Open Journal of Ecology
Vol.06 No.03(2016), Article ID:63582,13 pages
10.4236/oje.2016.63014
Mangrove Forest Characterization in Southeast Côte d’Ivoire
Isimemen Osemwegie1*, Dibi N’da Hyppolite1, Christine Stumpp2, Barbara Reichert3, Jean Biemi4
1WASCAL, UFR Biosciences, University of Felix Houphouet-Boigny, Abidjan, Côte d’Ivoire
2Institute of Groundwater Ecology, Helmholtz Zentrum München, German Research Center for Environmental Health (GmbH), Neuherberg, Germany
3Steinmann-Institut for Geology, Palaeontology and Mineralogy, University of Bonn, Bonn, Germany
4UFR Science de la Terre et Ressources Miniere, University of Felix Houphouet-Boigny, Abidjan, Côte d’Ivoire

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 17 December 2015; accepted 16 February 2016; published 19 February 2016
ABSTRACT
Mangrove ecosystems are faced with far more existential threats of erosion than their terrestrial counterparts. Consequences of their degradation vary from decline in edible aquatic stocks, coastal erosion and aquatic weeds invasion. Mangrove forest dynamics was assessed from multi-tem- poral analyses of remotely sensed satellite images (mosaics of 1989/90 and 2014/15) within 233,900 hectares. Ground-truthing was accompanied by field measurements in selected forest stands to characterize structure, estimate biomass and carbon pools. With conservation as overriding goal, a socio-economic survey was conducted to underpin the factors influencing mangrove forests over-exploitation and qualitatively assess the sensitivity of the locals to resources decline. The region recorded fifty percent loss of mangrove area during the 25-year period. Low leaf area index (1.02 - 2.52 m2∙m−2) confirms canopy openness. Above-ground root biomass (kg per root) ranged between 110.67 and 382.64. The roots demonstrate capacity to fix up to 176 Mg C ha−1 with average carbon content of 46 percent. Highest carbon pools were in the Eloka-To forest stands, in near natural conditions. Despite harsh environmental conditions, potential for natural regeneration was evidenced by seedlings density (individuals per m2) up to 76. Pilot survey revealed high dependence on mangrove resources for direct income (70 percent) and daily energy needs (60 percent). Despite the heightened awareness of the impending dangers posed by mangrove deforestation and willingness to conserve, riverine communities are incapacitated by lack of viable economic alternatives. External interventions are therefore imperative to achieve conservation goals with long-term implications for climate change adaptation and mitigation.
Keywords:
Carbon Pool, Climate Change, Conservation, Degradation, Mangrove Forest Resources

1. Introduction
Mangroves are trees and shrubs limited to tropical and subtropical coastlines between 25˚N and 38˚S [1] [2] , adapted to harsh (high salinity and anoxic) conditions of growth [2] [3] . They are key ecosystems within wetlands that make immense contributions to the wellbeing of societies by their ability to attenuate coastal waves, and provide households with clean water, food, recreation, and income sources [4] [5] . These sea-land boundary ecosystems support biodiversity conservation, climate change mitigation and adaptation measures. They form the core of primary productivity and constitute a large proportion of blue carbon sinks [6] [7] . They also play active roles in balancing global carbon budgets [8] . Their carbon storage potential is higher than that of phytoplankton [9] and fifty times more than that of terrestrial rainforests [10] . In pristine conditions, mangrove forests serve as soft coastal defense structures, reinforcing the resilience and adaptation of riverine rural communities to climate change. For instance, in Vietnam, 12,000 hectares of planted mangrove forest lands provided protection against a typhoon that devastated neighboring areas [11] . In spite of the heightened awareness of their economic and ecologic benefits [12] , globally increasing human pressures have resulted in loss of over fifty percent of mangrove area coverage, estimated at 165,000 hectares [13] [14] . Indiscriminate exploitation continues unabated at a rate of 0.1 percent per annum [5] , three to five times the values for terrestrial rainforests [5] . Different mangrove forest regions of the world have different forest structures and species composition. The primary uses are adapted to available mangrove species as well as the socio-economic structures and demands of the populations. On a regional basis, Asia suffered the largest net losses with a disappearance of over 1.9 million hectares between 1980 and 2005 [5] . The main threats arise from salt production and agriculture (rice, shrimp and pastoral farming). North and Central America recorded losses in forest area of approximately 690,000 hectares, while Africa recorded a loss of approximately 510,000 hectares during the 1980-2005 period. In North and Central America, the main threats are from real estate, eco-tourism development and aquaculture, while in Africa, urban encroachment, commercial exploitation and environmental pollution are the main culprits [15] .
