Advances in Biological Chemistry
Vol.4 No.2(2014), Article ID:44410,13 pages DOI:10.4236/abc.2014.42016
A New Kinetic Spectrophotometric Method for Determination of Cefadroxil in Pharmaceutical Formulations Using Lawsonia inermis (Henna) as Natural Reagent
Mohammed Hassan, Fathy A. Abeed, Bassam Saif
Department of Chemistry, Ibb University, Ibb, Yemen
Email: basamnagy@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 13 February 2014; revised 15 March 2014; accepted 22 March 2014
ABSTRACT
A new simple and sensitive kinetic spectrophotometric method has been proposed for the determination of cefadroxil in pure form and pharmaceutical formulations. The proposed method is based on the formation of yellow coloured product (λmax = 410 nm) that resulted after addition of the reagent, which is composed of a mixture of Henna extract, sodium hydroxide and potassium permanganate, to cefadroxil aqueous solution. The experimental conditions have been optimized. Beer’s law is obeyed over the concentrations of 100 - 35 μg/ml, with a linear regression correlation coefficient of 0.996. The proposed method has been successfully applied for the determination of cefadroxil in pharmaceutical dosage forms.
Keywords:Cefadroxil; Henna; Spectrophotometric Analysis; Pharmaceutical Analysis

1. Introduction
Cefadroxil an orally active cephalosporin (Figure 1) [1] , it is antibacterial used in treatment of mild to moderate infections caused due to susceptible microorganism [2] , and to treat bacterial infections of the skin, ear, soft tissues, strep throat and the urinary tract [3] .
Literature survey reveals that cefadroxil in its formulations has been estimated by number of methods such as spetcrophotometric [4] , fluorimetric [5] , polarographic [6] [7] , TLC [8] [9] and HPLC [10] . In the late 1900’s
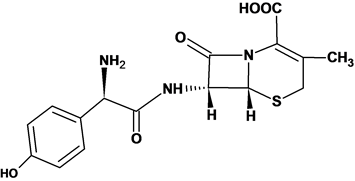
Figure 1. Chemical structure of cefadroxil.
and early 2000’s, a new concept has been introduced to the analytical chemistry technique that is green analytical chemistry by which natural or unrefined reagents are used as replacement for highly refined or purified reagents [11] . Since then, the use of this method has widely spread and has been used for quantitative analysis of many materials [12] -[16] . In analytical chemistry, green chemistry concepts have led to simpler analytical processes that are lower in operational cost and more rapid in order to reduce energy consumption [17] [18] .
In this work we aim to use a mixture of potassium permanganate and Henna extract as a reagent for determination of cefadroxil spectrophotometrically. Henna (Lawsonia inermis Linn) is a plant which grows wildly in abandoned areas. The dye derived from green leaves of henna is used to decorate the body with intricate designs and the principle coloring matter is lawsone, 2-hydroxy-1, 4-naphthoqunone [19] . Lawsone is chiefly responsible for the colorant property of Henna leaves [20] . This molecule has an affinity for bonding with protein and has been used to dye skin, fingernails, hair, leather, silk and wool [21] . This plant has been reported to have the antioxidative and anticarcinogenic effects [22] . No reports in literature have been found for the use of Henna extract as natural reagents in chemical analysis and hence this work might be the first attempt to do so. Various factors related to the proposed method have been tested to select the optimum conditions. The stoichoimetry and kinetics of the reaction have also been investigated.
2. Experimental
2.1. Apparatus
The absorbance measurements were performed on Spectronic 20 Visible spectrometer, Fisher Scientific, U. K. A Lutron digital pH-meter was used for pH adjustment, Jenway U. K.
2.2. Materials and Reagents
Potassium permanganate, sodium hydroxide and SDS were of analytical grade and obtained from Sigma Aldrich, USA. TX-100 GR grade was from Avonchem U. K. Starch Fisher Scientific, U. K, cellulose and arabinose BDH England, Pure Cefadroxil was obtained from Modern Pharma, Sana’a, Yemen. Double distilled water was used throughout all experiments. Solutions of Potassium permanganate (1.54 × 10−3 mol/dm−3) and sodium hydroxide (0.175 mol/dm−3) were always prepared freshly before the experiments. A stock solution of Cefadroxil (1000 μg/ml) was prepared by dissolving the required amount of Cefadroxil in double distilled water and shaking the solution until the complete dissolution. This solution was always kept refrigerated and used within 72 hours.
