Open Journal of Ecology
Vol.3 No.1(2013), Article ID:27679,4 pages DOI:10.4236/oje.2013.31004
Foraging habitat use of breeding barn swallow (Hirundo rustica) in farmland, estuary, and island
![]()
School of Renewable Natural Resources, Louisiana State University Agricultural Center, Baton Rouge, USA;
*Corresponding Author: skang562@gmail.com
Received 1 December 2012; revised 30 December 2012; accepted 7 January 2013
Keywords: Foraging Habitat; Foraging Distance; Farmland; Estuary; Island
ABSTRACT
The decline of barn swallow populations may be mainly caused by the reduction of their foraging habitat. A clear understanding of the links between proportions of available and used microhabitats of foraging barn swallows in farmland, estuary, and island habitats would enhance our understanding of the foraging habitat requirements of this species and on the effects of anthropogenic activities, such as habitat conversion (e.g., land to water, crop fields to non-arable land), on their distribution. We hypothesized that: 1) foraging swallows would be more abundant in the most common microhabitat; and 2) swallow abundance would decrease with increased foraging distance from the nest-site. As predicted by our first hypothesis, swallows were more abundant in the most common microhabitat (i.e., crop fields in farmland and non-arable land on the island). Our data also support our second hypothesis that increased foraging distances from the nest-site negatively affected foraging swallow abundance. In summary, barn swallows foraged in the habitats most convenient to nest-sites, however, management of agricultural lands should include non-arable lands in the composition of available foraging microhabitats.
1. INTRODUCTION
Conservation of animal populations requires information on where the populations are, why they are there, and where else they could be [1]. Therefore, temporal and spatial variation in habitat conditions may generate strong selective pressure for habitat selection [2], which in turn influences reproduction and survival of individual birds [3-5], and contributes to the regulation of bird populations [6,7].
Changes in agricultural land-use have been linked to significant declines of barn swallows in many parts of the world [8-14]. Over the last 70 years, agricultural intensification has divided historically multipurpose agriculture into specialization of livestock or row crop production [15]. Barn swallow populations have declined where livestock farming was replaced by row crop production [16,17]. The decline of barn swallow populations may have been caused by the reduction of its prey resources [18] and foraging habitat. Although foraging habitat use of barn swallows in livestock farmland has been described [19-21], to our knowledge, there have been no comparison studies of foraging habitat use among farmland and other habitat types (i.e., estuary, island).
Swallows are aerial insectivores, feeding singly or gathering in large aggregations to feed on swarms of insects. The birds feed in a given patch for several minutes and move to a new patch when insect swarms disperse. In areas of high insect density, foraging swallows approach swarms slowly and then accelerates from below to capture the insects, because insects cannot escape to higher altitudes, insects are more visible against the sky than against the ground, and the counter-shaded predators themselves are less visible to the prey [22].
A clear understanding of barn swallow habitat use patterns among microhabitat of farmlands, estuaries, and islands would enhance our understanding of foraging habitat requirements for this species as well as the effects of anthropogenic activities, such as habitat conversion (e.g., land to water, crop fields to non-arable land), on their distribution. The principal objectives of this study were to compare the patterns of foraging habitat use in different land-cover areas. We hypothesized that: 1) foraging swallows would be more abundant in the most common microhabitat; and 2) swallow abundance would decrease with increased foraging distance from the nest-site.
2. STUDY AREA AND METHODS
2.1. Study Area
This study was conducted in Geoje (34˚51'N, 128˚34'E) and Jeju (33˚57'N, 126˚17'E) island, Nakdong estuary (35˚7'N, 128˚56'E), and Kimhae plain (35˚13'N, 128˚52'E) in South Korea from March 2003 to September 2004. We quantified the surface area of the different microhabitats in a radius of 400 m around the island (4 foraging sites), estuary (10 foraging sites), and farmland (5 foraging sites) because we estimated that almost all foraging occurred within this range based on previous studies [18,19]. Each foraging site was divided into four microhabitat types: crop fields, non-arable land (ungrazed grassland, small woods), water (ditch, river, shore), and human settlements. All microhabitats were identified from field visits and aerial photography and then validated by the presence of barn swallows in each habitat type during data collection.
2.2. Data Collection
The use of foraging sites by adult barn swallows was quantified using focal-nest observations (15 minutes, [23]). One hundred individuals in farmland, estuary, and island sites were observed, respectively. During observations, the observers waited until an adult left the nest and recorded what habitat types were used by the foraging swallow. The observers continued to follow the focal bird until it returned to the nest or was lost from sight. Birds were excluded from analysis if observers lost sight of the swallow in the foraging habitat before 15 minutes had expired. To prevent pseudoreplication, foraging observations were calculated only once per day for each nesting pair. The number of swallows observed was foraging individuals at 50 m distance intervals from the nest. The foraging habitats and the distance ranges (e.g., <50 m, 50 - 100 m) from nest were marked before followed foraging swallows. The density of swallows was expressed as the accumulated number of swallows recorded by each habitat type (i.e., farmland, estuary, island).