In Côte d’Ivoire, a francophone country in West Africa, mangroves cover 0.3 percent of national landmass of 322,463 km2, 26.9 percent of which lies within RAMSAR sites, protected by the country’s Ministry of Water and Forestry [15] . They account for 0.02 percent of global mangrove forests and belong to the species-poor Atlantic East Pacific (AEP) group of mangroves [16] , represented by Rhizophora racemosa G.F.W. Meyer (Rhizophoraceae), Laguncularia racemosa, Avicennia germinans (Avicenniaceae), Conocarpus erectus (Combretaceae), Drepanocarpus lunatus G.F.W. Meyer (Papilionaceae) and Acrostichum aureum (Adiantaceae) [15] [17] . There are two broad categories: a western group extending from Liberia (western border) to Fresco, dominated by black mangroves, Avicennia germinans and an eastern group extending from Fresco to Axim (Ghana border), dominated by Rhizophora racemosa. These forests have undergone severe decline and recorded the highest annual rate of decline (−4.4%) amongst the African mangroves [15] . The socio-economic and ecological impacts of their degradation are widespread, reaching beyond the local communities to the entire country. Rural household protein intakes and incomes have greatly reduced as a result. There exist strong linkages between fisheries productivity and mangroves as studies have shown that every hectare of cleared forest results in the loss of between 100 - 600 kg of fisheries in nearby coastal waters [18] -[20] . Although details of mangrove patterns of distribution and uses can be found in the works of [15] [17] [21] , information regarding their structure, productivity and carbon pools are limited.
This study is a first attempt to bridge data gap and provide information on Rhizophora forest structure, above- ground root biomass and carbon storage potential. It also serves to document indigenous traditional knowledge and main uses of the mangrove plants from survey population and recommend ways to get the host population to actively participate in its conservation. This could aid to formulate, plan and execute restoration and conservation programs in communities with high dependence on a common pool resources.
2. Materials and Methods
2.1. Land Use Cover Change Detection
Land use cover change was assessed using remote sensing (ENVI 4.8, ITT Corporation) and GIS (ArcGIS 10.2, ESRI Inc.) techniques. The study window (longitudes 3˚15" and 3˚40"W and latitudes 6˚15" and 6˚40"N) encompasses an area of 236,842 hectares. Post-classification comparison was between mosaics of Lands at 5 Thematic Mapper (TM) images of January 1989 (path/row 195/56) & January 1990 (196/56) and Lands at 8 Operational Land Imager, OLI images of November 2014 (path/row 195/56) and January 2015 (196/56), on a 30 × 30 m spatial resolution. Therefore, land areas less than 3,000 hectares were not represented. Image pre- processing steps include layer stacking, cloud masking (cloud covers 7% of study area) and sub-setting. Image processing techniques made use of a combination of spectral signal analyses (principal component analysis (PCA), normalized difference vegetation index (NDVI) calculated as [Near Infrared (Band 4) − Red (Band 3)/Near Infrared (Band 4) + Red (Band 3)] and wetness index, WI = 0.1509ETM1 + 0.1973ETM2 + 0.3279ETM3 + 0.3406ETM4 − 0.7112ETM5 − 0.4572ETM7 (Tassel-cap transformation; [22] ). For both satellite images, supervised classification of land use/cover categories (mangroves, other forest types, settlements/bare soils, water bodies and agricultural lands) was based on maximum likelihood algorithm. Ground data was collected from eighty randomly selected locations within the study area. Result validation was with matrix of confusion and Kappa coefficient. Subsequently, a matrix of transition [23] was generated by the intersection of the two maps.
2.2. Field Measurements―Survey Sites
This study focused on the eastern group of mangroves, growing in the upland tidal areas of the Ébrié lagoon, the largest in West Africa. Further to ground-truthing activities, field measurements were carried out in four selected mangrove forest stands (Figure 1), subjected to different hydrological regimes: Eloka-To (longitude 3˚44'08"W and latitude 5˚18'04"N) and Agban (longitude 3˚18'38"W and latitude 5˚18'04"N) forest stands are located along the eastern fringes of the lagoon, while Audoin-Bégréto (longitude 4˚08'01"W and latitude 5˚17'16"N) and Mois (longitude 4˚14'42"W and latitude 5˚17'22"N) forest stands are located along the central areas of the lagoon. Mean annual (1970-2014) precipitation is 1704 mm and mean annual temperature for the same period is 26.8˚C. Relative air humidity is constant at an average of 83 percent [24] .
Eloka-To (hereinafter refer to as site A) has a minimally impaired forest with large continuous stands and continuous freshwater inputs, estimated annually at 5.0 × 109 cubic metre from the Comoé and La Mé Rivers. It is the most hydrodynamic area of the lagoon with up to 15 times annual renewal rates [25] . Tidal amplitudes in these parts can reach up to 2 meters. Rhizophora prop roots branch out from stems in near horizontal position to the ground. Adjacent to the mangroves are ephemeral, free-floating mats of water hyacinth Eichhornia crassipes (Pontederiaceae), water lilies, Pistiastratiotes (Araceae), and sea grass meadows. These are however restricted to mangrove stands at the eastern borders of the lagoon.
