied Microbiology, 106, 2086-2095.
- Iqbal, M., Sharma, S.D., Okazaki, Y., Fujisawa, M. and Okada, S. (2003) Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: Possible role in protection against chemical carcinogenesis and toxicity. Pharmacology & Toxicology, 92, 33-38. doi:10.1034/j.1600-0773.2003.920106.x
- Petri, N., Tannergren, C., Holst, B., Mellon, F.A., Bao, Y., Plumb, G.W., Bacon, J., O’Leary, K.A., Kroon, P.A., Knutson, L., Forsell, P., Eriksson, T., Lennernas, H. and Williamson, G. (2003) Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metabolism and Disposition, 31, 805-813. doi:10.1034/j.1600-0773.2003.920106.x
- Miyakoshi, M., Yamaguchi, Y., Takagaki, R., Mizutani, K., Kambara, T., Ikeda, T., Zaman, M.S., Kakihara, H., Takenaka, A. and Igarashi, K. (2004) Hepatoprotective effect of sesquiterpenes in turmeric. Biofactors, 21, 167- 170. doi:10.1002/biof.552210134
- Yoxall, V., Kentish, P., Coldham, N., Kuhnert, N., Sauer, M.J. and Ioannides, C. (2005) Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: Implications for ist chemopreventive activity. International Journal of Cancer, 117, 356-362. doi:10.1002/ijc.21191
- Hu, R., Xu, C., Shen, G., Jain, M.R., Khor, T.O., Gopalkrishnan, A., Lin, W., Reddy, B., Chan, J.Y. and Kong, A.N.T. (2006) Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in liver of C57BL/6J mice and C57BL/6J/Nrf2 (-/-) mice. Cancer Letters, 243, 170-192. doi:10.1016/j.canlet.2005.11.050
- Gowda, N.K.S, Ledoux, D.R., Rottinghaus, G.E., Bermudez, A.J. and Chen, Y.C. (2008) Efficacy of tumeric (Curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poultry Science, 87, 1125-1130. doi:10.3382/ps.2007-00313
- Hong, F., Freeman, M.L. and Liebler, D.C. (2005) Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chemical Research in Toxicology, 18, 1917-1926. doi:10.1021/tx0502138
- Abdull-Razis, A.F., Baratta, M., De Nicola, G.R., Iori, R. and Ioannides, C. (2010) Intact glucosinolates modulate hepatic cytochrome P450 and phase II conjugation activeties and may contribute directly to the chemopreventive activity of cruciferous vegetables. Toxicology, 277, 74-85. doi:10.1016/j.tox.2010.08.080
- Abdull-Razis, A.F., Bagatta, M., De Nicola, G.R., Iori, R. and Ioannides, C. (2011) Up-regulation of cytochrome P450 and phase II enzyme systems in rat precision-cut rat lung slices by the intact glucosinolates, glucoraphanin and glucoerucin. Lung Cancer, 71, 298-305. doi:10.1016/j.lungcan.2010.06.015
- Gao, S.S., Chen, X.Y., Zhu, R.Z., Choi, B.M. and Kim, B.R. (2010) Sulforaphane induces glutathione S-transferase isozymes which detoxify aflatoxin B(1)-8,9-epoxide in AML 12 cells. BioFactors, 36, 289-296. doi:10.1002/biof.98
- Gesellschaft für Ernährungsphysiologie (2006) Empfehlungen zur Energie und Nährstoffversorgung bei Schweinen. DLG-Verlag, ISBN-Nr.: 978-3-7690-0683-4.
- National Research Council (1998) Nutrient requirements of swine. 10th Revised Edition, The National Academies Press, Washington DC.
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)). Methods, 25, 402-408. doi:10.1006/meth.2001.1262
- Bustin, S.A., Benes, V., Garson, J.A., Hellemans, J., Huggett, J., Kubista, M., Mueller, R., Nolan, T., Pfaffl, M.W., Shipley, G.L., Vandesompele, J. and Wittwer, C.T. (2009) The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry, 55, 611-622. doi:10.1373/clinchem.2008.112797
- Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A. and Speleman, F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology, 3, Research0034.
- Hosseini, A., Sauerwein, H. and Mielenz, M. (2010) Putative reference genes for gene expression studies in propionate and β-hydroxybutyrate treated bovine adipose tissue explants. Journal of Animal Physiology and Animal Nutrition, 94, 178-184. doi:10.1111/j.1439-0396.2010.01002.x
- Vreeburg, R.A., Bastiaan-Net, S. and Mes. J.J. (2011) Normalization genes for quantitative RT-PCR in differentiated Caco-2 cells used for food exposure studies. Food & Function, 2, 124-129. doi:10.1039/c0fo00068j
- Miller, N.J., Rice-Evans, C., Davies, M.J., Gopinathan, V. and Milner, A. (1993) A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical Science, 84, 407-412.