2.3. Statistical Analysis
Analyses of variance (ANOVA: PROC MIXED SAS 9.3) were used to test for statistical differences in breeding swallow densities and the proportion of foraging microhabitats among habitat types. For both ANOVAs, data were tested for normality with the Shapiro-Wilks test. In the event that the residuals were not normally distributed, the data were natural log-transformed. Significant ANOVA effects were tested by post-hoc comparisons of Tukey adjusted least squared means. We also performed two generalized linear models (GLMs; PROC GLIMMIX: SAS 9.3). The first GLM compared the abundance of foraging swallows in different microhabitat types within the three larger habitat types with a cumulative logit link function and multinomial error distribution. The second GLM fit an exponential decay model’s (PROC GLIMMIX: SAS 9.3) assessing the relationship between distance and natural log-transformed abundance with an identity link function and normal error distribution. For all GLMs, alternative exponential family error distributions and link functions were explored (e.g., identity or log links and negative binomial or poisson distributions) and the final combination of link functions and error distributions were selected by inspecting the Pearson chisquare/degree of freedom statistic and mean-variance plots [24]. For all analyses, significance level was set at α = 0.05.
3. RESULTS
We found 219 breeding pairs and 921 barn swallows in 24 sites from March-September 2003 and 2004. Both breeding individual density (F2.3 = 1.50, P = 0.35) and breeding pair density (F2.3 = 2.57, P = 0.22) in farmland, estuary, and island did not statistically differ, respectively. The portion of crop fields was higher in farmland than in estuary and island sites (F2.21 = 14.95, P < 0.001). The proportion of human settlements and non-arable land in island was greater than farmland and estuary sites, respectively (human settlements: F2.21 = 8.72, P = 0.002, non-arable land: F2.21 = 17.62, P < 0.001). Water portion did not differ among farmland, estuary, and island (F2.21 = 2.56, P = 0.11).
In crop fields, foraging swallow abundance in farmland was higher than swallow abundance in estuary and island habitats but abundance over water areas was greater in island habitat than in farmland or estuary habitat (F = 24.77, df = 7, P < 0.001, Figure 1). Abundance in non-arable land and human settlements did not differ among farmland, estuary, and island habitats. The abundance of foraging barn swallows in farmland (F = 53.88, df = 6, P < 0.001), estuary (F = 54.47, df = 6, P < 0.001), and island (F = 268.58, df = 6, P < 0.001) decreased as distance from the nest-site increased (Figure 2).
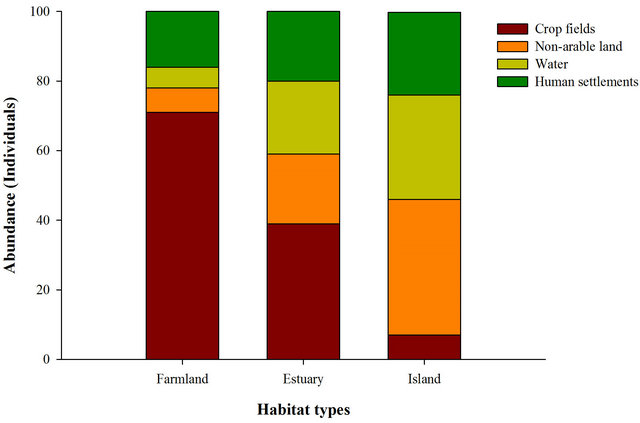
Figure 1. Distribution of 100 foraging barn swallows across four microhabitat types in three habitat types.
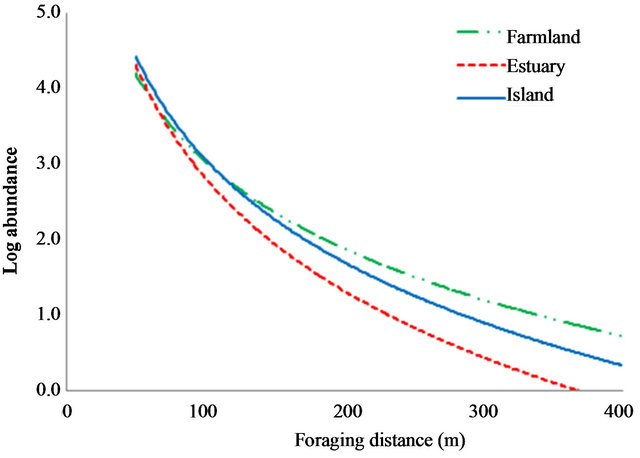
Figure 2. The log-transformed abundance of foraging swallows in relation to distance from the nest sites in farmland, estuary, and island. The log-transformed abundances were quantified using focal-nest observations.