Agban (hereinafter refer to as site B) has highly degraded forest stands, located 27 kilometres southwest of Eloka-To. Here freshwater supply is intermittent and tides can reach up to 0.6 metres after rainfall events.
Audoin-Bégréto (hereinafter refer to as site C) mangroves are subjected to seasonal salinity stress. Aquatic weeds are absent from these environment characterized by long island bars of oysters, Crassostrea agar contributing to the oxygenation of its soils.
Mois (hereinafter refer to as site D) hosts minimally impaired stands, subjected to seasonal salinity stress. It is located about 55 km southwest of Anna. Aquatic weeds are also conspicuously absent from this environment characterized by empty oyster shells, Crassostrea agar clinging to mangrove roots. The shorelines consist of coarse-grained sands unlike in the other forest stands with silty clay soils.
2.3. Environmental Variables
Physicochemical properties of the lagoon water were measured to characterize water sources and identify salinity impacts on the lagoon. Environmental variables (temperature, pH, dissolved oxygen and turbidity) were measured on the Ébrié lagoon using handheld sensors before (January) and after (October) rainfall events of 2014 to characterize the environment. In addition, water stable isotope (oxygen-18 and deuterium) analyses were carried on lagoon water samples using laser spectrometry (LS2120-i, Picarro Inc., Santa Clara, USA) at the Helmholtz Zentrum, München, Germany. δ values were normalized relative to Vienna-Standard Mean Ocean
Figure 1. 1989/90 (top) and 2014/15 (bottom) temporal patterns of land cover change as observed with remotely sensed images showing sampling sites. Seven percent cloud cover was masked from both images.
Water (V-SMOW):
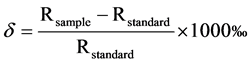
where δ (δ18O or δ2H) is the normalized difference of the isotope ratios R (18O/16O or 2H/1H) of the sample and the standard. Triplicate analyses indicate a precision of ±0.3‰ for δ18O and ±1.6‰ for δ2H.
2.4. Rhizophora Forest Characterization
Forest survey followed the procedures of [26] . Counts and measurements were random within ten, 1 m2 plots marked by PVC pipes, perpendicular to the shorelines. Maximum canopy height (m) was estimated using a clinometer. Canopy cover was by ocular estimation. Leaf area index, LAI (leaf area/ground area, m2∙m−2) was estimated from measurements of light absorption by the forest canopy [27] :
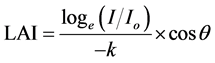
where  is ratio of photon flux density beneath the canopy and at ground level under direct sunlight. K, a light extinction coefficient was set as 0.5. For each sampled site, loge (
is ratio of photon flux density beneath the canopy and at ground level under direct sunlight. K, a light extinction coefficient was set as 0.5. For each sampled site, loge (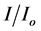 ) was calculated for pairs of simultaneous readings and averaged. Corrections were made for the angle of the sun from the vertical (cosθ). LAI was in turn used to estimate net canopy photosynthesis (PN) using the formula:
) was calculated for pairs of simultaneous readings and averaged. Corrections were made for the angle of the sun from the vertical (cosθ). LAI was in turn used to estimate net canopy photosynthesis (PN) using the formula:
Net carbon fixed, PN (Mg C ha−1 year−1), PN = A × d × LAI
where d is the day length (average of 12.4 hours) and A is the average rate of photosynthesis per unit leaf area (0.216 g C m−2 leaf area hr−1; [28] .
2.5. Above-Ground Root Biomass and Carbon Stock Estimation
Prop root diameter at 30 cm above ground was measured with a vernier caliper. Wood density was determined from fresh: dry weight ratio (oven drying at 70˚C for 72 hours) of disk samples. Carbon content of prop roots were determined by combusting 600 µg vacuum-dried wood chips from 3 centimeter thick sample disks in a EURO EA elemental Analyser at 1700˚C. The resulting carbon dioxide, CO2 was cryogenically separated using a manual extraction line and isotope ratios were determined on Isotope Ratio Mass Spectrometer, IRMS (Finnigan MAT 253; Thermo Electron). δ13C (13C/12C ratio) values were expressed as per mil relative to Vienna-Pee Dee Belemnite. Triplicate analyses indicate a precision of ±0.17‰. Above-ground roots biomass was estimated from species-specific allometric equation of [27] :
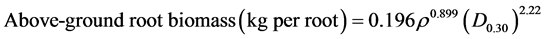
where ρ is root density (t∙m−3) and D0.3 is diameter at 30 cm for the Rhizophoraceae family. Results were multiplied by carbon content to determine carbon stocks.
2.6. Natural Regeneration Capacity
Rhizophora regenerative capacity was determined from seedlings (established propagules less than 1.3 m height) density. Supplementary information on density of periwinkles Pachymelania aurita were also used as metrics of ecosystem health. Collection was by hand picking within 0.25 m2 plots.
