Open Journal of Molecular and Integrative Physiology
Vol. 2 No. 4 (2012) , Article ID: 24578 , 12 pages DOI:10.4236/ojmip.2012.24018
Growth and differentiation factor-11 is developmentally regulated in skeletal muscle and inhibits myoblast differentiation
![]()
AgResearch Ltd., Ruakura Research Centre, Hamilton, New Zealand
Email: *frank.jeanplong@agresearch.co.nz
Received 23 August 2012; revised 25 September 2012; accepted 6 October 2012
Keywords: GDF-11; Developmental Expression; Post-Natal Muscle Growth; Sexual Dimorphism; Myoblast Differentiation
ABSTRACT
Growth and differentiation factor-11 (GDF-11) is a secreted protein that is closely related to myostatin, a known inhibitor of skeletal muscle development. The role of GDF-11 in regulating skeletal muscle growth remains unclear and the pattern of expression during post-natal growth has not been reported. Therefore, we sought to determine the expression of GDF-11 during post-natal growth and its effect on myoblast proliferation and differentiation. We collected gastrocnemius muscles from male and female mice at 2, 3, 4, 6, 12, 20 and 32 weeks of age (n = 6 per sex and age). In addition, gastrocnemius muscles were collected from male wild-type and myostatin knockout mice at 4, 6, 12 and 20 weeks of age (n = 6 per age and genotype). RNA was extracted and reverse transcribed. Northern analysis identified an expected 4.4 kb mRNA transcript for GDF-11 in gastrocnemius muscles of mice. The concentration of GDF-11 mRNA, as determined by quantitative PCR, was increased in gastrocnemius muscles from 2 to 6 weeks—a period of rapid postnatal muscle growth—and remained higher in male than female mice from 4 to 20 weeks of age (P < 0.05). Interestingly, the mRNA concentration of GDF-11 and its cognate receptors (ActRIIA, ActIIB and Alk5) were increased in gastrocnemius muscles of myostatin knockout compared with wild-type mice (P < 0.05), which may suggest a compensatory mechanism for the lack of myostatin. In support, recombinant GDF-11 inhibited differentiation of C2C12 murine myoblasts and those isolated from myostatin knockout and wild-type mice (P wang#Bracket## 0.05). Inhibited differentiation of C2C12 myoblasts was associated with decreased mRNA expression of early and late molecular markers of differentiation (MyoD, myogenin, IGF-II, desmin and MyHC, P < 0.05). Our results suggest that GDF-11 regulates growth of skeletal muscles by inhibiting myoblast differentiation in an autocrine/paracrine manner and, perhaps, also plays a role in regulating sexually dimorphic growth.
1. INTRODUCTION
Growth and differentiation factor-11 (GDF-11) is a secreted protein that belongs to the Transforming Growth Factor-β (TFG-β) superfamily and is known for its role in regulating anterior-posterior patterning [1-4] . However, the role of GDF-11 in the regulation of skeletal muscle development remains unclear.
GDF-11 is closely related to myostatin, a key inhibitor of muscle growth, and the two proteins share 90% amino acid sequence identity in their C-terminal receptor binding moieties. Therefore, the high level of structural similarity would predict a high degree of functional similarity between these two proteins. In support, GDF-11 inhibited muscle development in chick embryos and also inhibited the differentiation of myogenic cell lineages [4] . Furthermore, muscle-specific over-expression of follistatin, a common inhibitor of several TGF-β family members including myostatin and GDF-11, resulted in greater increases in skeletal muscle mass than observed in myostatin knockout mice, which suggested that follistatin was antagonizing an unknown factor that acted similarly to that of myostatin [5] . In further support for the existence of a myostatin-like factor, administration of a soluble form of the activin type IIB receptor (ActRIIB) [6] increased the mass of muscles by 20% - 30% in myostatin knockout mice. However, the identity of this factor has remained unknown.
Both GDF-11 and myostatin predominantly bind and signal through ActRIIB, but the actions of these two proteins differs quite markedly [7,8] . GDF-11 knockout mice die within 24 hours after birth of likely palate and renal abnormalities, which prevents the establishment of an adult muscle phenotype [1]. In contrast, myostatin knockout mice are healthy and exhibit a two to three fold increase in the size of individual skeletal muscles [9] . In a more recent study, GDF-11 and other TGF-β family members were identified in mouse and human sera using affinity purification with ActRII receptors [10] . The authors proposed that GDF-11 and activins are candidate novel muscle mass regulators [10] . Given the potential importance of GDF-11 in regulating development of skeletal muscle, it is intriguing to note that its expression in skeletal muscles during post-natal development has not been reported.
In this study, we sought to determine the expression and possible function of GDF-11 in skeletal muscles during post-natal development. To address this aim, we determined if: 1) expression of GDF-11 is associated with post-natal development of skeletal muscles, 2) expression of GDF-11 displays a sexually dimorphic pattern in skeletal muscles, 3) expression of GDF-11 is increased in myostatin knockout mice, 4) GDF-11 alters proliferation and/or differentiation of myoblasts.
2. MATERIALS & METHODS
2.1. Animals
In the first experiment, male and female mice (C57 strain) were killed at 2, 3, 4, 6, 12, 20 and 32 weeks of age (n = 6 of each sex per age), and the gastrocnemius muscles were dissected, weighed and snap frozen in liquid nitrogen and stored at −80˚C. In the second experiment, male wild-type (C57) and myostatin knockout mice (a gift from S.J. Lee of John Hopkins University, Baltimore, Maryland) were killed at 4, 8, 12 and 20 weeks of age (n = 6 of each genotype per age) and the gastrocnemius muscles were excised, weighed and snap frozen in liquid nitrogen and stored at −80˚C. All mice were maintained under a photoperiod of 14 h light: 10 h dark and were fed a standard rodent diet (Diet 86, Sharps grain and seed, Carterton, New Zealand) and food and water available adlibitum. The studies were approved by the Ruakura Animal Ethics Committee, Hamilton, New Zealand.
2.2. Isolation of Primary Mouse Myoblasts
Primary mouse myoblasts were isolated from hindlimb muscles of wild-type (C57) and myostatin knockout mice (5 - 6 weeks old) as we have previously described [11] . Briefly, hindlimb muscles were excised and minced finely with scalpel blades, then digested for 90 min in a PBS buffer (Oxoid, UK) containing 0.2% (m/v) collagenase type 1A (Sigma, St. Louis, MO, USA), triturated and filtered through a 100 μm membrane. The resulting cell suspension was resuspended in satellite cell medium (Dulbecco’s Modified Eagle’s Medium [DMEM; Invitrogen, CA] supplemented with 20% (v/v) Fetal Bovine Serum [FBS, Invitrogen, CA] and 10% (v/v) horse serum [HS, Invitrogen, CA]) and plated onto uncoated cell culture dishes (Nunc, Roskilde, Denmark) for 3 h to allow the preferential attachment of fibroblasts. Media containing unattached cells was then transferred to cell culture dishes that had been pre-coated with 10% (v/v) Matrigel (Becton Dickinson, NJ). Cells were then cultured in an atmosphere of 5% CO2 and 37˚C to increase cell number and subsequently plated into assays described below. To verify the efficiency of myoblast isolation, we carried out immunocytochemistry on isolated myoblast cultures with an antibody to Pax7 and found that >90% of the cells were positive for Pax7 (data not shown).