2.3. Preparation of Reagent
Fresh leaves of Lawsonia inermis L. (Henna) were collected from the garden of faculty of agriculture, Ibb University, Yemen. The leaves were washed with distilled water then sun dried and ground into powder using pestle and mortar. 0.032 g of the powdered leaves were mixed with 100 ml double distilled water in a flat bottom flask and boiled for 5 minutes. It was allowed to cool and then filtered using a Whatman filter paper no.1 [23] . The filtrate solution was used after diluting the volume up to 100 ml by double distilled water. The reagent solution was prepared by mixing proper volumes of Henna, sodium hydroxide and potassium permanganate stock solutions. The mixture was of green colour and used after standing time that is estimated experimentally.
2.4. Absorption Spectra
Figure 2 shows the spectra of both reagent solution and the reaction product. The reaction product shows a broad absorption band with λmax at 410 nm. The reagent solution showed no bands at this range of wavelength. Indicating that the band of the reaction product is distinguished. The absorbance at 410 nm was found to be increased with increasing drug concentration indicating that concentration of the drug can be followed spectrochemically at this wavelength.
2.5. General Procedure
Several volumes ranging from (0.35 - 1 ml) of cefadroxil stock solution (1000 µgmil−1), previously heated at 80˚C for five minutes and quenched to room temperature, were transferred to a series of 10 ml volumetric flask. Then 0.6 ml of the reagent solution was added to each flask with gently shaking and completed to volume with double distilled water. The absorbance of the resulting solution was measured at 410 nm after 10 min at 25˚C ± 1˚C against reagent blank. The variables affecting the reaction were carefully investigated and the conditions were optimized.
2.6. Mechanism of the Reaction
The possible mechanism of the reaction is depicted in Figure 3. As reported in literature, the main effective chromogen in Henna is lawsone, which belongs to a group of compounds called hydroxyquinones. These compounds undergo substitution in C3 to produce variety of derivatives under specific conditions [24] . Lawsone, 2-hydroxy-1, 4-napthoquinone, is oxidized by potassium permanganate in alkaline medium to produce product A and mangante. Mangante ion reacts further with cefadroxil to produce product B, probably after the pH of the medium turned to neutral after addition of cefadroxil to the reagent solution. The product A reacts with product B in the final step to form the final yellow product (product C) that absorbs at 410 nm. This mechanism was assured by estimating the stoichiometry between cefadroxil and potassium permanganate using Job’s method of continuous variation [25] . Figure 4 shows the relation between absorbance and mole fraction of cefadroxil with maximum absorbance occurs close to 0.6 mole fraction of cefadroxil, which indicates that the stoichiometry between cefadroxil and potassium permanganate is 1:2 in agreeing with that expected from the proposed mechanism.
3. Results and Discussion
3.1. The Optimum Conditions of the Reaction
The optimum conditions of the reaction were obtained by recording the absorbance as a function of one of factors that control the reaction and keeping other factors fixed. The value of factor that gives maximum and stable absorbance is considered to be the optimum. Figures 5-7 show the variation of absorbance with concentrations of KMnO4, NaOH and Henna respectively. The optimum values of these factors have been obtained from the corresponding figure and presented in Table 1. The first standing time (the time required for attaining constant

Figure 2. Absorption Spectra (−) reaction product; (.) reagent solution (1.54 × 10−3 mol/dm−3 potassium permanganate, 0.175 mol/dm−3 sodium hydroxide and 0.032 g/100 ml of Henna).

Figure 3. The proposed mechanism of the reaction.