2.7. Pilot Socio-Economic Survey
Social vulnerability of the riverine communities of Eloka-To, Anna and Mois to decline in mangrove forest resources was assessed by way of interview of 240 randomly selected and willing members of the survey population based on questionnaires that focuses on the uses, perception and conservation of mangrove forest resources. Eloka-To, Anna and Mois has 1021, 967 and 300 inhabitants respectively [29] . The choice of the number of respondents (sample size of 240) was based on the recommendations of [30] , for conducting a pilot social survey. This number represents about 10% of the target population.
3. Results and Discussions
3.1. Land Use Cover Change
Land use classification accuracy for both satellite images ranged between 61.9% and 97.6% with overall accuracy of 88.1% and 90.3% for 1989/90 and 2014/15 respectively. Confusion was between mangroves and other forest vegetation (Table 1). Kappa coefficient was excellent, greater than 0.8 for both maps, signifying few unclassified pixels. Generally, forested areas showed strong reduction in areal extent (Table 2). Mangrove forest cover decreased from 7863 hectares in 1989/90 to 3867 hectares in 2014/15 representing a net decrease of 3996 hectares or 50.8 (Figure 1). It is evidenced that only 18% of primary forest still exists from the matrix of transition (Table 3). Urbanization accounts for about 70% of the total loss in forest area. About 31.8% of forest land has been converted to settlements/bare soils, while a much higher percentage (43.8%) has been converted to
Table 1. Matrix of confusion (error matrix) for the different land use maps. Above, 1989/90 and below, 2014/15.
Table 2. Percentage change in land occupation for the different land use cover categories between 1989/90 and 2014/15.
Table 3. Matrix of transition. Shading intensity represents stability. Grey-colored areas are stable areas, while light-colored areas are percentage conversion to other land use cover types.
agricultural lands. This is to be expected as the Abidjan population increased from 2,102,000 inhabitants in 1990 [31] to 4,707,404 inhabitants in 2014 [29] . Land under permanent agriculture in the study area was about 50% during the investigation period. Concerning the water bodies, the position of the shoreline showed a 5% landward displacement during the investigation period. Mangroves thrive best with alternating rise and fall of sea level. Their biological response to permanent inundation of saline water resulting from sea level rise is landward migration [32] . However, land use modifications imposes migratory barriers, inhibiting propagation.
3.2. Habitat Characterization
The physicochemical parameters of the lagoon water indicated differences in water chemistry during the dry and wet season as well as between the different locations (Table 4). The lagoon was slightly alkaline (pH 7 - 7.6) except in site A, where the mangroves were exposed to weak acidic waters (pH 6.5 - 6.5) and after rainfall events in site B (pH 6.8). Temperatures were constant with lowest and highest values recorded in sites A and B respectively. Low salinity and water stable isotopes indicate the strong influence of freshwater at site A independent of the season, while other sites experienced seasonal salinity stresses. Site C has the highest fraction of saline water. All sites were oxic in the dry and wet season. At site B, dissolved oxygen levels were higher in the wet compared to the dry season; site C showed the opposite seasonal influences in dissolved oxygen levels. Highest turbidity was recorded in the highly degraded forest of site B after rainfall events.
3.3. Rhizophora Forest Characterization
Maximum canopy height ranged between 3.6 and 14.7 m (Table 5). Canopy cover ranged between 25% - 75%, 5% - 55%, 5% - 45% and 25% - 75% for sites A, B, C and D respectively. Canopy exposure as evidenced in
Table 4. Seasonal variations of the physicochemical parameters of mangrove standing waters, Ébrié lagoon.
Table 5. Mean and range (in parenthesis) of estimates of vegetation parameters for the different mangrove forest stands.
some plots of sites B and C leads to direct sunlight penetration, which in turn promotes high transpiration rates with consequences of decline in plant water use efficiency, net photosynthesis, stunted growth and die-off in extreme cases [9] . Light gaps are however advantageous to seedlings, as they are shade intolerant [33] . Rhizophora roots showed aggregate distribution with average root density of 22 individuals per m2 for surveyed sites. Lognormal plots [34] of diametric sizes of roots follows unimodal, negatively skewed distributions (Figure 2), reflect striking dissimilarities in root diameter, suggesting degraded forests. In a log-normal plot, undisturbed communities usually start high on the abscissa and flatten out towards higher classes. Conversely, disturbed communities start lower on the abscissa as observed in the different mangrove stands albeit with varying degrees of disturbances. The LAI values observed in these mangrove stands are similar to those of tropical savanna (mean ± S.D: 1.88 ± 1.81, [35] ). The amount of radiation transmitted from the top of the canopy to the forest ground are averages of 38, 59, 54 and 29 percent for sites A, B, C and D respectively. Lower amounts of radiation were transmitted to the forest floors in stands with relatively higher LAI values.