2.3. Northern Blot Analysis
Gastrocnemius muscles from male wild-type mice (C57, 8 weeks old) were homogenized on ice in Trizol reagent and total RNA was isolated following the manufacturer’s protocol (Invitrogen, CA). RNA was re-suspended in diethyl pyrocarbonate-treated water and the total RNA concentration was determined by measuring absorbance at 260 nm (Nanodrop spectrophotometer, Thermo Fisher Scientific, USA). Ten micrograms of total RNA were separated on a 1.2% formaldehyde-agarose gel with a 0.24-kb to 9.5-kb RNA molecular weight marker (Life Technologies GIBCO BRL) and transferred to an uncharged nylon membrane (Hybond-N, GE Healthcare, NJ) by capillary action. The membrane was cross-linked using ultra-violet radiation and stained with methylene blue to verify the integrity of RNA and the uniformity of transfer. To ensure that the probe does not detect myostatin or other members of the TGF-beta superfamily, the exon 1 sequence of GDF-11 was selected. A DNA probe was made using reverse transcription-polymerase chain reaction (RT-PCR). Five micrograms of total RNA from mouse gastrocnemius muscle was reverse transcribed using a Superscript III first strand cDNA synthesis kit (Invitrogen, CA) according to the manufacturer’s protocol. PCR was carried out with 1 µl of the reverse transcriptase reaction using AccuPrime GC-rich DNA polymerase (Invitrogen, CA) at 94˚C for 30 sec, 55˚C for 30 sec and 72˚C for 30 sec for 35 cycles and a final extension of 5 min at 72˚C. Oligonucleotide primers used were homologous to exon 1 of mouse GDF-11 (GenBank accession number: NM_010272): 5’-ATGGTGCTCGCG GCCCCGCT-3’ (forward) and 5’-CTGATGTTGGGCG CCTCCTTG-3’ (reverse). The PCR product was cloned into pCR4/TOPO vector (Invitrogen, CA) and its identity was confirmed by sequencing. Radio-labeling of the DNA probe and hybridization were carried out as previously described [12] .
2.4. Quantitative PCR (qPCR)
Total RNA was extracted from skeletal muscles or myoblasts using the Trizol reagent and 5 µg of total RNA was reverse transcribed into cDNA using the Superscript III first strand cDNA synthesis kit (Invitrogen, CA) according to the manufacturer’s instructions. All oligonucleotide primers used in this study span across exon-exon boundaries to avoid the amplification of genomic templates (Table 1). PCR was carried out with 2.5 ml of a 1:20 dilution of the reverse transcriptase reaction using the FastStart DNA Master Plus SYBR Green I reagent on a LightCycler 2.0 PCR machine (Roche Diagnostics). A dilution series of pooled reverse transcriptase reactions was used to create a standard curve. The PCR products were separated in a 1.5% agarose gel stained with SybrSafe (Invitrogen, CA) to confirm their size. Representative PCR products were extracted from the gel and directly sequenced to confirm DNA sequence identity. Data for each sample were normalized to the concentration of cDNA in each RT sample [13] .
2.5. Myoblast Proliferation Assays
Murine myoblasts (C2C12) were seeded onto uncoated 96-well tissue culture plates (Nunc, Denmark) at a cell density of 1000 cells per well in 200 µl of proliferation medium (DMEM supplemented with 10% (v/v) FBS), and incubated for 18 h at 37˚C with 5% CO2. Primary wild-type and myostatin knockout myoblasts were seeded at a cell density of 2000 cells per well in 200 µl of satellite cell medium. Medium was replaced with test medium containing 0, 3, 10, 30 and 100 nM of recombinant GDF-11 (R&D Systems, Inc., MN) in proliferation medium (n = 8). Plates were incubated at 37˚C with 5% CO2 for 48 h, and cells were washed and fixed. The extent of proliferation was measured using a methylene blue photometric end-point assay [14]. Each proliferation assay was repeated at least three times. To investigate the mRNA expression of ActRIIA, ActRIIB, activin-like kinase 4 (Alk4), Alk5 and GDF-11 during myoblast proliferation, myoblasts isolated from wild-type and myostatin knockout mice were seeded into uncoated 6-well tissue culture plates (Nunc, Denmark) at a cell density of 2000 cells per cm2 in 4 ml of satellite cell medium and incubated for 18 h at 37˚C with 5% CO2 (n = 3 per genotype). Medium was replaced with 4 ml of proliferation medium and incubated at 37˚C with 5% CO2 for 48 h, and cells were collected with 1 ml of Trizol reagent (Invitrogen, CA).
2.6. Myoblast Differentiation Assays
To investigate the effect of GDF-11 in myoblast differentiation, the fusion index (the number of myosin heavy chain [MyHC]-positive myotube nuclei expressed as a percentage of the total number of nuclei per field) was determined. To do this, C2C12 myoblasts, or primary myoblasts obtained from wild-type and myostatin knockout mice were seeded at 50,000 cells per well on 13-mm diameter coverslips (Thermanox, Nunc) in proliferation medium for C2C12 or satellite cell medium for primary myoblasts and allowed to attach overnight at 37˚C, 5% CO2. Medium was replaced with differentiation medium (DMEM with 2% [v/v] HS supplemented with 0, 10, 30 and 100 nM recombinant GDF-11 [R&D Systems, Inc., MN; n = 3]), and the plates were incubated at 37˚C, 5% CO2 for 72 h. Coverslips were fixed, blocked and incubated with 1:200 dilution of a monoclonal anti-MyHC (MF-20, DSHB, University of Iowa, Iowa, USA) primary antibody at 4˚C overnight. Coverslips were washed
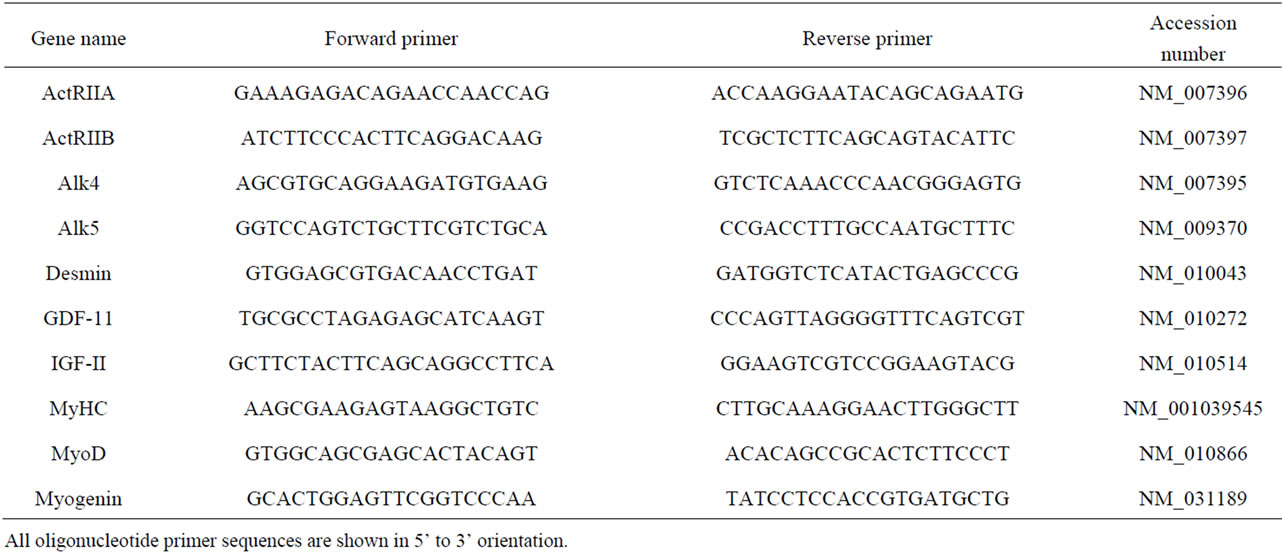
Table 1. Oligonucleotide primers used in qPCR.