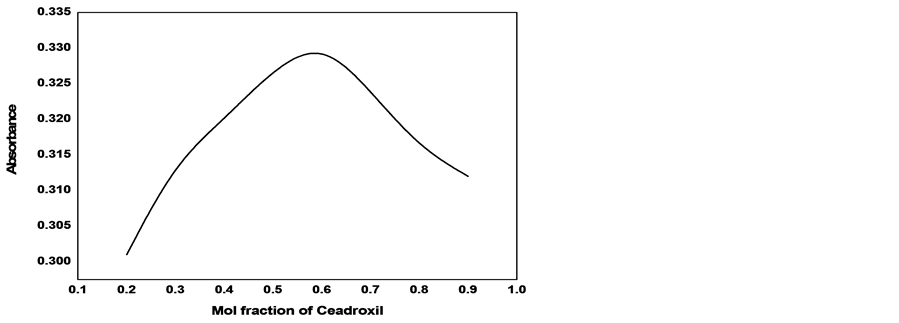
Figure 4. Continuous variations graph for the reaction between cefadroxil and KMnO4.

Figure 5. Effect of volume of KMnO4 on the absorbance of cefadroxil at 410 nm.
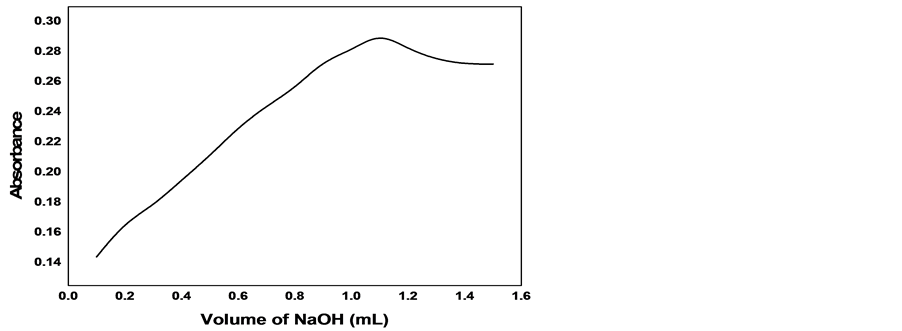
Figure 6. Effect of volume of NaOH on the absorbance of cefadroxil at 410 nm.
Figure 7. Effect of volume of Henna on the absorbance of cefadroxil at 410 nm.
Table 1. The optimum conditions for determination of cefadroxil.
absorbance after mixing the components of the reagent) and second standing time (the time required for attaining constant absorbance after mixing the reagent and cefadroxil solutions, were estimated from Figure 8, where absorbance has been plotted against time. The estimated first and second standing times were 6 and 10 min respectively. In Figure 9 the absorbance was plotted against the volume of the reagent to estimate the optimum volume of the reagent (mixture of KMnO4, NaOH and henna). The value of 0.6 ml was found to be the optimum volume of the reagent. The various optimised values for the reaction are presented in Table 1.
3.2. Calibration Curve
Aliquot volumes of standard stock solutions, containing 350 - 1000 μg/ml of cefadroxil, were transferred to 10 ml calibrated flasks. 0.6 ml of reagent solution was added to each flask with gently shaking and completed to volume with double distilled water. The absorbance of yellow coloured chromogen was measured at 410 nm after 10 min at 25˚C ± 1˚C against the reagent blank. This process has been repeated three times for each solution. The calibration curve was constructed by plotting the mean absorbance for each solution against its concentration and the regression equation was calculated and presented in Figure 10.
3.3. Interference Study
The interference from other possible excipients and diluents was tested by carrying out the test for a solution of 100 µg/ml of pure cefadroxil in presence of some of these materials (starch, cellulose and L-arabinose) in a ratio of 10:1 relative to cefadroxil. The resultant recoveries were presented in Table 2 that shows that the error from interference remains in the minimum and this method can be applied for determination of cefadroxil in its formulations.
3.4. Beer-Lambert’s Law
Figure 10 shows the calibration curve, which resulted from the application of this method in concentrations range 35 - 100 µg/ml (Table 3). The curve shows that Beer-Lambert’s law is obeyed in cefadroxil concentrations range of 40 - 100 µg/ml with correlation coefficient 0.994.

Figure 8. Plots of Absorbance vs. time of (a) after addition of cefadroxil (100 μgml−1) to the reagent; (b) before addition of cefadroxil to the reagent at 410 nm.

Figure 9. Effect of volume of reagent on the absorbance at 410 nm.