3.4. Carbon Storage Potentials
The lowest carbon influx rates were recorded in site B. Assuming the average annual net primary productivity of 22.28 t C ha−1 (Table 5), prop roots within the investigated area are capable of fixing a crude estimate of 0.86 Gt CO2 annually. Carbon contents of roots constitute an average of 44.9% of the oven-dry mass (Table 5). δ13Cmangrove were isotopically lighter compared to standards and ranged between −26.09 and −29.08, suggesting a Calvin mechanism (C3) of photosynthesis. These values are comparable to those of the Rhizophora mangroves of Malaysia [36] , Sri Lanka [37] and Tanzania [38] . Carbon pools, on per hectare basis were highest in site A, the freshwater stands, while lowest values were in the degraded forests of site B. Stored carbon values fell into the range (160 - 200 Mg∙ha−1) estimated by [39] , except those of sites B and D that were lower.
3.5. Regenerative Capacity
A common feature of these Rhizophora forests is the absence in their under-storey of other vegetation types. Their seedlings constitute the ground-storey. The studied forests showed potential for natural unaided regeneration with average seedlings density of 10 (Figure 3). Site D demonstrates the highest rates of survival of propagules
Figure 2. Lognormal plots of mangrove prop root density in 1 × 1 m plots at the different localities.
with seedlings density up to 73 roots per m2. This might be probably due to the firm sandy layers that facilitates the successful establishment of propagules. Conversely, site A recorded the lowest seedlings density. Dense canopy cover coupled with water-logged soils that prevent solar radiation and dissolved oxygen from reaching the forest floors are likely causes of low survival rates of propagules.
3.6. Supplementary Ecological Data
Litter fall (materials on forest floor, 5 mm below ground layer) density was on average 3.2, 1.5, 2.0, and 4.7 kg∙m−2 for sites A, B, C and D respectively. These were mostly from dried leaves, stems and fresh and dried propagules. Highest litter fall density (6.1 kg∙m−2) was recorded at site D, while the lowest (1.5) was recorded at site C. Conclusions cannot however be drawn from this ecological data as more information are needed on sedimentation rates, burial rates and nutrient cycling.
The distribution of a gastropod mollusc, Pachymelania aurita increases with decreasing water salinity (Figure 4). Mangrove crabs were common features in all forest stands.
3.7. Pilot Socio-Economic Survey
The diverging views and knowledge of mangrove forest resources stem from ethnic diversity. Eloka-To is a homogenous population of indigenous Ébriés, Anna is a community with indigenous population of Ébriés mixed with nationals of neighboring countries like the Republic of Benin and Togo. Mois is an encampment largely
Figure 3. Box plots of seedlings density on 1 × 1 m plots in the different localities. Values are means ± range.
Figure 4. Box plots of population density (individuals per 1/4 m2) of benthic grazer, Pachymelania aurita. Values are means ± range.
populated by nationals from neighboring Republic of Benin. More than 70% of the survey population can identify mangroves species. In the local Ébrié dialect Rhizophora racemosa is referred to as “n’tagbagna” meaning legged tree. They are also commonly called “palétuviers rouge”. Raphiahookeri and Dreparnocarpuslunatus are locally referred to as, “palmiers” and “griffes des leopards” respectively. Other mangrove species including the mangrove fern, Acrostichum aureum are regarded as weeds. All respondents, except those from Mois opined that there has been a decrease in areal extent of the mangrove forest and that the trend will continue. Vulnerability is assessed based on a triad of exposure, sensitivity and adaptive capacity [40] . In this regard, Anna with a slope of 1.5% is geographically most vulnerable to coastal barrier degradation and therefore the most prone to coastal hazards, followed by Mois encampment (slope: 3.7%) and Eloka-To (6%). Sensitivity of the locals to mangrove forests resource decline was assessed by their dependence on the resource. Polls reveal high dependence on mangrove timber and non-timber forest products (crabs, fishes, shrimps and birds). The key areas of use are as direct income sources (70%) and domestic energy needs (60%). Figure 5 highlights anthropogenic activities in some of the mangrove forest stands. There is unhindered access to forest resources in all communities except in Eloka-To, where community management committee organizes and supervises logging activities. In spite of these laudable initiatives, there are still several reported cases of illegal and indiscriminate logging.
Adaptive Capacity
According to [41] , more than half of the rural population in Côte d’Ivoire lives below the poverty line (less than US $1 per day). Faced with steady decline of mangrove resources, survey results show that the capacity of the population to adapt to alternative economic activities is low due to lack of alternative revenue sources. In


Figure 5. Anthropogenic activities in selected mangrove forests: (a) Logged timbers on the Ébrié lagoon in Eloka-To; (b) Mangrove forest land reclaimed for construction in Anna; (c) Mangrove forest lands re-claimed for community extension in Audoin-Bégréto; (d) Mangrove forest lands reclaimed for agriculture in Mois (bottom right). Photos by Osemwegie I.