- Wang, C.C., Chu, C.Y., Chu, K.O., Choy, K.W., Khaw, K.S., Rogers, M.S. and Pang, C.P. (2004) Trolox-equivalent antioxidant capacity assay versus oxygen radical absorbance capacity assay in plasma. Clinical Chemistry, 50, 952-954. doi:10.1373/clinchem.2004.031526
- Wong, S.H., Knight, J.A., Hopfer, S.M., Zaharia, O., Leach, C.N. Jr. and Sunderman, F.W. Jr. (1987) Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clinical Chemistry, 33, 214-220.
- Bradford, M.M. (1976) A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248-254. doi:10.1016/0003-2697(76)90527-3
- Jugl-Chizzola, M., Ungerhofer, E., Gabler, C., Hagmüller, W., Chizzola, R., Zitterl-Eglseer, K. and Franz, C. (2006) Testing of the palatability of Thymus vulgaris L. and Origanum vulgare L. as flavouring feed additive for weaner pigs on the basis of a choice experiment. Berliner und Munchener Tierarztliche Wochenschrift, 119, 238-243.
- Ilsley, S.E., Miller, H.M. and Kamel, C. (2005) Effects of dietary quillaja saponin and curcumin on the performance and immune status of weaned piglets. Journal of Animal Science, 83, 82-88.
- Wenk, C. (2005) Einsatz von Kräutern und deren Extrakten in der Tierernährung: Erwartungen und Möglichkeiten. 4th BOKU-Symposium Tierernährung, 17-27.
- Shapiro, T.A., Fahey, J.W., Dinkova-Kostova, A.T., Holtzclaw, W.D., Stephenson, K.K., Wade, K.L., Ye, L. and Talalay, P. (2006) Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutrition and Cancer, 55, 53-62. doi:10.1207/s15327914nc5501_7
- McMillan, M., Spinks, E.A. and Fenwick, G.R. (1986) Preliminary observations on the effect of dietary brussels sprouts on thyroid function. Human Toxicology, 5, 15-19. doi:10.1177/096032718600500104
- Papatsiros, V.G., Tzika, E.D., Papaioannou, D.S., Kyriakis, S.C., Tassis, P.D. and Kyriakis, C.S. (2009) Effect of Origanum vulgaris and Allium sativum extracts fort the control of proliferative enteropathy in weaning pigs. Polish Journal of Veterinary Sciences, 12, 407-414.
- Manzanilla, E.G., Perez, J.F., Martin, M., Kamel, C., Baucells, F. and Gasa, J. (2004) Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs. Journal of Animal Science, 82, 3210-3218.
- McCue, P., Vattem, D. and Shetty, K. (2004) Inhibitory effect of clonal oregano extracts against porcine pancreatic amylase in vitro. Asia Pacific Journal of Clinical Nutrition, 13, 401-408.
- Prathapkumar, S.H., Rao, V.S., Paramkishan, R.J. and Bhat, R.V. (1997) Disease outbreak in laying hens arising from the consumption of fumonisin-contaminated food. British Poultry Science, 38, 475-479. doi:10.1080/00071669708418024
- Gowda, N.K.S, Ledoux, D.R., Rottinghaus, G.E., Bermudez, A.J. and Chen, Y.C. (2008) Efficacy of tumeric (Curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poultry Science, 87, 1125-1130. doi:10.3382/ps.2007-00313
- Yarru, L.P., Settivari, R.S., Gowda, N.K.S., Antoniou, E., Ledoux, D.R. and Rottinghaus, G.E. (2009) Effects of tumeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poultry Science, 88, 2620-2627. doi:10.3382/ps.2009-00204
- Giannenas, I., Florou-Paneri, P., Papazahariadou, M., Christaki, E., Botsoglou, N.A. and Spais, A.B. (2003) Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Archiv fur Tierernahrung, 57, 99-106. doi:10.1080/0003942031000107299
- El-Nekeety, A.A., Mohamed, S.R., Hathout, A.S., Hassan, N.S., Aly, S.E. and Abdel-Wahhab, M.A. (2011) Antioxidant properties of Thymus vulgaris oil against aflatoxininduce oxidative stress in male rats. Toxicon, 57, 984-991. doi:10.1016/j.toxicon.2011.03.021
- Offord, E.A., Macé, K., Avanti, O. and Pfeifer, A.M. (1997) Mechanisms involved in the chemoprotective effects of rosemary extract studied in human liver and bronchial cells. Cancer Letters, 114, 275-281. doi:10.1016/S0304-3835(97)04680-6
- Costa, S., Utan, A., Speroni, E., Cervellati, R., Piva, G., Grandini, A. and Guerra, M.C. (2007) Carnosic acid from rosemary extracts: A potential chemoprotective agent against aflatoxin B1. An in vitro study. Journal of Applied Toxicology, 27, 152-159. doi:10.1002/jat.1186
- Fiala, J.L., Egner, P.A., Wiriyachan, N., Ruchirawat, M., Kensler, K.H., Wogan, G.N., Groopman, J.D., Croy, R.G. and Essigmann J.M. (2011) Sulforaphane-mediated reduction of aflatoxin B1-N7-guanine in rat liver DNA: Impacts of strain and sex. Toxico, 121, 57-62. doi:10.1093/toxsci/kfr026
- Blomberg, L., Henriksson, A. and Conway, P.L. (1993) Inhibition of adhesion of Escherichia coli K88 to piglet ileal mucus by Lactobacillus spp. Applied and Environmental Microbiology, 59, 34-39.