and incubated with 1:300 dilution of biotinylated antimouse secondary antibody (RPN1001, GE Healthcare), followed by 1:300 dilution of streptavidin biotinylated horseradish peroxydase complex (RPN1051V, GE Healthcare), and developed with 3,3’-diaminobenzidine tetrachloride (D-5637, Sigma). Coverslips were counterstained with Gill’s haematoxylin, dried and mounted onto glass slides for microscopic analysis. The total number of nuclei and those in MyHC-positive myotubes were counted in three randomly selected fields per coverslip using Image-J software (NIH). The fusion index was determined by averaging nine different counts (a total number of 2500 - 5000 nuclei) for each treatment and the index was re- peated at least three times.
To examine the mRNA expression of ActRIIA, ActRIIB, Alk4, Alk5 and GDF-11 during myoblast differentiation, myoblasts isolated from wild-type and myostatin knockout mice were seeded into uncoated 6-well tissue culture plates (Nunc, Denmark) at a cell density of 25,000 cells per cm2 in 4 ml of satellite cell medium and incubated for 18 h at 37˚C with 5% CO2 (n = 3 per genotype). Medium was replaced with 4 ml of differentiation medium and incubated at 37˚C with 5% CO2 for 72 h, and then collected with 1 ml of Trizol reagent. To determine the effect of recombinant GDF-11 on the mRNA expression of Desmin, IGF-II, MyHC, MyoD and Myogenin during the course of myoblast differentiation, C2C12 myoblasts were seeded into uncoated 6-well tissue culture plates at a cell density of 25,000 cells per cm2 in 4 ml of proliferation medium and incubated for 18 h at 37˚C with 5% CO2. Medium was replaced with 1.5 ml of differentiation medium supplemented with 0 or 300 nM GDF-11 (R & D Systems, Inc., MN; n = 3) and incubated at 37˚C with 5% CO2 for 48 or 72 h, and then cells were collected with 1 ml of Trizol reagent.
2.7. Statistical Analysis
For in vitro studies, data were analyzed by ANOVA with dose of GDF-11 and genotype, and their interaction included as treatment terms. For in vivo studies, data were analyzed by ANOVA with sex, age or genotype and their interaction included as treatment terms. Post hoc analysis among groups was performed using the method of Tukey. Data are presented as means ± standard error of the mean (S.E.M.).
3. RESULTS
3.1. Body and Muscle Masses
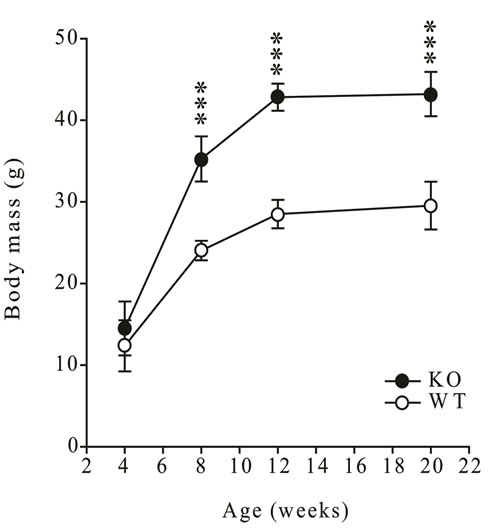
The sexually dimorphic difference in body mass and the mass of the gastrocnemius muscles was evident from six weeks of age (P < 0.001, first animal experiment) during post-natal development, and was published previously [15] . In addition, the body mass and gastrocnemius mass of myostatin knockout male mice were greater than wildtype from eight weeks of age (P < 0.001, second animal experiment, Figure 1).
3.2. GDF-11 Is Transcribed in Skeletal Muscle
Northern blot analysis using an exon 1-specific probe for GDF-11 detected a single, high intensity hybridization signal at 4.4 kb in gastrocnemius muscles of adult male mice (Figure 2, a representative blot for one muscle is shown).
 (a)
(a)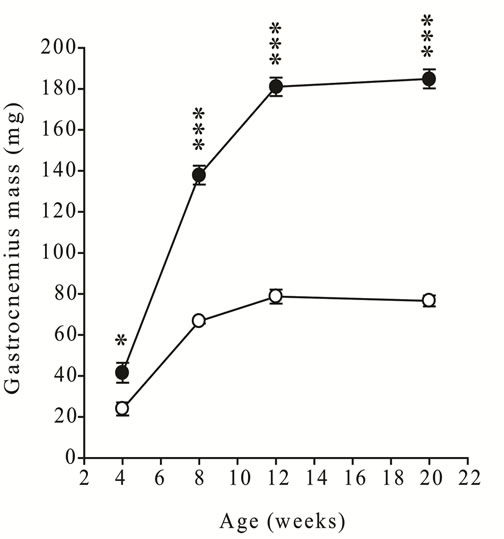 (b)
(b)
Figure 1. (a) Body mass and (b) gastrocnemius muscle mass in male wild-type (C57 strain) and myostatin knockout mice from 4 to 20 weeks of age. Values are mean ± S.E.M. (n = 6 per age and genotype). Asterisks indicate significance between genotypes at each age (*P < 0.05, ***P < 0.001).
3.3. GDF-11 Expression Is Developmentally Regulated in a Sexually Dimorphic Manner
The concentration of GDF-11 mRNA peaked at 3 - 4 weeks of age in gastrocnemius muscles of both sexes and was higher in males than females, then steadily declined to adulthood, while remaining higher in males (P < 0.05, Figure 3).
3.4. GDF-11 Inhibits Myoblast Differentiation
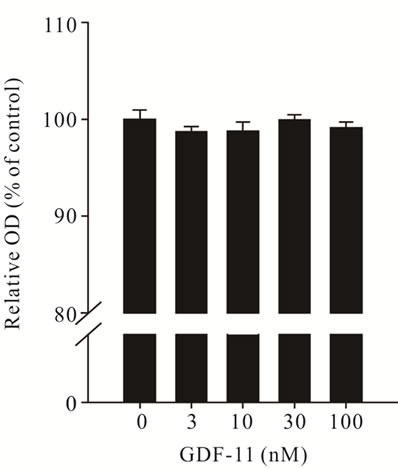
GDF-11 had no effect on proliferation (Figure 4(a)), but
 (a) (b)
(a) (b)
Figure 2. Identification of GDF-11 mRNA in gastrocnemius muscle of an adult male wild-type mice using Northern blot analysis. (a) Total RNA was isolated from gastrocnemius muscle, separated in a formaldehyde gel, blotted to a nylon membrane and hybridized with a radiolabeled DNA probe complementary to the exon 1 sequence of mouse GDF-11. (b) Methylene blue stained nylon membrane shows the loading and integrity of total RNA. Estimated size of GDF- 11 mRNA (4.4 kb), the positions of an RNA molecular weight marker and the location of 18S and 28S ribosomal bands are indicated.