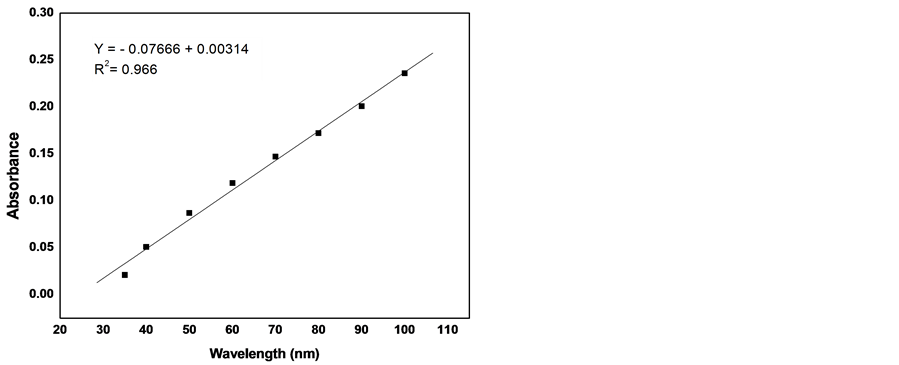
Figure 10. Calibration curve of cefadroxil at 410 nm using the proposed method.
Table 2. Interference study.
Table 3. Calibration curve of cefadroxil at 410 nm.
3.5. Pharmaceuticals Formulation
The following available commercial preparations were analyzed:
1) Cefadroxil capsules (CEFAROL, MODERN PHARM FOR GLOBAL PHARMA CO. REPUBLIC OF YEMEN), labelled to contain 500 mg per capsule.
2) Cefadroxil capsules (IBIDROXIL, GLAXOSMITHKLINE S. A. E. EL SALAM CITY, CAIRO, A.R.E.E), labelled to contain 500 mg per capsule.
3) Cefadroxil capsules (cefadroxil, MIDICO LABS. HOMS-SYRIA), labelled to contain 500 mg per capsule.
3.6. Sample Solutions
The pharmaceutical formulations of cefadroxil in the form of capsule were procured from local market. Three capsules were weighed, finely powdered and mixed thoroughly. The average weight for capsule was determined. An accurately weighed amount of the powder obtained from capsules equivalent to 0.1 g of pure drug was dissolved in 100 ml double distilled water. The contents of the flask were swirled, sonicated for 5 min, and then completed to 100 ml volume with double distilled water. The contents were mixed well and filtered. This prepared solution was diluted quantitatively with double distilled water to obtain a suitable sample solution of 100 μg/ml for the analysis. About 1 ml of cefadroxil (35 - 100 µgml−1) was transferred into 10 ml calibrated volumetric flask subsequently.
3.7. Kinetic Studies
The kinetics of the reaction has been investigated using pseudo-first order method . The concentration of the cefadroxil was assumed to be in excess over that of the reagent and its concentration can be considered unaltered during the reaction. Figure 11 shows the plot between ln(A-A∞) and time at different concentrations of cefadroxil and fixing other conditions. The curves were successfully fitted to straight lines (r ˃ 0.99) that indicates the accurate applicability of pseudo-first order method for this reaction. The same procedure has been repeated at different concentrations of Henna, sodium hydroxide and potassium permanganate. The observed rate constants, kobs, were calculated from slopes of such lines in each experimental run and presented in Table 4. The relation between kobs value and cefadroxil concentration was plotted in Figure 12, which shows a trend of straight line with intercept close to zero confirming the first order kinetics with respect to cefadroxil . Increasing the concentration of Henna solution also resulted in increase of kobs linearly as shown in Figure 13 with intercept close to zero that confirms that the order of the reaction with respect to Henna is unity. The observed rate constant was found to increase with increasing the concentration of KMnO4 (Figure 14). Variation of NaOH concentration does not result in an appreciable change of kobs value, which can be explained by that the concentration of NaOH is relatively so large that any minimal change is not effective, or that NaOH does not participate in the rate determining step of the reaction leading to zero order with respect to NaOH .
