Open Journal of Molecular and Integrative Physiology
Vol.3 No.4(2013), Article ID:39566,5 pages DOI:10.4236/ojmip.2013.34023
Endothelium derived relaxation factors reduce sulfur dioxide-induced aortic relaxation
![]()
1Biology Department, Faculty of Science, University of Zakho, Duhok, Iraq
2Biology Department, College of Science, University of Salahaddin, Erbil, Iraq
Email: abbas.salihi@uni-sci.org
Copyright © 2013 Omar A. M. Al-Habib, Abbas B. Q. Salihi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 30 July 2013; revised 30 August 2013; accepted 6 September 2013
Keywords: Sulfur Dioxide; Endothelium; Endothelium Derived-Relaxation Factors; Organ Bath; PowerLab System; Aorta
ABSTRACT
The endothelium plays a key role in the control of vascular patency and tone. Thus, the main objective of the study was to determine the role of endothelium and its derived relaxation factors in mediating relaxation of rat thoracic aorta, in response to sulfur dioxide (SO2) derivatives “1:3 M/M sodium bisulfite (NaHSO3) and sodium sulfite (Na2SO3)” using PowerLab tissue bath system. Endothelial denudation enhanced relaxation responses of SO2 derivatives with an IC50 of 6.11 mM as compared to control rings with an IC50 of 6.21 mM, as well as the maximum relaxation (Emax) was increased from 62.026% ± 6.527% to 83.13% ± 14.755%. Furthermore, the relaxation responses to SO2 derivatives in aortic rings were significantly enhanced by indomethacin, clotrimazole and methylene blue with IC50’s of 4.8 mM, 5.33 mM and 4.01 mM, and Emax were raised to 101.1% ± 6.537%, 66.92 ± 7.538 and 104.68 ± 3.575, respectively. Meanwhile, L-NAME did not alter dose-dependent relaxation of SO2 derivatives in comparison to control aortic rings. The results of this study had shown that endothelium denudation and blocking of endothelium derived-relaxation factors enhanced vasodilator effect of SO2; this may clarify the role of endothelium in the vasodilatory mechanism of SO2.
1. INTRODUCTION
The vascular endothelium is strategically located at the interface between the circulating blood and vessel wall, providing a permeability barrier to the movement of cell metabolites and nutrients, while allowing the transfer of electrical signals within the intact tissue [1]. Endothelial cells synthesize and release various factors that modulate in short terms vascular tone. The vasoactive factors include relaxing substances, prostaglandin I2 (PGI2), NO, endothelium-derived hyperpolarizing factors (EDHF), Cnatriuretic peptide, and contracting substances, such as, thromboxane A2 (TXA2), endothelin-1, angiotensin II, superoxide anion [2].
Sulfur dioxide is a common air pollutant released into the atmosphere from the combustion of fossil fuel [3]. Inhaled SO2 is hydrated to produce sulfurous acid in the respiratory tract, which subsequently dissociates to form its derivatives, bisulfite and sulfite (1:3 M/M in neutral fluid). The derivatives can be absorbed into the blood or other body fluids [4]. In addition, bisulfite/sulfite enters the body via foods, beverages and drugs, because of sulfiting agents, such as SO2, metabisulfite, NaHSO3, and Na2SO3 [5]. Endogenously produced gaseous SO2 is hypothesized to fulfill a physiological role in regulating cardiovascular function, distinctive from its toxicological effects [6]. Vasodilatory effect of SO2 derivatives on isolated rat aortic rings first reported by Meng and his team in 2005, although the exact mechanism of vasodilatation is still unknown [7].
Sulfur dioxide could relax vascular smooth muscle cells (VSMCs), and the mechanism might be associated with the activation of calcium (Ca++) channels and ATPdependent potassium (KATP) channels [8]. Further more, [9] found that SO2 derivatives can alter cell’s excitability by increasing the firing frequency of potassium (K+) channels in hippocampal neurons and ventricular myocytes. While, [10] showed that SO2 derivatives significantly enhanced voltage-gated sodium channels in a concentration-dependent manner in isolated adult rat cardiomyocytes. On the other hand, [11] recently found that rat blood pressure could be lowered by SO2 and its derivatives, also they were demonstrated that the vasorelaxant effect of SO2 at basal and low concentrations might be mediated by NO and/or cGMP pathway. NO mediates vasorelaxation by increasing the cellular cGMP level and/or stimulating Ca++-dependent K+ channels (KCa) in VSMCs. So the mechanism of SO2-induced vasorelaxation should partially involve the contribution of KCa channels. Because there is only little information about the mechanism of action of SO2, therefore, this study is one of our attempts to detect the role of endothelium in the relaxatory pathway of SO2.
2. MATERIALS AND METHODS
The animal experimental procedures conformed to the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (NIH) in the United States and was approved by the Animal Research Committee of Zakho University. Adult male Wistar rats (Rattus norvegicus) were used for this study. The animals were kept under standard laboratory conditions. After anaesthetizing, the chest cavity was opened, after removal of excess tissue and fat, thoracic aorta was isolated and transferred to beaker containing Krebs solution (composition in mM: NaCl—136.9, KCl—5.4, Glucose—5.5, NaHCO3—23.8, MgCl2—1, CaCl2—1.5, and EDTA—0.003), equilibrated with 95% O2 and 5% CO2. The beaker was placed in the water bath at 37˚C.
The procedure of [12] and coworkers (2009) with some modifications was followed to study the vascular reactivity in the isolated aorta. Two stainless steel wires were carefully inserted into lumen of the aortic rings. One wire was anchored to the hook at the base of an organ bath (Model 166051, Radnoti, Monrovia Ca, USA) and other wire was connected to force transducer (MLT0201/RAD 5 mg - 25 mg, AD instruments, Sydney, Australia) coupled to the transbridge amplifier (ML 224, Quad Bridge Amp, AD instruments). Data was acquired with a PowerLab Data Acquisition System (ML 870, Power Lab, AD instruments) using the chart software (Version 7) for measurement of isometric tension. The degree of contraction and relaxation were indicated by the tension development in the recording system and expressed in gram.