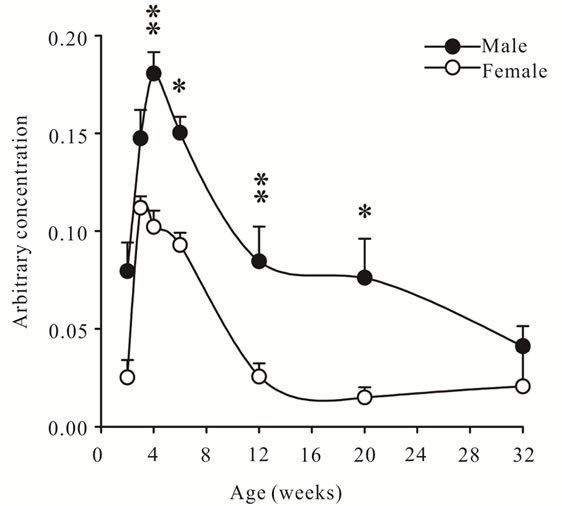
Figure 3. Arbitrary concentrations of GDF-11 mRNA (mean ± S.E.M.) in gastrocnemius muscles of wild- type male and female mice at 2, 3, 4, 6, 12, 20 and 32 weeks of age (n = 6 per age and sex). Asterisks indicate significance (*P < 0.05, **P < 0.01).
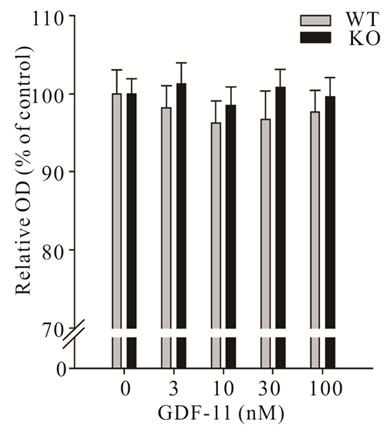
inhibited differentiation of C2C12 myoblasts (P < 0.05, Figure 4(b)). Next, we wanted to determine if GDF11 equally affects primary myoblasts isolated from either wild-type or myostatin knockout mice. Consistent with the action on C2C12 myoblasts, GDF-11 did not affect proliferation of primary muscle cells from either genotype (Figure 5(a)). To check the possibility that proliferating myoblasts (C2C12, primary wild-type and myostatin knockout) might lack the cognate receptors of GDF-11 for signalling, we determined the mRNA concentration of ActRIIA (also known as ActRII), ActRIIB, Alk4 (also known as ActRIB) and Alk5 (also known as
 (a)
(a)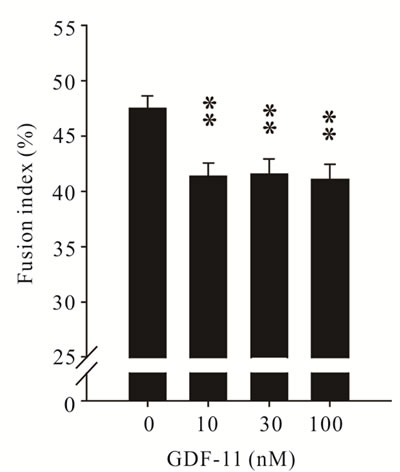 (b)
(b)
Figure 4. Effect of recombinant GDF- 11 (mean ± S.E.M.) on the proliferation and differentiation of C2C12 myoblasts. (a) Myoblasts were treated with increasing concentration of GDF- 11 (n = 8) in proliferation medium for 48 h or (b) The fusion index was measured after 72 h in differentiation medium supplemented with GDF-11 (n = 3). Asterisks indicate significance (**P < 0.01).
TGFβR1) during the course of proliferation in all types of myoblasts using qPCR. All receptors were expressed at comparable concentrations in C2C12 and primary proliferating and differentiated myoblasts (data not shown).
In agreement with our finding with C2C12 myoblasts, GDF-11 inhibited differentiation of both wild-type and myostatin knockout myoblasts in a dose-dependent manner without an effect of genotype (P < 0.05, Figure 5(b)). In addition, GDF-11 decreased the number of MyHC-positive myotubes but did not cause a noticeable change in cell morphology in all differentiated myoblasts (Figure 6).
 (a)
(a) (b)
(b)
Figure 5. Effect of recombinant GDF- 11 (mean ± S.E.M.) on the proliferation and differentiation of myoblasts isolated from wild-type and myostatin knockout mice. a) Myoblasts were treated with increasing concentrations of GDF-11 (n = 8 per dose and genotype) in proliferation medium for 48 h or b) the fusion index was measured after 72 h in differentiation medium supplemented with GDF-11 (n = 3 per dose and genotype). Asterisks indicate significance to controls (**P < 0.01, ***P < 0.001).
3.5. GDF-11 Down-Regulates Early and Late Markers of Myoblast Differentiation
To examine whether decreased differentiation of myoblasts, as a result of GDF-11 treatment, was associated with a decrease in the mRNA expression of early and late markers of differentiation, we determined the concentration of MyoD, myogenin, IGF-II, desmin and MyHC mRNAs using qPCR. The concentration of MyoD, Myogenin and IGF-II mRNA, early markers of myoblast differentiation, decreased in differentiating C2C12 myoblasts treated with GDF-11 compared with that of controls at 48 h, but not at 72 h (P < 0.05, Figure 7). In addition, mRNA expression of desmin and MyHC, late markers of myoblast differentiation, were altered in response to exogenous GDF-11 treatment. Firstly, concentrations of desmin mRNA were decreased at 48 h, and tended to be decreased at 72 h in GDF-11 treated differentiating C2C12 myoblasts compared with controls (P < 0.01 and P = 0.065, respectively, Figure 7). Secondly, the concentration of MyHC mRNA decreased in differentiating C2C12 myoblasts treated with GDF-11 for 72 h (P < 0.05, Figure 7).
3.6. Concentrations of GDF-11 and Receptor mRNAs Are Up-Regulated in Muscles of Myostatin Knockout Mice
The concentration of GDF-11 mRNA was higher in gastrocnemius muscles of myostatin knockout mice than in those from wild-type mice (P < 0.05, Figure 8). This observation prompted us to investigate whether the signalling of GDF-11 was affected by the absence of myostatin at the level of receptors. The concentrations of ActRIIA, ActRIIB and Alk5 mRNA were increased in myostatin knockout compared with wild-type mice, while concentrations of mRNA for Alk4 were not different between the two genotypes (P < 0.05, Figure 8). Despite increased concentrations of GDF-11, ActRIIA, ActRIIB and Alk5 in muscles of myostatin knockout mice, there were no differences in the concentrations of mRNA of these genes and of Alk4 between myoblasts isolated from wild-type and myostatin knockout mice either in proliferation or differentiation (data not shown).