The effect of temperature has been investigated by performing experimental runs at various temperatures in temperature range 290 - 320 K. Figure 15 shows the graphitic representation of lnkobs versus temperature inverse, which has been fitted to straight line satisfactorily. The activation parameters Ea, ∆H#, ∆S# and ∆G# have been calculated from the slope of the straight line and found to be 0.401 kJmol−1, 2.47 kJmol−1, −28.38 Jmol−1K−1 and 10.93 kJmol−1 respectively. The negative value of entropy indicates that the structure of the activated complex is more ordered those of the reactants . The value of activation Gibbs energy is positive that indicates that the formation of the activated complex is not spontaneous which results in slowing down the reaction.

Figure 11. Pseudo first-order plots at following conditions: [cefadroxil] = 2.75 × 10−4 moldm−3, [KMnO4] = 1.54 × 10−3 moldm−3, [NaOH] = 0.175 moldm−3, [Henna] = 0.032 g/100ml.
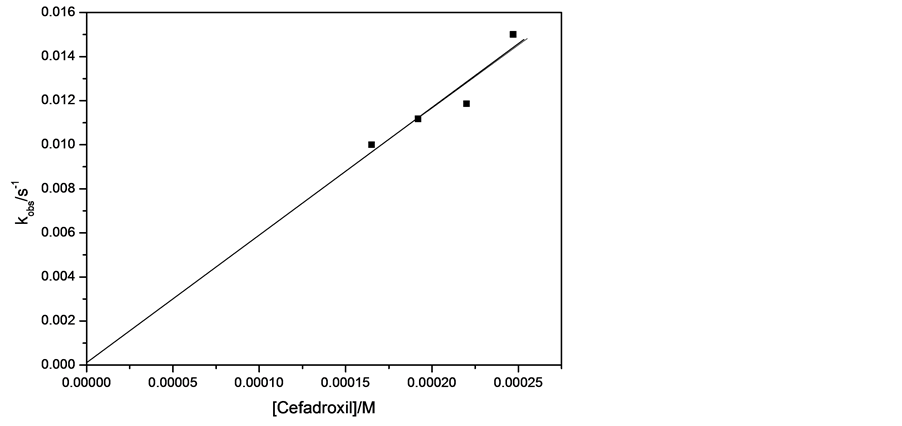
Figure 12. kobs vs. concentration of cefadroxil at 297 K and the following conditions: [KMnO4] = 1.54 × 10−3 moldm−3, [NaOH] = 0.175 moldm−3, [Henna] = 0.032 g/100ml.
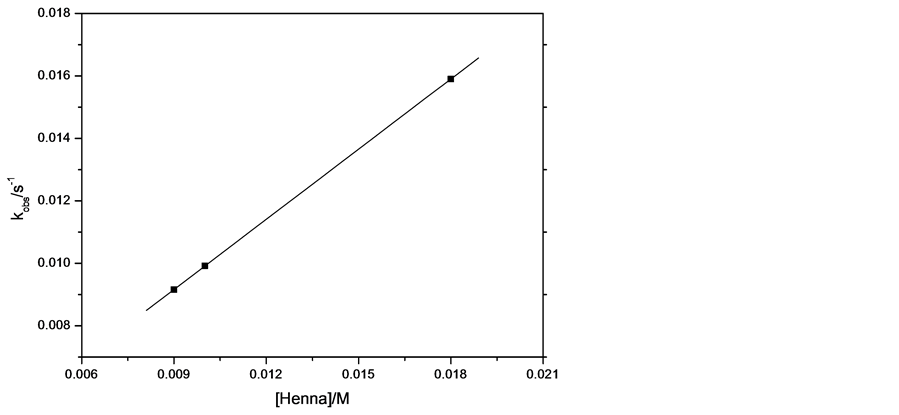
Figure 13. kobs vs. concentration of Henna at 297 K and the following conditions: [cefadroxil] = 2.75 × 10−4 moldm−3, [KMnO4] = 1.54 × 10−3 moldm−3, [NaOH] = 0.175 moldm−3.
3.8. Recovery Studies
The accuracy of the proposed methods was checked by recovery studies with the addition of standard drug solution to pre-analyzed sample solution at two different concentration levels (80 and 40) within the range of linearity for the drugs. The recovery from these two pure samples was around 99%, which is a reasonable value when compared with other spectrometric methods that has been suggested for determination of this drug .