Eloka-To, 95% are fishermen with 65% involved in subsistence farming and 5% into commerce. In Anna, over 75% are fishermen, even though quite a number of them have given up this occupation due to dwindling aquatic stocks, 40% are subsistence farmers and 45% traders. In Mois, 69% of the respondents are fishermen, 30% of which are directly involved in the sales of their produces.
Prior to investigation, it was hypothesized that locals will willingly refrain access and make contributions to conservation as they are the primary beneficiary. However, results show otherwise. Restoration plans and corrective measures aimed at conservation will depend largely on grassroots involvement [42] [43] . In order for conservation efforts to be effective, it is imperative to address the peculiar socio-economic needs of the host communities. The provision of social amenities and services (Figure 6) will be crucial motivating factors in ensuring local participation in mangrove forest restoration programs.
Livelihood diversification will help reduce human pressures on these ecosystems. Micro projects such as aquaculture and establishment of skills acquisition centers should be encouraged with a view to diversifying sources of income for the riverine population.
Unsustainable agribusiness and other human activities pose considerable threat to mangrove conservation. Nature plays only secondary roles. For instance, in Anna, mangroves are threatened by urban encroachment and sand mining/dredging activities that results in drained soils. Mangroves stands in Eloka-To are threatened by commercial exploitation and low survival rates of juveniles owing to increased tidal amplitudes, the result of the silting up of the mouth of the Comoé River. That of Mois is threatened by strong wave actions that hinder the successful establishment of propagules and forest land reclamation for agricultural purposes. The high demand for Rhizophora timber as fuel wood lies in its unique hard wood structure, high calorific value and ease of acquisition. The prices are comparable to those of other forest woods. At current exchange rate of US $1 dollars to 598.5 West African CFA franc, a bundle of ten pieces of chopped wood is sold for between 200 CFA franc (US $0.33) and 500 CFA franc (US $0.84) and a twenty kilogram bag of wood charcoal is sold for 3000 CFA franc (US $5). Bakeries and eateries in nearby urban areas spend between 60,000 CFA Franc (US $100.26) and 400,000 CFA Franc (US $668.34) monthly on fuel woods. There is an organized supply chain structure from producers to end users. In order to discourage demand, pigouvian taxes needs to be levied on end users. Existing national anti-logging legislations should be enforced and offenders sanctioned to serve as deterrent. Incentives; monetary and materials should be provided towards the empowerment of existing local environmental protection committees to patrol and protect the forests. Reforestation projects―manual establishment of propagules, temporal restraint of access to forest and the exploitation of other fuel sources such as agricultural wastes and timber woods like Albiziazygia (leguminosae) and Acacia magnum (Fabaceae) with fast growth, and good regeneration capacity (Centre National de Recherche Agronomique, CNRA, 2013) should be encouraged.
4. Conclusion
It is clear from the foregoing that the integrity of the Rhizophora mangrove forests in the studied regions has
Figure 6. Forms of indemnity respondents are willing to accept from the government in order to restrain temporary use of mangrove forest resources.
been compromised. Their geographic form and occurrence are such that interference with their functionality has generated ripple effects on inherent aquatic biodiversity and adjoining terrestrial ecosystem. The theory of the tragedy of the commons is more exemplified in these mangrove forests today than ever before. The dangers of mangrove forest degradation are not restricted to carbon dioxide emissions, but can generate socio-economic displacements due to loss of biodiversity and ecosystem services. Although, deforestation is a global threat, solutions and reforestation programs must take into consideration socio-economic peculiarities of different host communities in order to be sustainable and successful.
Acknowledgements
This work was carried out under the framework of the West African Science Service Centre on Climate Change and Adapted Land Use, (WASCAL) programme, funded by the German Ministry of Education and Research (BMBF), Germany. The authors also acknowledge the Rufford Small Grants for Nature Conservation (grant no. 14352-1) for funding. The authors are also grateful to Ms. Petra Seibel and Ms. Ramona Brejcha for stable isotope analyses and the chiefs of the participating rural communities, the interviewers and all volunteered respondents.
Cite this paper
IsimemenOsemwegie,Dibi N’daHyppolite,ChristineStumpp,BarbaraReichert,JeanBiemi, (2016) Mangrove Forest Characterization in Southeast Côte d’Ivoire. Open Journal of Ecology,06,138-150. doi: 10.4236/oje.2016.63014
References
- 1. Tomlinson, P.B. (1986) The Botany of Mangroves. Cambridge University Press, Cambridge, 419.
- 2. Spalding, M., Kainuma, M. and Collins, L. (2010) World Atlas of Mangroves. Earthscan, London, Washington DC, 319.