4. DISCUSSION
In this study, we aimed to systematically examine whether GDF-11 has a role in the regulation of myogenesis by measuring its expression in vivo and analysing the effect of exogenous GDF-11 treatment in the proliferation and differentiation of cultured muscle cells in vitro. We show here for the first time that the expression of GDF-11 is developmentally regulated in skeletal muscles in a sexually dimorphic manner. Furthermore, GDF- 11 inhibits differentiation of myoblasts via down-regu-
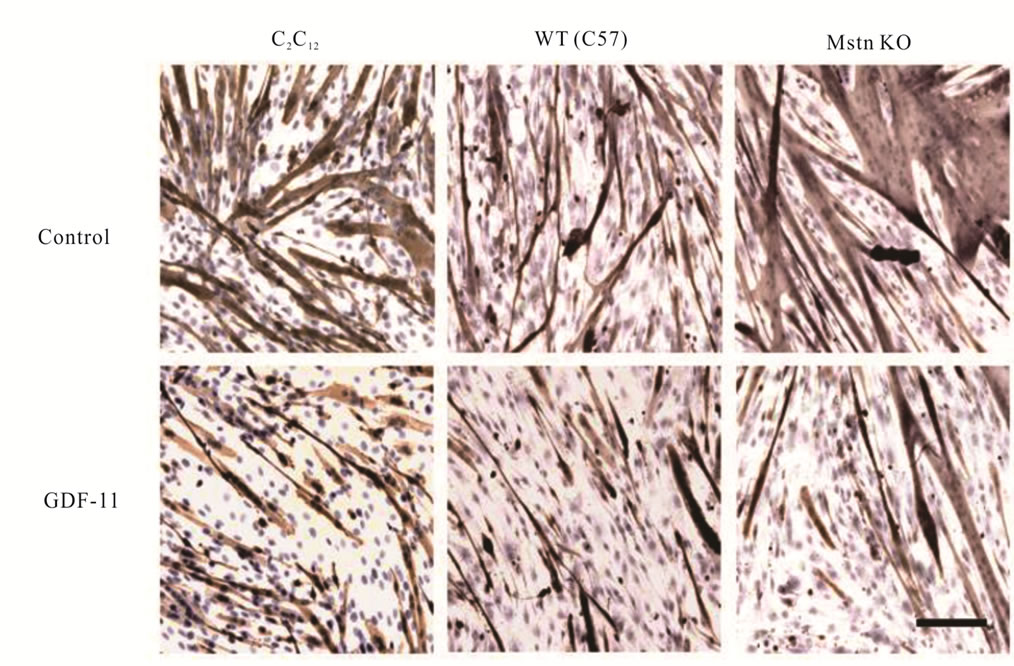
Figure 6. Inhibition of myoblast differentiation by GDF-11. C2C12, and primary myoblasts isolated from wild-type (WT, C57) and myostatin knockout (Mstn KO) mice were cultured in differentiation medium with and without GDF-11 (100 nM) for 72 h. Myoblasts were fixed and immunostained using an anti-MyHC antibody, and counterstained with Gill’s hematoxylin. Bar is 100 μm.
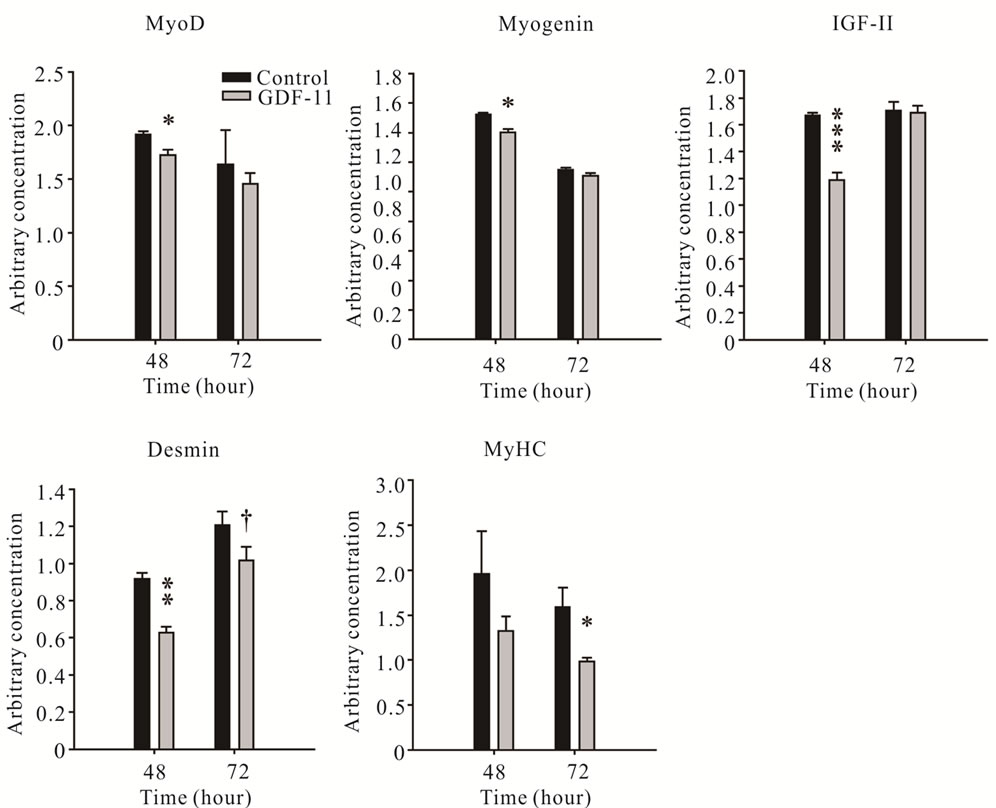
Figure 7. Arbitrary concentrations (mean ± S.E.M.) of MyoD, Myogenin, IGF-II, Desmin and MyHC mRNAs measured using qPCR during differentiation of C2C12 myoblasts treated with GDF-11 or vehicle at 48 h and 72 h (n = 3). Asterisks indicate significance (†P < 0.1, *P < 0.05, **P < 0.01 and ***P < 0.001).
lating muscle-specific genes required for myogenic differentiation. Our data lend support to the notion that GDF-11 does play a role in regulating post-natal development of skeletal muscle.
GDF-11 and myostatin are the most closely related members of the TGF-β superfamily of ligands and receptors suggesting a functional redundancy between these secreted proteins [3] . This redundancy was confirmed by a recent study in which it was shown that the GDF-11/myostatin double knockout mice had more severe homeotic transformations of the axial skeleton than the GDF-11 knockout mice [16]. In that study, deletion of GDF-11 specifically in skeletal muscles in either myostatin knockout or wild-type mice had no effect on muscle mass, fibre number or type [16]. However, the authors acknowledged that the animal model for the muscle-specific deletion of GDF-11 had a number of shortcomings: 1) a possible incomplete deletion of the gene, 2) that the MLC-Cre transgene was switched on just before birth allowing GDF-11 to function normally during the critical, early stages of foetal myogenesis [17] , 3) that GDF-11 was expressed in tissues other than muscle and was acting in an endocrine manner to regulate muscle mass [2,10,16] . Despite those shortcomings, it is hard to dismiss the 20% - 30% increase in muscle mass in myostatin knockout mice after blocking myostatin signallingwhich suggests that a myostatin-like factor exists ADDIN EN.CITE ADDIN EN.CITE.DATA [6] . Whether or not this factor is GDF-11 has remained unclear and cannot be fully resolved in the current study.