3.9. Precision and Accuracy
The accuracy parameter of this method such as regression coefficient of standard curve, standard deviation, LOD and LQD shows in Table 5. These values were compared with those found in other suggested spectrometric methods in Table 6. The comparison shows that the proposed method is accurate in the range 35 - 100 µgl−1
Table 4. kobs values at various concentrations of Cefadroxil, KMnO4, Henna and NaOH.
Table 5. Results of optimization, precision and accuracy.
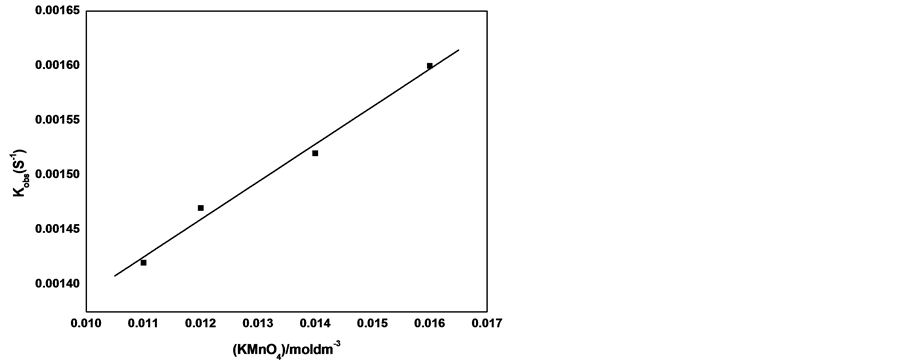
Figure 14. kobs vs. concentration of KMnO4 at 297 K and the following conditions [cefadroxil] = 2.75 × 10−4 moldm−3, [NaOH] = 0.175 moldm−3, [Henna]= 0.032 g/100ml.
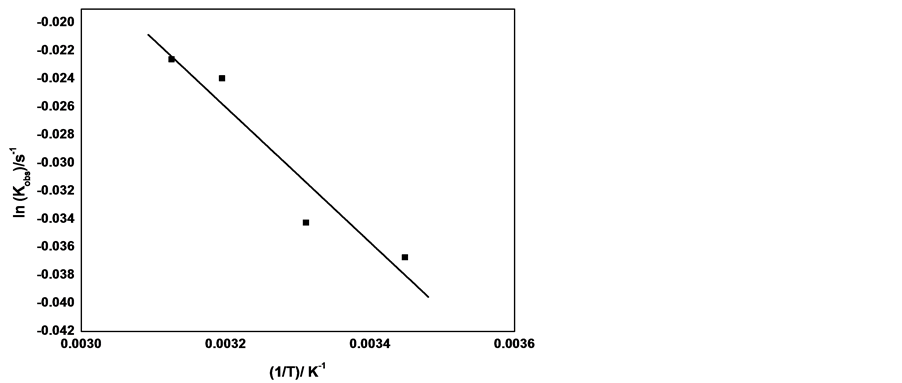
Figure 15. Plots of lnkobs vs. temperature inverse at following conditions: [cefadroxil] = 2.75 × 10−4 moldm−3, [KMnO4] = 1.54 × 10−3 moldm-3, [NaOH] = 0.175 moldm−3, [Henna]= 0.032 g/100ml.
Table 6. Comparing of some spectrophotometric methods used in analysis of cefadroxil.
Table 7. Analysis of cefadroxil from pharmaceutical preparations.
but the sensitivity is less than those of other methods. However, the involvement of natural product in this method is still an advantage.
3.10. Analysis of Pharmaceutical Preparations
Table 7 shows the results of analysis of some pharmaceutical preparations collected from Yemeni markets. The reproducibility of the method was found to be acceptable as can be concluded from the reasonable range of relative standard deviation (%RSD).
4. Conclusion
The proposed method is simple, accurate, low cost, and precise. Therefore, it can be used for the routine analysis of cefadroxil in pure form and pharmaceutical formulations.
Acknowledgements
The authors are thankful to Ibb University, Ibb, Yemen for providing the chemicals and instruments for this study. We are grateful to the Modern Pharma, Sana’a, Yemen for providing the pure cefadroxil antibiotic.