- Boddupalli, S., Mein, J.R., Lakkanna, S. and James, D.R. (2012) Induction of phase 2 antioxidant enzymes by broccoli sulforaphane: Perspectives in maintaining the antioxidant activity of vitamins A, C, and E. Frontiers in Genetics, 3, 7.
- Li, F., Hullar, M.A., Beresford, S.A. and Lampe, J.W. (2011) Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. British Journal of Nutrition, 106, 408-416. doi:10.1017/S0007114511000274
- Campbell, F.C. and Collett, G.P. (2005) Chemopreventive properties of curcumin. Future Oncology, 1, 405-414. doi:10.1517/14796694.1.3.405
- Garg, R., Gupta, S. and Maru, G.B. (2008) Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: Mechanism of its anti-initiating action. Carcinogenesis, 29, 1022-1032. doi:10.1093/carcin/bgn064
- Joshi, J., Ghaisas, S., Vaidya, A., Vaidya, R., Kamat, D.V., Bhagwat, A.N. and Bhide, S. (2003) Early human safety study of turmeric oil (Curcuma longa oil) administered orally in healthy volunteers. Journal of the Association of Physicians of India, 51, 1055-1060.
- Miyakoshi, M., Yamaguchi, Y. and Takagaki, R. (2004) Hepatoprotective effect of sesquiterpenes in turmeric. Biofactors, 21, 167-170. doi:10.1002/biof.552210134
- Jäger, S., Scheffler, A. and Schmellenkamp, H. (2011) Pharmakologie ausgewählter Terpene. Pharmazeutische Zeitung online. http://www.pharmazeutische-zeitung.de/index.php?id=1354
- Keum, Y.S. (2011) Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: Implications of posttranslational modifications. Annals of the New York Academy of Sciences, 1229, 184-189. doi:10.1111/j.1749-6632.2011.06092.x
- Meesters, R.J., Duisken, M. and Hollender, J. (2009) Cytochrome P450-catalysed arene-epoxidation of the bioactive tea tree oil ingredient p-cymene: Indication for the formation of a reactive allergenic intermediate? Xenobiotica, 39, 663-671. doi:10.1080/00498250902989094
- Wang, X., Tomso, D.J., Chorley, B.N., Cho, H.Y., Cheung, V.G., Kleeberger, S.R. and Bell, D.A. (2007) Identification of polymorphic antioxidant response elements in the human genome. Human Molecular Genetics, 16, 1188- 1200. doi:10.1093/hmg/ddm228
- Zhao, J., Zhang, J.S., Yang, B., Lv, G.P. and Li, S.P. (2010) Free radical scavenging activity and characterization of sesquiterpenoids in four species of Curcuma using a TLC bioautography assay and GC-MS analysis. Molecules, 15, 7547-7557. doi:10.3390/molecules15117547
- Wingler, K., Müller, C. and Brigelius-Flohé, R. (2001) Stability of gastrointestinal glutathione peroxidase mRNA in selenium deficiency depends on its 3’UTR. Biofactors, 14, 43-50. doi:10.1002/biof.5520140107
- Wingler, K., Müller, C., Schmehl, K., Florian, S. and Brigelius-Flohé, R. (2000) Gastrointestinal glutathione peroxidase prevents transport of lipid hydroperoxides in CaCo-2 cells. Gastroenterology, 119, 420-430. doi:10.1053/gast.2000.9521
- Simitzis, P.E., Symeon, G.K., Charismiadou, M.A., Bizelis, J.A. and Deligeorgis, S.G. (2010) The effects of dietary oregano oil supplementation on pig meat characteristics. Meat Science, 84, 670-676. doi:10.1016/j.meatsci.2009.11.001
- Janz, J.A., Morel, P.C., Wilkinson, B.H. and Purchas, R.W. (2007) Preliminary investigation of the effects of low-level dietary inclusion of fragrant essential oils and oleoresins on pig performance and pork quality. Meat Science, 75, 350-355. doi:10.1016/j.meatsci.2006.06.027
- Lahucky, R., Nuernberg, K., Kovac, L., Bucko, O. and Nuernberg, G. (2010) Assessment of the antioxidant potential of selected plant extracts—In vitro and in vivo experiments on pork. Meat Science, 85, 779-784. doi:10.1016/j.meatsci.2010.04.004