Rings were allowed to equilibrate for 60 minutes at a resting tension of 2 grams with changes of buffer every 15 minutes. When the isometric tension had stabilized, inhibitory concentration-response curves of the SO2 derivatives “1:3 M/M NaHSO3 and Na2SO3” (3 mM - 9 mM) were constructed against contractions induced with phenylephrine (PE; 1 × 10−6 M).
To detect the role of endothelial cells in the relaxant effect of SO2 derivatives, sandwich preparations were made similar to those described by [13], in which the endothelial layer in a long segment of the aorta was removed by gently rubbing the intimal surface of the rings with a syringe needle covered by a piece of cotton. The endothelium-denuded segment was then cut into several pieces; each piece was tested to confirm the removal of the endothelium by the lack of any response to acetylcholine (1 × 10−5 M) following the pre-constriction with PE (1 × 10−6 M).
The role of endothelium/NO, cGMP, PGI2 and epoxyeicosatreinoic acid (EET) in association with vasorelaxation induced by SO2 derivatives were evaluated following incubation of endothelium-intact rings with, LNAME (3 × 10−4 M), methylene blue (3 mM), indomethacin (3 × 10−5 M) or clotrimazole (3 × 10−5 M), blockers of above mentioned factors respectively, for 10 minutes prior to application of PE.
The concentration-response curves were fitted with a Hill equation, from which the half maximal inhibitory concentration (IC50) values were given as geometric mean with 95% confidence intervals (95% CI). Maximum contractile responses to SO2 derivatives were calculated as a percentage of the contraction produced by PE and were expressed as the means ± standard error of the mean (SEM). The tension produced by PE was defined as 0% relaxation, and the baseline tension before addition of vasoconstrictors were defined as 100% relaxation.
Statistical Analysis
The statistical analysis was performed using two-way analysis of variance (ANOVA) supported by Bonferroni test when carrying out pair wise comparison between the same doses of different groups [14]. P-value less than 0.05 (P < 0.05) were considered as statistically significant. All the graph, calculation and statistical analyses were performed using GraphPad Prism software version 5.0 for Windows (GraphPad Software, San Diego, California, USA).
3. RESULTS
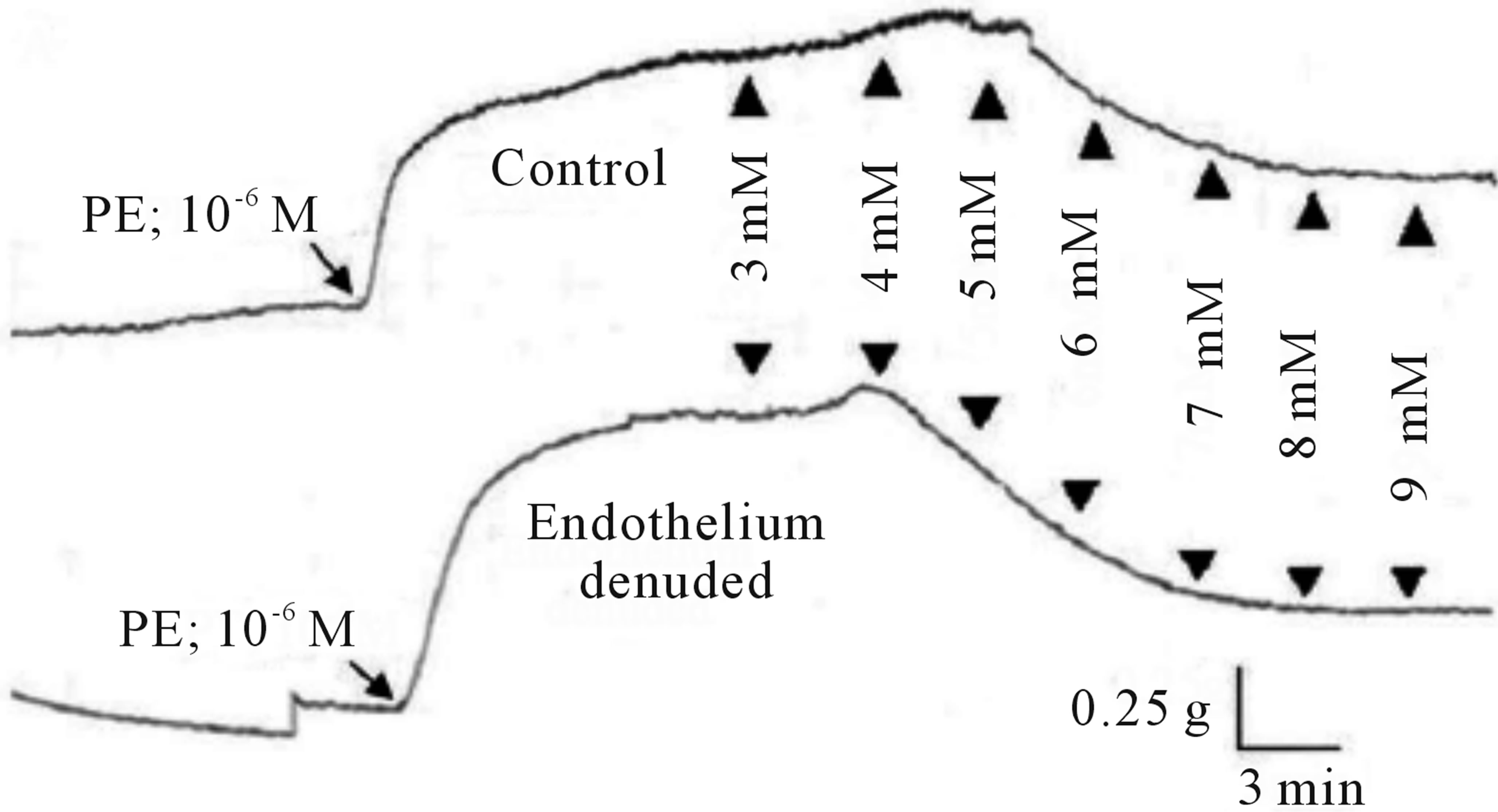
The cumulative addition of SO2 derivatives, at the plateau phase of the contraction induced by PE (10−6 M) in rats thoracic aortic rings caused contraction-dependent inhibition of the PE-induced contraction, in both, endothelium-intact and endothelium-denuded preparation (Figure 1). Endothelium denudation significantly (P < 0.05) induced relaxation only at a dose (7 mM) with IC50 6.11 mM (with IC50 of CI 95% 5.302 to 6.919 mM) and 6.21 mM (with IC50 of CI 95% 5.845 to 6.574 mM) in the denuded and intact endothelial rings, respectively. The Emax for endothelium denuded and intact rings were 83.13% ± 14.755% and 62.026% ± 6.527%, respectively, as shown in (Table 1).