We have shown here that there is a temporal pattern of expression of GDF-11 in skeletal muscles and concentrations of mRNA are higher in males than in females. Moreover, the concentrations of GDF-11 mRNA were increased in the gastrocnemius muscles from 2 to 6 weeks, the period of greatest growth velocity, in both sexes. This observation suggests that GDF-11 may have a role in regulating post-natal growth of skeletal muscles. The concentrations of GDF-11 mRNA continuously declined from 4 to 32 weeks of age in both sexes which is in contrast to the expression of myostatin mRNA, which steadily increased in post-natal gastrocnemius muscle and was maintained in adults in both sexes ADDIN EN.CITE ADDIN EN.CITE.DATA [15] . Therefore, the stages at which GDF-11 and myostatin influence muscle growth may differ during post-natal development. The 4.4 kb hybridization signal detected by Northern blot analysis is consistent with the size of GDF-11 mRNA identified in other tissues such as brain, dental pulp and lungs of mice [2]. Furthermore, the expression of GDF-11 in skeletal muscles of mice, as we have shown here, is consistent with the expression in embryonic muscles of Xenopus, chickens and fish [3, 4,18] .
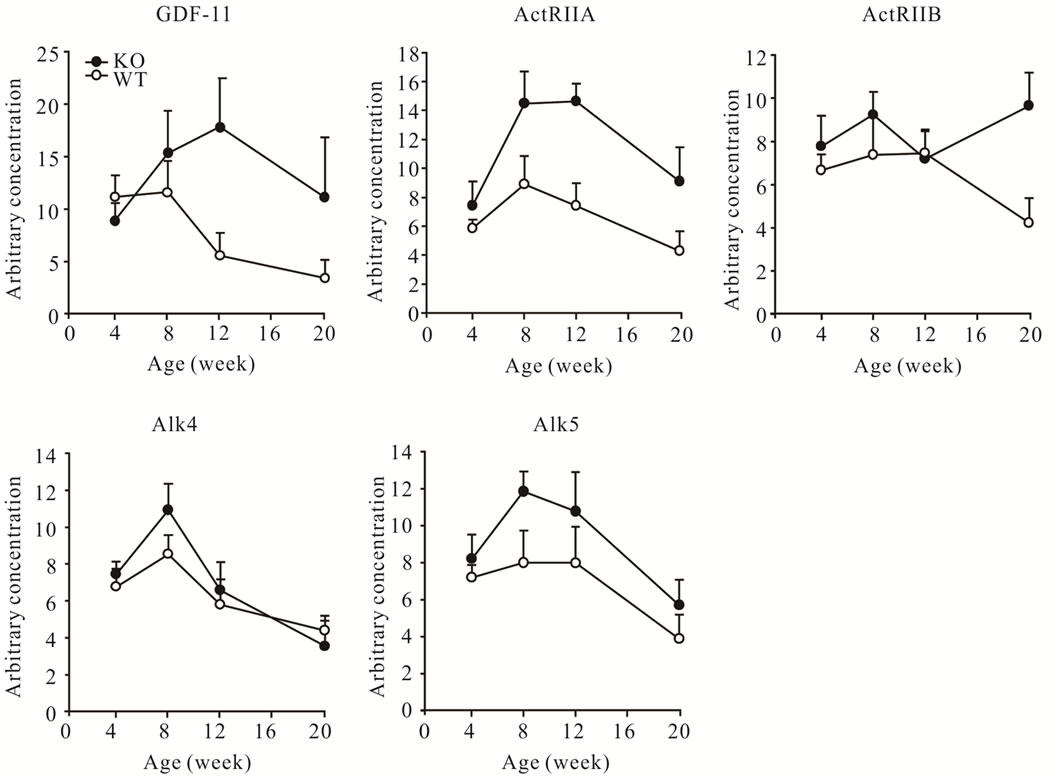
Figure 8. Arbitrary concentrations (mean ± S.E.M.) of GDF-11, ActRIIA, ActRIIB, Alk4 and Alk5 mRNAs in wild-type (WT) and myostatin knockout (KO) male mice at 4, 8, 12, and 20 weeks of age (n = 6 per age and genotype).
The sexually dimorphic difference in GDF-11 expression coincides with the divergent growth between sexes which occurs from about six weeks of age in mice [15]. In comparison, we have shown that there are higher concentrations of mRNA and decreased mature myostatin protein in male than in female muscles and have shown that these differences are developmentally regulated by growth hormone [15,19] . The concentration of GDF-11 mRNA is higher in males than females which would seem to be inconsistent with the greater muscle mass in males than females if we assume that GDF-11 is an inhibitor of muscle development. Perhaps, there is a post-transcriptional regulation of GDF-11, similar to that of myostatin. To resolve this, however, will require antibodies that discriminate GDF-11 and myostatin. To the best of our knowledge, all commercially available antiGDF-11 antibodies cross-react with myostatin. In support, we have previously shown that commercially available and our in-house anti-myostatin antibodies detect a faint band at the expected size of mature myostatin protein (26 kDa) in skeletal muscles of myostatin knockout mice and we speculated that this band is GDF-11 [20] . It has been assumed that the abundance of GDF-11 protein is lower than myostatin in skeletal muscles of adult mice, but it would require an independent investigation to verify this postulate. The possibility that GDF-11 may have a paracrine/endocrine role in the innervation of muscle fibres and/or the development of motoneurons should not be dismissed because sexually dimorphic differences in the number and size of motoneurons have been demonstrated in rodents and GDF-11 has been identified as a regulator of neurogenesis [21- 24] .
We show in the current study that the concentrations of GDF-11 mRNA were increased in the gastrocnemius muscles of myostatin knockout compared with those in wild-type mice. These data may suggest that GDF-11 is induced to compensate for the absence of functional myostatin. However, the greater rate of growth and mass of skeletal muscles in myostatin knockout mice suggests that GDF-11 is unable to act effectively or to fully compensate for the absence of myostatin. Therefore, while redundancy between GDF-11 and myostatin has been suggested, there must be specific functions that cannot be replaced by either factor [16]. To determine if there were changes in the expression of receptors that might explain a difference in function, we measured the expression of receptors known to mediate the signalling of GDF-11 and myostatin. We observed increased concentrations of ActRIIA, ActRIIB and Alk5 mRNA, while Alk4 remained unchanged in muscles of myostatin knockout compared to those in wild-type mice. These data suggest that it is not the insufficient expression of any of the cognate receptors that results in the inability of the increased concentrations of GDF-11 to efficiently compensate for myostatin signalling. However, we must acknowledge that differences between the downstream signalling of myostatin and GDF-11 are likely and are unclear at present. It is also equally likely that other TGF-β ligands such as activin A, BMP-2 or TGF-β1 play a role in regulating skeletal myogenesis as it has been suggested by others [25-27] .