4. DISCUSSION
The relationship between microhabitat proportion of foraging area and foraging barn swallow abundance in each microhabitat varied among farmland, estuary, and island habitat types. As predicted by our first hypothesis, crop fields (i.e., dominant microhabitat) in farmland and non-arable land (i.e., the highest portion) in island had larger foraging swallow abundance than in other microhabitat types. Previous study [20] noted that foraging habitat selection of breeding barn swallows followed broadly similar patterns to the distribution of aerial invertebrates because invertebrates are aggregated within fields. In this sense, our finding suggests that the higher abundances of foraging swallows in a higher proportion (i.e., more available) microhabitat among different habitat types may reflect the variation of their available prey resources.
Despite different foraging swallow abundance in a microhabitat across three habitat types, the positive association between the abundance of foraging swallows and most available microhabitat portion may be associated with vegetated boundaries (e.g., hedgerows or vegetated fence lines around crop fields or separating non-arable land), which were a consistent feature among these seemingly disparate microhabitat types. Previous study noted that the increased proportion of invertebrates occurred along hedgerows presumably because these boundaries reduce wind speed, which has been negatively correlated with aerial invertebrate densities [25]. Conversely, in open areas, greater wind speed disperses aerial invertebrates increasing difficulty in capture by foraging swallows. In addition, higher wind speeds are more energetically costly for swallow foraging [26], therefore, it is possible that swallows target vegetated boundaries with higher densities of aerial invertebrates and lower flight energy expense to reduce the metabolic costs of foraging [27].
Our data also support second hypothesis that increased foraging distances from the nest-site negatively affects foraging swallow abundance. Analysis of foraging distance indicated that barn swallows prefer to forage within 100 m from their nest in all habitat types. Similar patterns of foraging behavior (i.e., foraging distance from the nest) in farmland, estuary, and island may be a result of co-varying prey distribution and energy expenditure by searching flight. When foraging barn swallows require a longer distance from the nest to search their prey due to lack of prey resources, daily expenditure rates can increase due to search flight [28].
The comparison of foraging swallow abundance with microhabitat composition and foraging behavior in different habitat types can be used to assess habitat requirements for barn swallow population. Our data suggested that non-agricultural land cover types (i.e., estuary, island) are also important habitat as foraging site to breeding barn swallow population. If sustained protection of the breeding barn swallow population based on foraging habitat availability is desired, maintenance of non-agricultural lands in farmland, estuary, and island should be incorporated into a comprehensive conservation and management plan.
5. ACKNOWLEDGEMENTS
This project was supported by a Dong-A University in South Korea. We thank C. J. Kwon, S. King, R. Safran, and B. Pickens for their critical insights. We are grateful to the farmers who gave us permission to repeatedly visit their farms. We also appreciate the comments of the anonymous reviewers, whose suggestions improved this manuscript.
REFERENCES
- Aarts, G., MacKenzie, M., McConnell, B., Fedak, M. and Matthiopoulos, J. (2008) Estimating space-use and habitat preference from wildlife telemetry data. Ecography, 31, 140-160. doi:10.1111/j.2007.0906-7590.05236.x
- Cody, M.L. (1985) Habitat selection in birds. Academic Press, Gainesville.
- Brown, J.L. (1969) Territorial behavior and population regulation in birds. Wilson Bulletin, 81, 293-329.
- Fretwell, S.D. and Lucas, H.L. (1970) On territorial behavior and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheoretica, 19, 16-36. doi:10.1007/BF01601953
- Sutherland, W.J. and Parker, G.A. (1985) Distribution of unequal competitors. In: Sibly, R.M. and Smith, R.H. Eds., Behavioural Ecology: Ecological Consequences of Adaptive Behavior, Blackwell Scientific, Oxford, 224-274.
- Newton, I. (1998) Population limitation in birds. Academic Press, California.