Inclusion of aortic rings with L-NAME did not alter dilation from control, however this response tended towards significance in precontracted rings with indomethacin, clotrimazole and methylene blue with an IC50’s of 4.801 mM (with an IC50 of CI 95% between 4.523 to 5.079), 5.331 mM (4.868 to 5.795) and 4.011 mM (3.846 to 4.176), and Emax were increased to 101.1% ± 6.537%, 66.92 ± 7.538 and 104.68 ± 3.575 respectively, as shown in (Figures 2-6 and Table 1).
4. DISCUSSION
It is well known that the endothelium plays an important role in the regulation of vascular tone by synthesis and release of endothelium-derived relaxing factors, including NO, PGI2 and EDHF. Thus, it was decided to investigate role of endothelium and EDRFs involved in SO2- induced responses.
The results of the present study demonstrated that removal of functional endothelium increased the relaxation response to SO2 only at high dose (7 mM), indicating that vasorelaxation of SO2 may be inhibited by substances released by endothelium. This effect may be due to antagonistic actions of PGI2, EET and soluble gualanyl cyclase, because when aortic rings preincubated with either blockers an increment of SO2-induced vasorelaxation were observed. In this experiment, after the aortic rings were preincubated with indomethacin, the vasodilator effect caused by exogenous SO2 derivatives was enhanced in part. The PGI2 route might be one of the antagonistic mechanisms of the vasorelaxation caused by SO2 derivatives, because the preincubation with indomethacin caused only in part an enhancement of the aortic relaxation. Although, their was no previous study to explain the relation between SO2 and EET, [15] reported that SO2 might be a type of EDHFs, because it shares several features with EDHFs, furthermore, [16] concluded that within a physiological relevant concentration range, might induce a release of EDHF from vascular endothelium, but they didn’t explained the effect of EET on SO2.
On the other hand, the results showed that the vasodilatory effects of SO2 derivatives could be not inhibited by treating aortic rings with L-NAME, it proved that the vasodilatory effect of SO2 was not mediated by NO. The same results concerning the relation between SO2 and NO were observed by [7]. The results of this study concluded that endothelium denudation and blocking of endothelium derived-relaxation factors enhanced vasodilator effect of SO2; this may elucidate the role of en-
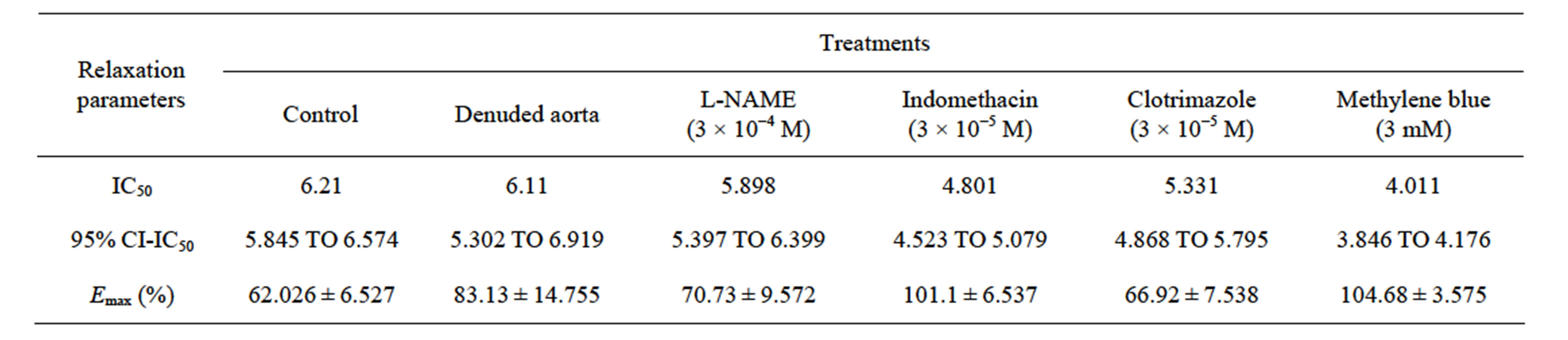
Table 1. The IC50 (IC50 of CI 95%) and Emax ± SEM for the effect of SO2 derivatives on control and preincubated aortic rings with L-NAME, indomethacin, clotrimazole and methylene blue.