We have demonstrated that recombinant GDF-11 protein had no effect on the proliferation of C2C12 or primary myoblasts isolated from wild-type and myostatin knockout mice. The lack of response to GDF-11 was not due to the lack of expression of cognate receptors in proliferating myoblasts because our qPCR analysis showed that the mRNA was present for these receptors in proliferating and differentiated myoblasts. Our data contrast with those of others who used muscle-derived progenitor cells (MDPCs), a subpopulation of muscle derived stem cells capable of differentiating into functional cardiomyocytes, to show that either knockdown of GDF-11 using siRNA or blockade with follistatin increases the proliferation of MDPCs [28] . The source of the discrepancy is likely to stem from the inherent differences in gene expression and activation of pathways between the immortalized C2C12 cell line, primary myoblasts and the ex vivo maintained MDPCs. In support, myostatin was not detectable in MDPCs but was abundant in myogenic cells (such as C2C12 myoblasts) and skeletal muscle [28,29] . Furthermore, the inhibition of GDF-11 expression in MDPCs increased the concentration of cyclin D1 through the inactivation of Smad2/3 signalling as opposed to a recent study demonstrating that myostatin signalling enhanced cyclin D1 degradation independently from a Smad3-mediated signalling in C2C12 myoblasts [30] . Therefore, it was proposed that GDF-11, which is expressed in MDPCs and skeletal muscle tissue, is a logical candidate for an inhibitor of MDPCs self-replication [28] .
In contrast to the ineffectiveness of GDF-11 on proliferation, we show here that GDF-11 inhibited the differentiation of C2C12 and primary myoblasts isolated from wild-type and myostatin knockout mice, which is consistent with and extends the findings of others [10,31] . We measured a decrease in concentrations of MyoD and IGF-II mRNA in GDF-11 treated C2C12 myoblasts to that of controls at 48 hours, a time at which myoblasts withdraw from the cell cycle and subsequently fuse to form myotubes. Autonomous differentiation of myoblasts is under the mutual positive control of MyoD and IGF-II [32]. Therefore, downregulation of these factors by GDF-11 signalling contributes to the reduced myoblast differentiation. We also observed a decrease in the mRNA expression of myogenin in response to GDF-11 treatment. Myogenin has a critical role in the transactivation of multiple muscle-specific genes that are required for the execution of the muscle differentiation programme [33]. In comparison, myostatin has been reported to inhibit MyoD and myogenin mRNA expression during myoblast differentiation, which further underlines functional redundancy between myostatin and GDF-11 [34,35] . Additional evidence for a role of GDF-11 in inhibiting differentiation is the downregulation of desmin and MyHC mRNAs, molecular markers of fully differentiated muscle cells. Furthermore, treatment with a soluble ActRIIB receptor, which increased the mass of skeletal muscles of myostatin knockout mice, was able to neutralize the inhibitory effect of exogenous GDF-11 in myoblast differentiation [6,10] . In further support, differentiation of rat myoblasts (L6E9), which are deficient in myostatin but not in GDF-11, are enhanced by the delivery of a dominant-negative ActRIIB suggesting that TGF-β ligands such as activins and GDF-11 regulate the size of differentiated myotubes [29]. These observations suggest that GDF-11 inhibits terminal differentiation and fusion of myogenic cells, which are required for the accretion of myonuclei into and subsequent hypertrophy of myofibres during skeletal muscle development.
We also explored whether muscle satellite cell derived proliferating and differentiated myoblasts isolated from myostatin knockout and wild-type mice retain a similar profile of mRNA expression for GDF-11 and receptors found in skeletal muscle tissue. We found no difference in the concentration of GDF-11 or receptor mRNAs between genotypes. The possible explanations for these findings are: 1) the ratio of satellite cell nuclei to total number of nuclei is rapidly decreasing in skeletal muscles after birth and, therefore, the gene expression profile in a given muscle is determined by muscle fibre nuclei not the satellite cell nuclei, 2) satellite cell derived myoblasts removed from their original niche and placed into a high serum medium which contains circulating myostatin would actively respond to the environmental signals and re-adjust their mRNA expression accordingly.
In summary, we have shown that GDF-11 is expressed in skeletal muscles of mice in a sexually dimorphic manner during post-natal growth. The concentration of GDF-11 is developmentally regulated and increases in the absence of myostatin. Furthermore, GDF-11 inhibits the differentiation of muscle cells in culture by the down-regulation of muscle-specific factors required for myogenic differentiation. Therefore, we propose that GDF-11 acts in an autocrine/paracrine and/or endocrine manner to regulate myogenesis in a similar fashion to that of myostatin.
5. ACKNOWLEDGEMENTS
We thank Dr Harold Henderson for his help with statistical analysis. This study was supported by the Foundation of Research Science and Technology New Zealand.
REFERENCES
- McPherron, A.C., Lawler, A.M. and Lee, S.J. (1999) Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nature Genetics, 22, 260-264. doi:10.1038/10320
- Nakashima, M., Toyono, T., Akamine, A. and Joyner, A. (1999) Expression of growth/differentiation factor 11, a new member of the BMP/TGFbeta superfamily during mouse embryogenesis. Mechanisms of Development, 80, 185-189.
- Gamer, L.W., Wolfman, N.M., Celeste, A.J., Hattersley, G., Hewick, R. and Rosen, V. (1999) A novel BMP expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in Xenopus embryos. Developmental Biology, 208, 222-232. doi:10.1006/dbio.1998.9191
- Gamer, L.W., Cox, K.A., Small, C. and Rosen, V. (2001) GDF-11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb. Developmental Biology, 229, 407-420. doi:10.1006/dbio.2000.9981
- Lee, S.J. and McPherron, A.C. (2001) Regulation of myostatin activity and muscle growth. Proceedings of the National Academy of Sciences of the United States of America, 98, 9306-9311. doi:10.1073/pnas.151270098
- Lee, S.J., Reed, L.A., Davies, M.V., Girgenrath, S., Goad, M.E., Tomkinson, K.N., Wright, J.F., Barker, C., Ehrmantraut, G., Holmstrom, J., Trowell, B., Gertz, B., Jiang, M.S., Sebald, S.M., Matzuk, M., Li, E., Liang, L.F., Quattlebaum, E., Stotish, R.L. and Wolfman, N.M. (2005) Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proceedings of the National Academy of Sciences of the United States of America, 102, 18117-18122. doi:10.1073/pnas.0505996102
- Rebbapragada, A., Benchabane, H., Wrana, J.L., Celeste, A.J. and Attisano, L. (2003) Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Molecular and Cellular Biology, 23, 7230-7242. doi:10.1128/MCB.23.20.7230-7242.2003
- Oh, S.P., Yeo, C.Y., Lee, Y., Schrewe, H., Whitman, M. and Li, E. (2002) Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes and Development, 16, 2749-2754. doi:10.1101/gad.1021802
- McPherron, A.C., Lawler, A.M. and Lee, S.J. (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature, 387, 83-90. doi:10.1038/387083a0
- Souza, T.A., Chen, X., Guo, Y., Sava, P., Zhang, J., Hill, J.J., Yaworsky, P.J. and Qiu, Y. (2008) Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Molecular Endocrinology, 22, 2689-2702. doi:10.1210/me.2008-0290
- McCroskery, S., Thomas, M., Maxwell, L., Sharma, M. and Kambadur, R. (2003) Myostatin negatively regulates satellite cell activation and self-renewal. Journal of Cell Biology, 162, 1135-1147. doi:10.1083/jcb.200207056
- Jeanplong, F., Bass, J.J., Smith, H.K., Kirk, S.P., Kambadur, R., Sharma, M. and Oldham, J.M. (2003) Prolonged underfeeding of sheep increases myostatin and myogenic regulatory factor Myf-5 in skeletal muscle while IGF-I and myogenin are repressed. Journal of Endocrinology, 176, 425-437. doi:10.1677/joe.0.1760425
- Lundby, C., Nordsborg, N., Kusuhara, K., Kristensen, K.M., Neufer, P.D. and Pilegaard, H. (2005) Gene expression in human skeletal muscle: Alternative normalization method and effect of repeated biopsies. European Journal of Applied Physiology and Occupational Physiology, 95, 351-360. doi:10.1007/s00421-005-0022-7
- Oliver, M.H., Harrison, N.K., Bishop, J.E., Cole, P.J. and Laurent, G.J. (1989) A rapid and convenient assay for counting cells cultured in microwell plates: Application for assessment of growth factors. Journal of Cell Science, 92, 513-518.