- 3. Alongi, D.M. (2002) Present State and Future of the World’s Mangrove Forests. Environmental Conservation, 29, 331-349.
http://dx.doi.org/10.1017/S0376892902000231 - 4. Millennium Ecosystem Assessments (2005) Summary for Decision Makers. In: Ecosystems and Human Wellbeing: Synthesis, Island Press, Washington DC, 1-24.
- 5. Food and Agriculture Organization of the United Nations (2007) The World’s Mangroves 1980-2005. Forestry Paper 153.
- 6. Twilley, R.R., Chen, R. and Hargis, T. (1992) Carbon Sinks in Mangroves and Their Implication to Carbon Budget of Tropical Ecosystems. Water Air Soil Pollution, 64, 265-288.
http://dx.doi.org/10.1007/BF00477106 - 7. Donato, D.C., Kauffman, J.B., Murdiyarso, D., Kurnianto, S., Stidham, M. and Kanninen, M. (2011) Mangroves among the Most Carbon-Rich Forests in the Tropics. Nature Geoscience, 4, 293-297.
http://dx.doi.org/10.1038/ngeo1123 - 8. Bouillon, S. and Connolly, R.M. (2009) Carbon Exchange among Tropical coastal Ecosystems. In: Nagelkerken, I., Ed., Ecological Connectivity among Tropical Coastal Ecosystems, Springer, Berlin, 45-70.
http://dx.doi.org/10.1007/978-90-481-2406-0_3 - 9. Kathiresan, K. and Bingham, B.L. (2001) Biology of Mangroves and Mangrove Ecosystems. Advances in Marine Biology, 40, 81-251.
http://dx.doi.org/10.1016/S0065-2881(01)40003-4 - 10. Kathiresan, K. (2012) Importance of Mangrove Ecosystem. International Journal of Marine Science, 2, 70-89.
- 11. Reid, H. and Huq, S. (2005) Climate Change-Biodiversity and Livelihood Impacts. In: Robledo, C., Kanninen, M. and Pedroni, L., Eds., Tropical Forests and Adaptation to Climate Change: In Search of Synergies, CIFOR, Bongor, 57.
- 12. Murray, B.C., Pendleton, L., Jenkins, W.A. and Sifleet, S. (2011) Green Payments for Blue Carbon Economic Incentives for Protecting Threatened Coastal Habitats. Nicholas Institute for Environmental Policy Solutions, Duke University, Durham.
- 13. Kelleher, G., Bleakley, C. and Wells, S. (1995) A Global Representative System of Marine Protected Areas. Vol. 1, World Bank, Washington DC.
- 14. Saenger, P. and Bellan, M.F. (1995) The Mangrove Vegetation of the Atlantic Coast of Africa: A Review. University of Toulouse, Toulouse.
- 15. United Nations Environment Programme (2007) Mangroves of Western and Central Africa. UNEP-Regional Seas Programme/UNEP-WCMC.
http://www.unep-wcmc.org/resources/publications/UNEP_WCMC_bio_series/26.htm - 16. Duke, N.C., Ball, M.C. and Ellison, J.C. (1998) Factors Influencing Biodiversity and Distributional Gradients in Mangroves. Global Ecology and Biogeography, 7, 27-47.
http://dx.doi.org/10.2307/2997695 - 17. Nicole, M., Egnankou, W.M. and Schmidt, M. (1994) A Preliminary Inventory of Coastal Wetlands of Côte d’Ivoire. IUCN, Gland, 80.
- 18. MacKinnon, J. and MacKinnon, K. (1986) Review of the Protected Areas System of the Indo-Malayan Realm. World Conservation Union (IUCN), Gland. Cited in FAO & Wetlands International, 2006.
- 19. Naylor, R.L., Goldburg, R.J., Primavera, J.H., Kautsky, N., Beveridge, M.C.M., Clay, J., Folke, C., Lubchenco, J., Mooney, H. and Troell, M. (2000) Effect of Aquaculture on World Fish Supplies. Nature, 405, 1017-1024.
http://dx.doi.org/10.1038/35016500 - 20. Primavera, J.H. (2005) Global Voices of Science: Mangroves, Fishponds, and the Quest for Sustainability. Science, 310, 57-59.
http://dx.doi.org/10.1126/science.1115179 - 21. Egnankou, W.M. (1985) étude des mangroves de Côte d’Ivoire. Aspect écologique et recherches sur les possibilités de leur aménagement. PhD Thesis, University of Toulouse, Toulouse.
- 22. Crist, E.P. and Cicone, R.C. (1984) A Physically-Based Transformation of Thematic Mapper Data—The TM Tasseled Cap. IEEE Transactions on Geoscience and Remote Sensing, 22, 256-263.
http://dx.doi.org/10.1109/TGRS.1984.350619 - 23. Inoussa, M.M., Mahamane, A., Mbow, C., Saadou, M. and Yvonne, B. (2011) Dynamique spatio-temporelle des forets claires dans le Parc national du W du Niger (Afrique de l’Ouest). Science et Changements Planétaires/Sécheresse, 22, 108-116.