 (a)
(a)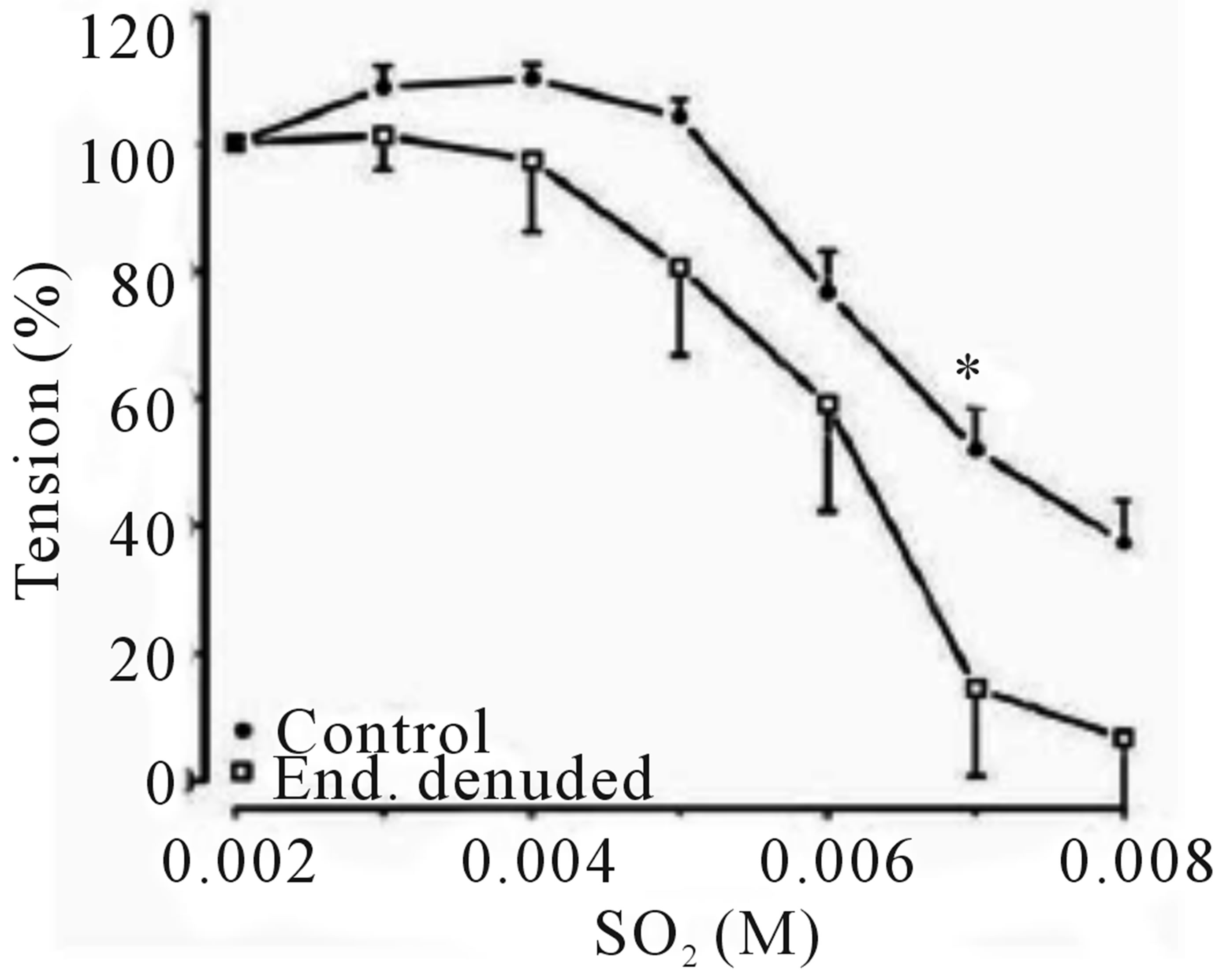 (b)
(b)
Figure 1. Concentration-response effects of SO2 on PE (1 μM)-induced vasoconstriction. (a) Typical chart view trace; and (b) Dose-response curve showing comparative vasorelaxation effects of SO2 on PE-induced vasoconstriction (control) and endothelium denuded aortic rings. ▲ indicates addition of SO2 (mM) in cumulative manner. (*p < 0.05; compared to control; Two-way ANOVA, Bonferroni post test).
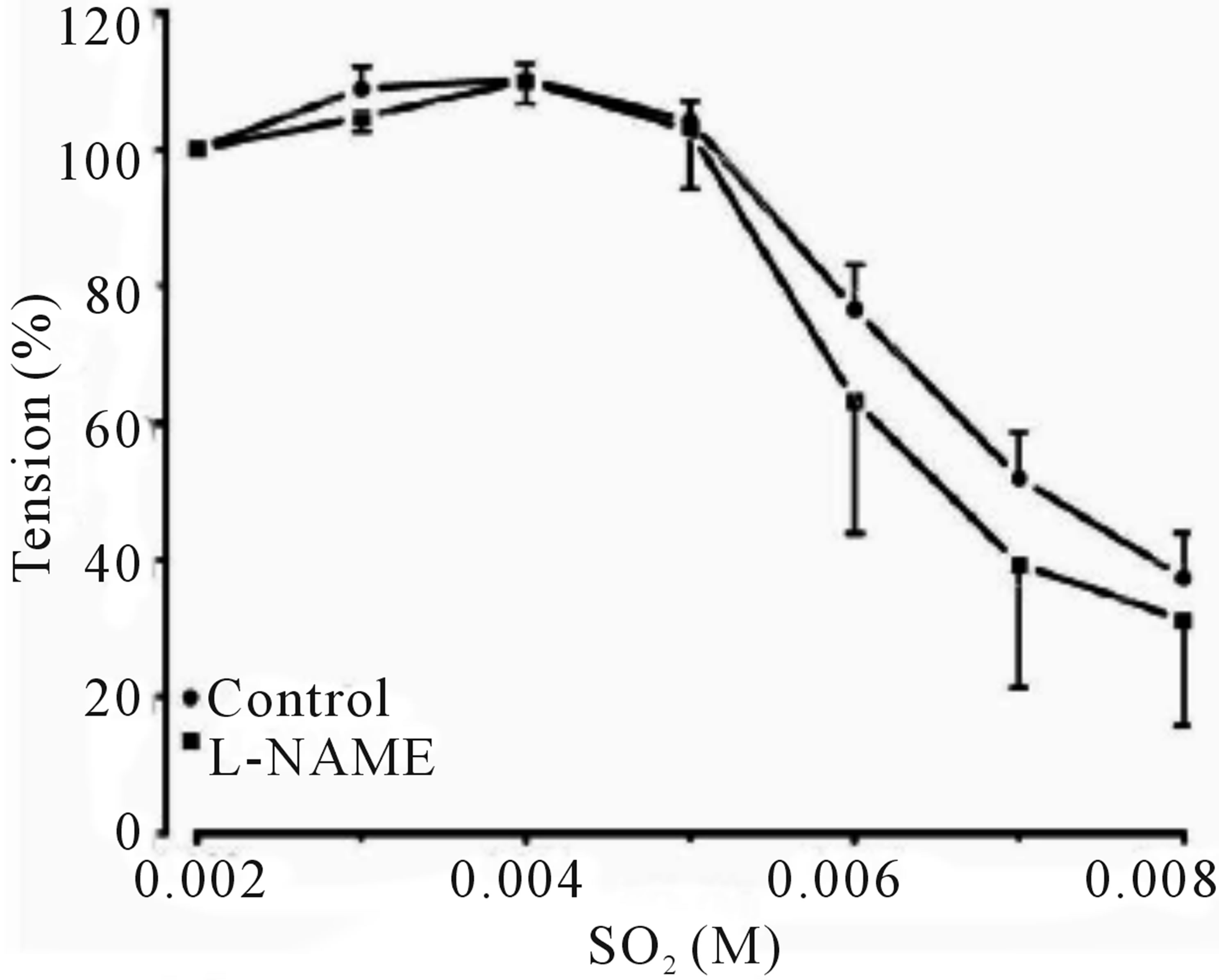