- Oldham, J.M., Osepchook, C.C., Jeanplong, F., Falconer, S.J., Matthews, K.G., Conaglen, J.V., Gerrard, D.F., Smith, H.K., Wilkins, R.J., Bass, J.J. and McMahon, C.D. (2009) The decrease in mature myostatin protein in male skeletal muscle is developmentally regulated by growth hormone. Journal of Physiology, 587, 669-677. doi:10.1113/jphysiol.2008.161521
- McPherron, A.C., Huynh, T.V. and Lee, S.J. (2009) Redundancy of myostatin and growth/differentiation factor 11 function. BMC Developmental Biology, 9, 24. doi:10.1186/1471-213X-9-24
- Rosenthal, N., Kornhauser, J.M., Donoghue, M., Rosen, K.M. and Merlie, J.P. (1989) Myosin light chain enhancer activates muscle-specific, developmentally regulated gene expression in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America, 86, 7780-7784. doi:10.1073/pnas.86.20.7780
- Funkenstein, B. and Olekh, E. (2010) Growth/differentiation factor-11: An evolutionary conserved growth factor in vertebrates. Development Genes and Evolution, 220, 129-137. doi:10.1007/s00427-010-0334-4
- McMahon, C.D., Popovic, L., Jeanplong, F., Oldham, J.M., Kirk, S.P., Osepchook, C.C., Wong, K.W., Sharma, M., Kambadur, R. and Bass, J.J. (2003) Sexual dimorphism is associated with decreased expression of processed myostatin in males. American Journal of Physiology Endocrinology and Metabolism, 284, E377-E381.
- McFarlane, C., Langley, B., Thomas, M., Hennebry, A., Plummer, E., Nicholas, G., McMahon, C., Sharma, M. and Kambadur, R. (2005) Proteolytic processing of myostatin is auto-regulated during myogenesis. Developmental Biology, 283, 58-69. doi:10.1016/j.ydbio.2005.03.039
- Fraley, G.S. and Ulibarri, C. (2001) Sexual dimorphism in the number and size of SNB motoneurons: Delayed development during normal ontogeny. Brain Research Developmental Brain Research, 126, 57-64.
- Wu, H.H., Ivkovic, S., Murray, R.C., Jaramillo, S., Lyons, K.M., Johnson, J.E. and Calof, A.L. (2003) Autoregulation of neurogenesis by GDF11. Neuron, 37, 197-207.
- Liu, J.P. (2006) The function of growth/differentiation factor 11 (Gdf11) in rostrocaudal patterning of the developing spinal cord. Development, 133, 2865-2874. doi:10.1242/dev.02478
- Liu, J.P., Laufer, E. and Jessell, T.M. (2001) Assigning the positional identity of spinal motor neurons: Rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron, 32, 997-1012.
- Link, B.A. and Nishi, R. (1997) Opposing effects of activin A and follistatin on developing skeletal muscle cells. Experimental Cell Research, 233, 350-362. doi:10.1006/excr.1997.3575
- Chalaux, E., Lopez-Rovira, T., Rosa, J.L., Bartrons, R. and Ventura, F. (1998) JunB is involved in the inhibition of myogenic differentiation by bone morphogenetic protein-2. Journal of Biological Chemistry, 273, 537-543. doi:10.1074/jbc.273.1.537
- Heino, J. and Massague, J. (1990) Cell adhesion to collagen and decreased myogenic gene expression implicated in the control of myogenesis by transforming growth factor beta. Journal of Biological Chemistry, 265, 10181-10184.
- Nomura, T., Ueyama, T., Ashihara, E., Tateishi, K., Asada, S., Nakajima, N., Isodono, K., Takahashi, T., Matsubara, H. and Oh, H. (2008) Skeletal muscle-derived progenitors capable of differentiating into cardiomyocytes proliferate through myostatin-independent TGFbeta family signaling. Biochemical and Biophysical Research Communications, 365, 863-869. doi:10.1016/j.bbrc.2007.11.087
- Rossi, S., Stoppani, E., Gobbo, M., Caroli, A. and Fanzani, A. (2010) L6E9 myoblasts are deficient of myostatin and additional TGF-beta members are candidates to developmentally control their fiber formation. Journal of Biomedicine and Biotechnology, 2010, 326909. doi:10.1155/2010/326909
- Yang, W., Zhang, Y., Li, Y., Wu, Z. and Zhu, D. (2007) Myostatin induces cyclin D1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/ AKT/GSK-3 beta pathway and is antagonized by insulinlike growth factor 1. Journal of Biological Chemistry, 282, 3799-3808. doi:10.1074/jbc.M610185200
- Trendelenburg, A.U., Meyer, A., Rohner, D., Boyle, J., Hatakeyama, S. and Glass, D.J. (2009) Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. American Journal of Physiology Cell Physiology, 296, C1258-C1270. doi:10.1152/ajpcell.00105.2009
- Montarras, D., Aurade, F., Johnson, T., J, I.I., Gros, F. and Pinset, C. (1996) Autonomous differentiation in the mouse myogenic cell line, C2, involves a mutual positive control between insulin-like growth factor II and MyoD, operating as early as at the myoblast stage. Journal of Cell Science, 109, 551-560.
- Molkentin, J.D. and Olson, E.N. (1996) Defining the regulatory networks for muscle development. Current Opinion in Genetics and Development, 6, 445-453.
- Joulia, D., Bernardi, H., Garandel, V., Rabenoelina, F., Vernus, B. and Cabello, G. (2003) Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Experimental Cell Research, 286, 263-275.
- Langley, B., Thomas, M., Bishop, A., Sharma, M., Gilmour, S. and Kambadur, R. (2002) Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. Journal of Biological Chemistry, 277, 49831-49840. doi:10.1074/jbc.M204291200
NOTES
*Corresponding author.