- 24. National Meteorological Station (2014) SODEXAM. Côte d’Ivoire.
- 25. Durand, J.R. and Guiral, D. (1994) Hydroclimat et hydrochimie. In: Environnement et ressources aquatiques de Côte d’Ivoire. Les milieuxlagunaires. ORSTOM, 2, 59-90.
- 26. Kathiresan, K. 3.3. Methods of Studying Mangrove.
http://dev.ourworld.unu.edu - 27. Komiyama, A., Poungparn, S. and Kato, S. (2005) Common Allometric Equations for Estimating the Tree Weight of Mangroves. Journal of Tropical Ecology, 21, 471-477.
http://dx.doi.org/10.1017/S0266467405002476 - 28. English, S.C., Wilkinson, C.R. and Basker, V.J. (1994) Survey Manual for Tropical Marine Resources. 2nd Edition, Australian Institute of Marine Science, Townsville.
- 29. Recensement General de la Population et de l’habitat Institute National de la Statistique (INS), RGPH, 1998. Abidjan, Côte d’Ivoire.
- 30. Baker, T.L. (1994) Doing Social Research. 2nd Edition, McGraw-Hill Inc., New York.
- 31. United Nations Human Settlements Programme (2014) The State of African Cities.
- 32. Alongi, D.M. (2008) Mangrove Forests: Resilience, Protection from Tsunamis, and Responses to Global Climate Change. Estuarine Coastal and Shelf Science, 76, 1-13.
http://dx.doi.org/10.1016/j.ecss.2007.08.024 - 33. Smith-III, T.J. (1992) Forest Structure. In: Robertson, A.I. and Alongi, D.M., Eds., Tropical Mangrove Ecosystems, American Geophysical Union, Washington DC, 101-136.
- 34. Gray, J.S. and Pearson, T.H. (1982) Objective Selection of Sensitive Species Indicative of Pollution-Induced Change in Benthic Communities. 1. Comparative Methodology. Marine Ecology Progress Series, 9, 111-119.
http://dx.doi.org/10.3354/meps009111 - 35. Asner, G.P., Scurlock, J.M.O. and Hicke, J.A. (2003) Global Synthesis of Leaf Area Index Observations: Implications for Ecological and Remote Sensing Studies. Global Ecology & Biogeography, 12, 191-205.
http://dx.doi.org/10.1046/j.1466-822X.2003.00026.x - 36. Rodelli, M.R., Gearing, J.N., Gearing, P.J., Marshall, N. and Sasekumar, A. (1984) Stable Isotope Ratio as a Tracer of Mangrove Carbon in Malaysian Ecosystems. Oecologia, 61, 326-333.
http://dx.doi.org/10.1007/BF00379629 - 37. Bouillon, S., Dahdouh-Guebas, F., Rao, A.V.V.S., Koedam, N. and Dehairs, F. (2003) Sources of Organic Carbon in Mangrove Sediments: Variability and Possible Ecological Implications. Hydrobiologia, 495, 33-39.
http://dx.doi.org/10.1023/A:1025411506526 - 38. Muzuka, A.N.N. and Shunula, J.P. (2006) Stable Isotope Compositions of Organic Carbon and Nitrogen of Two Mangrove Stands along the Tanzanian Coastal Zone. Estuarine, Coastal and Shelf Science, 66, 447-458.
http://dx.doi.org/10.1016/j.ecss.2005.10.007 - 39. Hutchinson, J., Manica, A., Swetnam, R., Balmford, A. and Spalding, M. (2014) Predicting Global Patterns in Mangrove Forest Biomass. Conservation Letters, 7, 233-240.
http://dx.doi.org/10.1111/conl.12060 - 40. Wongbusarakum, S. and Loper, C. (2011) Indicators to Assess Community-Level Social Vulnerability to Climate Change: An Addendum to SocMon and SEM-Pasifika Regional Socioeconomic Monitoring Guidelines. National Oceanic and Atmospheric Administration (NOAA) and Apia, Samoa: Secretariat of the Pacific Regional Environmental Programme (SPREP).
http://socmon.org - 41. International Fund for Agricultural Development (2015).
http://www.ruralpovertyportal.org/country/home/tags/cote_divoire - 42. Vigdis, F. (1992) Report of the United Nations Conference on Environment and Development, UNCED, Rio de Janeiro.
- 43. Field, C.D. (1999) Rehabilitation of Mangrove Ecosystems: An Overview. Marine Pollution Bulletin, 37, 383-392.
http://dx.doi.org/10.1016/S0025-326X(99)00106-X
NOTES
*Corresponding author.