Figure 2. Cumulative dose-response curve for the vasorelaxant effects of SO2 on control and preincubated aortic rings with L-NAME (3 × 10−4 M), precontracted with PE (10−6 M).
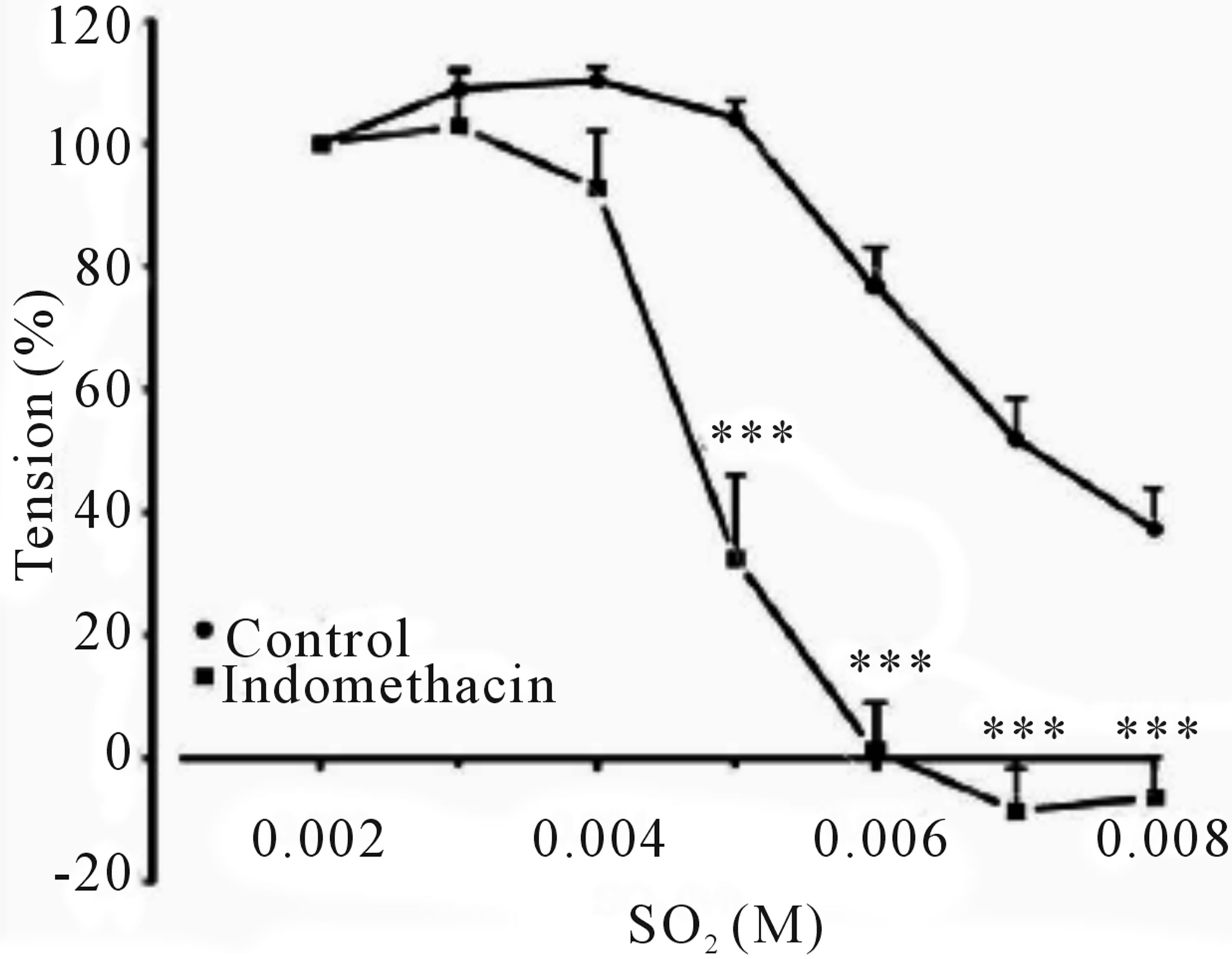
Figure 3. Cumulative dose-response curve for the vasorelaxant effect of SO2 on control and preincubated aortic rings with indomethacin (3 × 10−5 M), precontracted with PE (10−6 M). (***p < 0.001, compared to control; Two-way ANOVA, Bonferroni post test).