References
- Buck, R.E. and Price, K.E. (1977) Cefadroxil, a New Broad-Spectrum Cephalosporin. Antimicrobial Agents and Chemotherapy, 11, 324-330. http://dx.doi.org/10.1128/AAC.11.2.324
- Sweetman, S.C. (1999) Martindale: The Complete Drug Reference. Pharmaceutical Press, London, 165-166.
- Keynolds, J.E.F. (1999) Matrindale. The Extra Pharmacopoeia. 32nd Edition, The Pharmaceutical Press, London, 163.
- Sastry, C.S., Rao, K.R. and Prasad, D.S. (1997) Spectrophotometric Determination of Cephradine and Cefadroxil Using 2,3-Dichloro-5,6-Dicyano-1,4-Benzoquinonereagent. Acta, 126, 167-172.
- Yang, J.H., Zhou, G.J., Tie, N.Q., Han, R.J., Lin, C.G. and Hu, J.T. (1996) Simultaneous Determination of Cephalexin and Cephadroxil by Using the Coupling Technique of Synchronous Fluorimetry and H-Point Standard Additions Method. Analytica Chimica Acta, 325, 195-200. http://dx.doi.org/10.1016/0003-2670(96)00032-3
- AbdelGaber, A.A., Ghandour, M.A. and Al-Said, H.S. (2003) Polarographic Studies of Some Metal(II) Complexes with Cephalosporins Selected from the First Generation. Analytical Letters, 36, 1245-1260. http://dx.doi.org/10.1081/AL-120020156
- Ivaska, A. and Nordstrom, F. (1983) Determination of Some Cephalosporins by Differential Pulse Polarography and Linear Scan Voltammetry. Analytica Chimica Acta, 146, 87-95. http://dx.doi.org/10.1016/S0003-2670(00)80595-4
- Fabre, H., Blanchin, M.D., Lerner, D. and Mandrou, B. (1985) Determination of Cephalosporins Utilising Thin-Layer Chromatography with Fluorescamine Detection. Analyst, 110, 775. http://dx.doi.org/10.1039/an9851000775
- Shabadi, C.V., Shelar, B.A. and Shelab, A.R. (1998) Simultaneous Determination of Cephalexin Acid Cefadoxil in Pharmaceutical Preparation by Quantitative Thin Layer Chromatography. Indian Drugs, 35, 766-770.
- Patel, Y.P., Dhorda, U.J., Sundaresan, M. and Bhagwat, A.M. (1997) Isocratic, Simultaneous HPLC Determination of Five Antibiotics by Ion-Pair Chromatography. Indian Drugs, 34, 43-47.
- Anastas, P.T. (1999) Green Chemistry and the Role of Analytical Methodology Development. Critical Reviews in Analytical Chemistry, 29, 167-175. http://dx.doi.org/10.1080/10408349891199356
- Tontrong, S., Khonyoung, S. and Jakmunee, J. (2012) Flow Injection Spectrophotometry Using Natural Reagent from Morinda citrifolia Root for Determination of Aluminium in Tea. Food Chemistry, 123, 624-629. http://dx.doi.org/10.1016/j.foodchem.2011.10.100
- Grudpan, K., Hartwell, K.S., Wongwilai, H.W. and Grudpan, S. (2011) Exploiting Green Analytical Procedures for Acidity and Iron Assays Employing Flow Analysis with Simple Natural Reagent Extracts. Talanta, 84, 1396-1400. http://dx.doi.org/10.1016/j.talanta.2011.03.090
- Grudpan, K., Kradtap, S., Lapanantnoppakhun, S. and McKelvie, I. (2010) The Case for the Use of Unrefined Natural Reagents in Analytical Chemistry-A Green Chemical Perspective. Analytical Methods, 2, 1651-1661. http://dx.doi.org/10.1039/c0ay00253d
- Hartwell, K.S. (2012) Exploring the Potential for Using Inexpensive Natural Reagents Extracted from Plants to Teach Chemical Analysis. Chemistry Education Research and Practice, 13, 135-146.