- Johnson, M.D. (2007) Measuring habitat quality: A review. Condor, 109, 489-507. doi:10.1650/8347.1
- Vickery, J.A., Tallowin, J.R., Feber, R.E., Asteraki, E.J., Atkinson, P.W., Fuller, R.J. and Brown, B.K. (2001) The management of lowland neutral grasslands in Britain: Effects of agricultural practices on birds and their food resources. Journal of Applied Ecology, 38, 647-664. doi:10.1046/j.1365-2664.2001.00626.x
- Benton, T.G., Bryant, D.M, Cole, L. and Crick, H.Q.P. (2002) Linking agricultural practice to insect and bird populations: A historical study over three decades. Journal of Applied Ecology, 39, 673-687. doi:10.1046/j.1365-2664.2002.00745.x
- Benton, T.G., Vickery, J.A. and Wilson, J.D. (2003) Farmland biodiversity: Is habitat heterogeneity the key? Trends in Ecology and Evolution, 18, 182-188. doi:10.1016/S0169-5347(03)00011-9
- Burfield, I. and Van Bommel, F. (2004) Birds in Europe: Population estimates, trends and conservation status. Bird Life International, Cambridge.
- Newton, I. (2004) The recent declines of farmland bird populations in Britain: An appraisal of causal factors and conservation actions. Ibis, 146, 579-600. doi:10.1111/j.1474-919X.2004.00375.x
- Donald, P.E., Sanderson, F.J., Burfield, I.J. and Bommel, F.P.J. (2006) Further evidence of continent-wide impacts of agricultural intensification on European farmland birds, 1990-2000. Agriculture, Ecosystems and Environment, 116, 189-196. doi:10.1016/j.agee.2006.02.007
- Grüebler, M.U., Komer-Nievergelt, F. and Hirschheydt, J.V. (2010) The reproductive benefits of livestock farming in barn swallows Hirundo rustica: Quality of nest site or foraging habitat? Journal of Applied Ecology, 47, 1340- 1347. doi:10.1111/j.1365-2664.2010.01873.x
- Robinson, R.A. and Sutherland, W.J. (2002) Post-war changes in arable farming and biodiversity in Great Britain. Journal of Applied Ecology, 39, 157-176. doi:10.1046/j.1365-2664.2002.00695.x
- Robinson, R.A., Crick, H.Q.P. and Peach, W.J. (2003) Population trends of swallows Hirundo rustica breeding in Britain. Bird Study, 50, 1-7. doi:10.1080/00063650309461283
- Evans, K.L. and Robinson, R.A. (2004) Barn swallows and agriculture. British Birds, 97, 218-230.
- Møller, A.P. (2001) The effect of dairy farming on barn swallow Hirundo rustica abundance, distribution and reproduction. Journal of Applied Ecology, 38, 378-389. doi:10.1046/j.1365-2664.2001.00593.x
- Ambrosini, R., Bolzern, A.M., Canoval, L., Arieni, S., Møller, A.P. and Saino, N. (2002) The distribution and colony size of barn swallows in relations to agricultural land use. Journal of Applied Ecology, 39, 524-534. doi:10.1046/j.1365-2664.2002.00721.x
- Evans, K.L., Wilson, J.D. and Bradbury, R.B. (2007) Effects of crop type and aerial invertebrate abundance on foraging barn swallows Hirundo rustica. Agriculture, Ecosystems and Environment, 122, 267-273. doi:10.1016/j.agee.2007.01.015
- Henderson, I., Holt, C. and Vickery, J. (2007) National and regional patterns of habitat association with foraging barn swallows Hirundo rustica in the UK. Bird Study, 54, 371-377. doi:10.1080/00063650709461497
- Warrick, D.R. (1998) The turningand linear-maneuvering performance of birds: The cost of efficiency for coursing insectivores. Canadian Journal of Zoology, 76, 1063-1079. doi:10.1139/z98-044
- McCarty, J.P. and Winkler, D.W. (1999) Foraging ecology and diet selectivity of tree swallows feeding nestlings. Condor, 101, 246-254. doi:10.2307/1369987
- Zuur, F.F., Ieno, E.N., Walker, N.J., Savelley, A.A. and Smith, G.M. (2009) Mixed effects models and extensions in ecology with R statistics for biology and health series. Springer Science + Business Media, LLC, New York.
- Peng, R.K., Fletcher, C.R. and Sutton, S. (1992) The effect of microclimate on flying Depterans. International Journal of Biometeorology, 36, 69-76. doi:10.1007/BF01208916
- Norberg, U.M. (1990) Vertebrate flight: Mechanics, physiology, morphology, ecology and evolution. SpringerVerlag, Berlin.
- Evans, K.L., Bradbury, R.B. and Wilson, J.D. (2003) Selection of hedgerows by barn swallows Hirundo rustica foraging on farmland: The influence of local habitat and weather. Bird Study, 50, 8-14. doi:10.1080/00063650309461284
- Schmidt-Nielsen, K. (1972) Locomotion: Energy cost of swimming, flying, and running. Science, 177, 222-228. doi:10.1126/science.177.4045.222