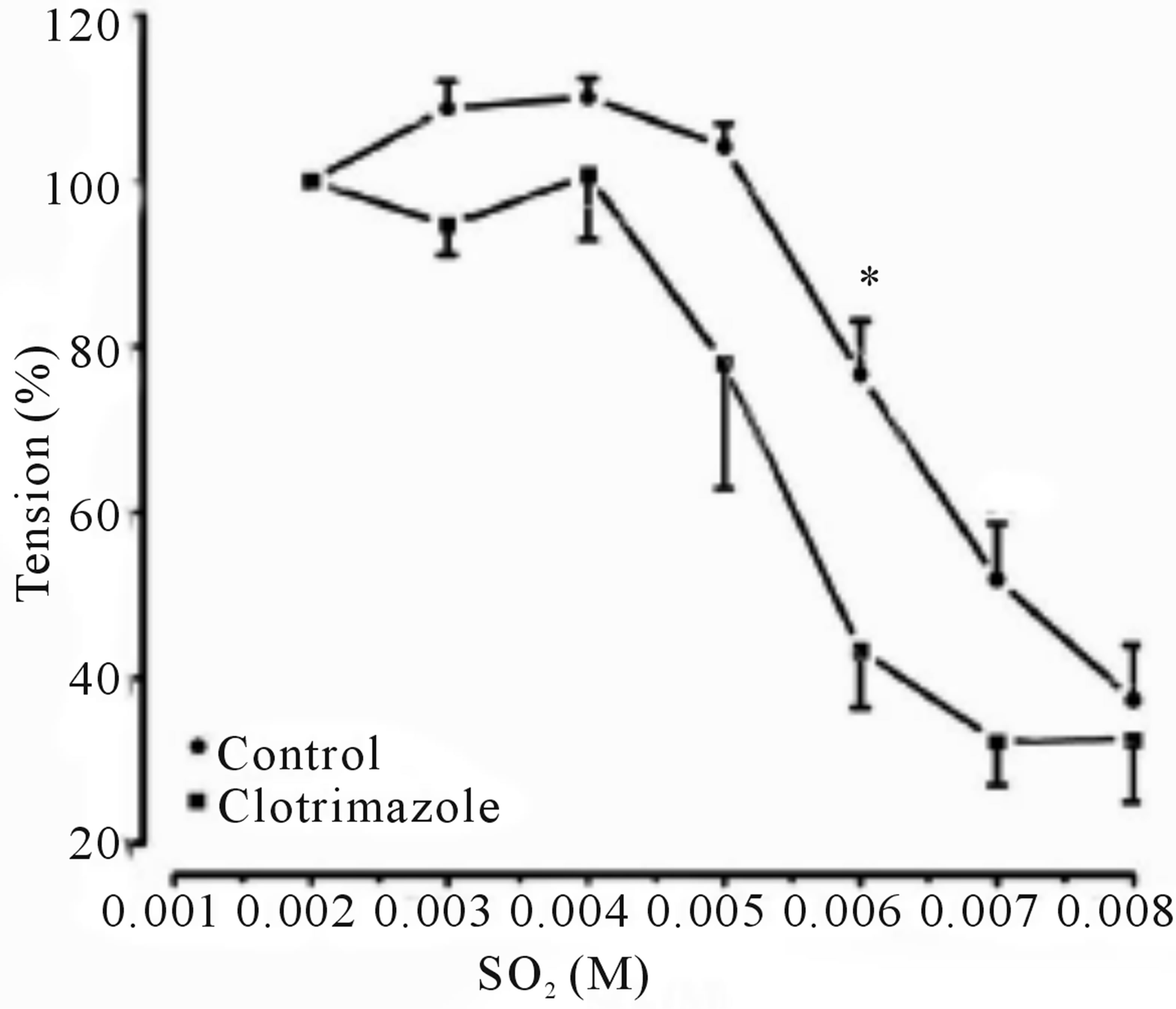
Figure 4. Cumulative dose-response curve for the vasorelaxant effects of SO2 on control and preincubated aortic rings with clotrimazole (3 × 10−5 M), precontracted with PE (10−6 M). (*p < 0.05; compared to control; Two-way ANOVA, Bonferroni post test).