- Anastas, P.T. and Warner, J.C. (1998) Green Chemistry: Theory and Practice. Oxford University Press, New York, 29-56.
- Wang, J. (2002) Real-Time Electrochemical Monitoring: Toward Green Analytical Chemistry. Accounts of Chemical Research, 35, 811-816. http://dx.doi.org/10.1021/ar010066e
- Armenta, S., Garrigues, S. and de la Guardia, M. (2008) Green Analytical Chemistry. TrAC Trends in Analytical Chemistry, 27, 497-511. http://dx.doi.org/10.1016/j.trac.2008.05.003
- Prosen, H., Antonic, J. and Klobcar, A. (2005) Determination of Some Organochlorine Compounds in Herbal Colouring Agent Henna (Lawsonia inermis) and in Tea (Thea sinensis). Archives of Industrial Hygiene and Toxicology, 56, 1-7.
- Chadwick, A.F. and Craker, L.E. (1984) The Scientific Literature on Selected Herbs, and Aromatic and Medicinal Plants of the Temperate Zone, Herbs: An Indexed Bibliography; 1971-1980. Archon Books, 770.
- Singh, Y.V., Kumar, S. and Singh, M. (2005) Agro-History, Uses, Ecology and Distribution of Henna (Lawsonia inermis L. syn. Alba Lam). Henna Cultivation, Improvement and Trade. Central and Arid Zone Research Institute, Jodhpur, 11-12.
- Endrini, S., Rahmat, A., Ismail, P. and Yun, H.T. (2000) Anticarcinogenic Properties and Antioxidant Activity of Henna (Lawsonia inermis). Journal of Medical Sciences, 2, 194-197.
- Mittal, G.C. and Aguwa, C.N. (1983) Abortifacient Effects of the Roots of Momodica Charantia Angustisepala. Journal of Ethnopharm, 7, 169-173. http://dx.doi.org/10.1016/0378-8741(83)90018-1
- Spyroudis, S. (2000) Hydroxyquinones: Synthesis and Reactivity. Molecules, 5, 1291-1330.
- David, H. (2000) Modern Analytical Chemistry. McGraw-Hill, New York, 404.
- Laidler, K.J. (1987) Chemical Kinetics. 3rd Edition, Pearson Education, Singapore City, 197.
- Adari, K.K., Nowduri, A. and Parvataneni, V. (2008) Kinetics and Mechanism of Oxidation of L-Cystine by Cerium (IV) in Sulphuric Acid Medium. Acta Chimica Slovenica, 55, 425-429.
- Dhillon, S. and Gill, K. (2006) Basic Pharmacokinetics. Clinical Phrmokinetics, Pharmaceutical Press, London.
- Patwari, S.B., Khansole, S.V. and Vibhute, Y.B. (2000) Kinetics and Mechanism of Oxidation of Aniline and Substituted Anilines by Isoquinolinium Bromochromate in Aqueous Acetic Acid. Journal of the Iranian Chemical Society, 6, 399-404.
- Patel, S. and Patel, N.J. (2001) Visible Spectrophotometric Method for Estimation of Cefadroxil in Tablet Dosage Form. International Research Journal of Pharmacy, 2, 73-76.
- El-Shaboury, S.R., Mohamed, F.A., Saleh, G.A. and Rageh, A.H. (2010) Kinetic Spectrophotometric Determination of Certain Cephalosporins Using Iodate/Iodide Mixture. Natural Science, 2, 432-443. http://dx.doi.org/10.4236/ns.2010.25053
- Rageh, A.H., El-Shaboury, S.R., Saleh, A.G. and Mohamed, F.A. (2010) Pectophotometric Method for Determination of Certain Cephalosporins Using 4-Chloro-7-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl). Natural Science, 2, 828-840. http://dx.doi.org/10.4236/ns.2010.28104
- Espinosa-Manisilla, A., Acedo-Valenzuela, I.M., Salinas F. and Canada, F. (1998) Kinetic Determination of Ansamicins in Pharmaceutical Formulations and Human Urine. Manual and Semiautomatic (Stopped-Flow) Procedures. Analytica Chimica Acta, 376, 365-375. http://dx.doi.org/10.1016/S0003-2670(98)00537-6