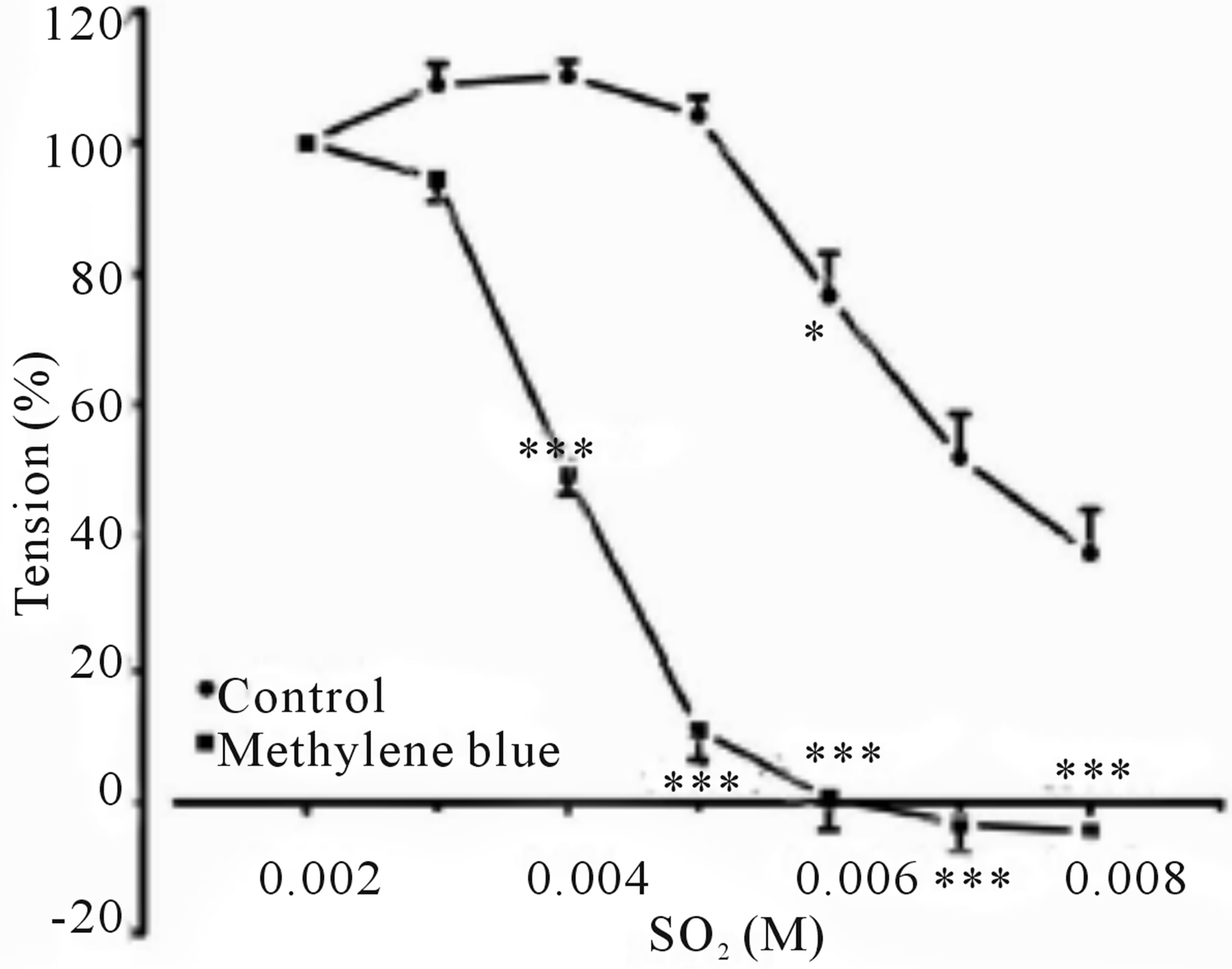
Figure 5. Cumulative dose-response curve for the vasorelaxant effects of SO2 on control and preincubated aortic rings with Methylene blue (3 mM), precontracted with PE (10−6 M). (***p < 0.001; compared to control; Two-way ANOVA, Bonferroni post test).
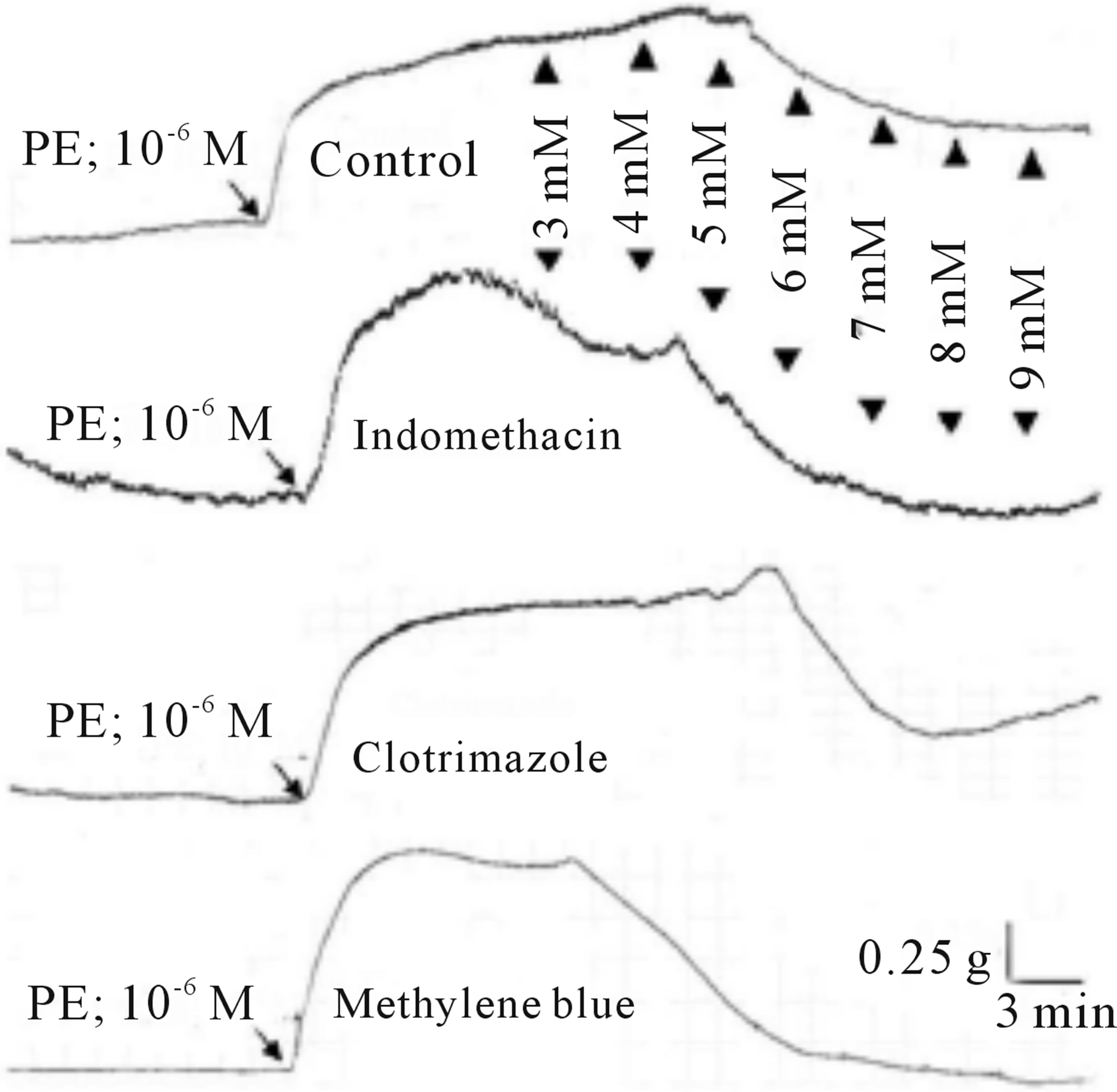
Figure 6. Typical chart view trace of concentration-response effects of SO2 on PE (1 μm)-induced vasoconstriction, showing comparative vasorelaxant effects of SO2 (control) and indomethacine (3 × 10−5 M), clotrimazole (3 × 10−5 M), and methylene blue (3 mM), preincubated aortic rings, respectively. ▲ indicates addition of SO2 (mM) in cumulative manner.
dothelium in the vasodilatory mechanism of SO2.
REFERENCES
- Adams, D.J. and Hill, M.A. (2004) Potassium channels and membrane potential in the modulation of intracellular calcium in vascular endothelial cells. Journal of Cardiovascular Electrophysiology, 15, 13. http://dx.doi.org/10.1046/j.1540-8167.2004.03277.x
- Zhang, D. (2007) Hydroperoxide-induced oxidative stress in the arterial wall: Pharmacological characterization of the effects on arterial contractility. Academic Thesis, Eberhard Karls Universität Tübingen.
- Nie, A. and Meng, Z. (2005) Study of the interaction of sulfur dioxide derivative with cardiac sodium channel. Biochimica et Biophysica Acta, 1718, 7. http://dx.doi.org/10.1016/j.bbamem.2005.09.020
- Meng, Z., Qin, G., Zhang, B. and Bai, J. (2004) DNA damaging effects of sulfur dioxide derivatives in cells from various organs of mice. Mutagenesis, 19, 4. http://dx.doi.org/10.1093/mutage/geh058
- Nie, A. and Meng, Z. (2007) Sulfur dioxide derivatives modulate Na/Ca exchange currents and cytosolic [Ca2+]i in rat myocytes. Biochemical and Biophysical Research Communications, 358, 6. http://dx.doi.org/10.1016/j.bbrc.2007.05.008
- Li, J. and Meng, Z. (2009) The role of sulfur dioxide as an endogenous gaseous vasoactive factor in synergy with nitric oxide. Nitric Oxide, 20, 166-174. http://dx.doi.org/10.1016/j.niox.2008.12.003
- Meng, Z., Li, Y. and Li, J. (2007) Vasodilatation of sulfur dioxide derivatives and signal transduction. Archives of Biochemistry and Biophysics, 467, 291-296. http://dx.doi.org/10.1016/j.abb.2007.08.028
- Du, X., Zhang, C., Jin, H., Du, J. and Tang, C. (2006) Vasorelaxant effect of sulfur dioxide derivatives on isolated aortic rings of rats and its mechanisms. Journal of Peking University, 38, 581-585.
- Du, Z., Zhou, Y. and Yang, P. (2007) Sulfur dioxide derivatives increase a hyperpolarization-activated inward current in dorsal root ganglion neurons. Toxicology, 239, 180-185. http://dx.doi.org/10.1016/j.tox.2007.07.005
- Nie, A. and Meng, Z. (2005) Sulfur dioxide derivative modulation of potassium channels in rat ventricular myocytes. Archives of Biochemistry and Biophysics, 442, 187-195. http://dx.doi.org/10.1016/j.abb.2005.08.004
- Zhang, Q. and Meng, Z. (2009) The vasodilator mechanism of sulfur dioxide on isolated aortic rings of rats: Involvement of the K+ and Ca2+ channels. European Journal of Pharmacology, 602, 117-123. http://dx.doi.org/10.1016/j.ejphar.2008.11.030
- Aziz, N., Malik, H., Safur, R., Samra, B., Sidra, R. and Anwar, H. (2009) Antihypertensive, antioxidant, antidyslipidemic and endothelial modulating activities of a polyherbal formulation (POL-10). Vascular Pharmacology, 50, 8. http://dx.doi.org/10.1016/j.vph.2008.09.003
- Dong, H., Gareth, J., Denise, G., William, C. and Christopher, R. (1997) NO/PGI2-independent vasorelaxation and the cytochrome P450 pathway in rabbit carotid artery. British Journal of Pharmacology, 120, 695-701. http://dx.doi.org/10.1038/sj.bjp.0700945
- Motulsky, H. and Christopolous, A. (2003) GrdaphPad Prism: Fitting models to biological data using linear and non-linear regression; a practical guide to curve fitting. GraphPad Software, Inc.
- Liu, D., Jin, H., Tang, C. and Du, J. (2010) Sulfur dioxide: A novel gaseous signal in the regulation of cardiovascular functions. Mini-Reviews in Medicinal Chemistry, 10, 1039- 1045. http://dx.doi.org/10.2174/1389557511009011039
- Meng, Z., Li, J., Zhang, Q., Bai, W., Yang, Z., Zhao, Y. and Wang, F. (2009) Vasodilator effect of gaseous sulfur dioxide and regulation of its level by Ach in rat vascular tissues. Inhalation Toxicology, 21, 1223-1228.

