Advances in Biological Chemistry
Vol.3 No.3A(2013), Article ID:33594,14 pages DOI:10.4236/abc.2013.33A004
Phosphoinositide and phospholipid phosphorylation and hydrolysis pathways. Organophosphate and organochlorine pesticides effects
![]()
1Escuela de Ciencia y Tecnología. Universidad Nacional de San Martín, San Martín, Buenos Aires, Argentina
2Instituto Multidisciplinario de Investigación y Desarrollo de la Patagonia Norte (IDEPA), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional del Comahue, Neuquén, Argentina
3Facultad de Ciencias Médicas, Universidad Nacional del Comahue, Cipolletti, Río Negro, Argentina
Email: tfonovic@unsam.edu.ar
Copyright © 2013 Teresa Fonovich, Gladis Magnarelli. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 25 April 2013; revised 30 May 2013; accepted 10 June 2013
Keywords: Phosphatidylinositol-4,5-biphosphate; Phosphoinositide; Phospholipid; Phosphatidylcholine; Organophosphate Pesticides; Organochlorine Pesticides
ABSTRACT
Phospholipid and phosphoinositide phosphorylation pathways have been shown to be of crucial importance on producing lipid mediators. The earlier findings reported on lipid molecules playing roles in different metabolic pathways used to assign them the exclusive role of second messenger generators. Several researchers have recently described how direct interaction of phospholipids and phosphoinositides with molecules or organelles, without the need for producing second messenger molecules, is responsible for their mechanism of action. Organophosphate and organochlorine pesticide toxicity mechanisms have been extensively studied in relation to their well known effects on cholinesterase activities and on the alterations of electric activity in the nervous system of different organisms respectively. There is little but consistent evidence that some compounds, including in both groups of pesticides, are also able to interact with phospholipid and phosphoinositide phosphorylation pathways in several organisms and tissues. The present review consists of an actualization of basic research on phospholipid and phosphoinositide phosphorylation and hydrolysis pathways, as well as a description of some reported evidences for the effects of the above mentioned pesticides on them.
1. INTRODUCTION
Phosphoinositides are the phosphorylated derivatives of phosphatidylinositol (PtdIns). They consist of negatively charged molecules whose amounts present in eukaryotic cells represent less than 10% of the total phospholipids. Phosphhoinositides include phosphatidylinositol-3-phosphate (PtdIns-3-P), phosphatidylinositol-4-phosphate (PtdIns-4-P), phosphatidylinositol-5-phosphate (PtdIns-5-P), phosphatidylinositol-3,5-bisphosphate (PtdIns-3,5-P2), phosphatidylinositol-4,5-bisphosphate (PtdIns-4,5-P2), phosphatidylinositol-3,4-bisphosphate (PtdIns-3,4-P2), and phosphatidylinositol-3,4,5-trisphosphate(PtdIns-3,4,5- P3). These molecules are involved in different cellular processes as signal transduction (which was the one discovered in the first place), intracellular membrane trafficking, exoand endo-cytosis, nuclear events, cytoskeleton remodeling, cell growth, cell polarity, etc. [1,2]. Major similarities between mammalian, yeast and higher plant phosphoinositide signaling reveal the early appearance and evolutionary conservation of this language. However, there have also been found major differences that suggest that organisms may have evolved different PtdIns-4-P and PtdIns-4,5-P2 functions [3]. The aim of the present review is to report an actualization of both the basic research on phospholipid and phosphoinositide phosphorylation and hydrolysis pathways and some effects of organophosphate and organochlorine pesticides that have been described on these pathways.
2. PHOSPHOLIPIDS AND PHOSPHOINOSITIDES
2.1. Pathways Involving Phosphorylation and Hydrolisis
The enzymes responsible for the phosphorylation and dephosphorylation of phosphoinositides are different kinases and phosphatases. PtdIns-5-kinase and PtdIns-4- kinase catalize the phosphorylation of PtdIns-4-P and PtdIns-5-P thus producing PtdIns-4,5-P2. On the other hand, phospholipase C (PLC) is the phosphatase which substrate is PtdIns-4,5-P2 and produces, as the conesquence of an appropriate stimulus, the second messenger molecules: diacylglycerol (DAG) and Inositol-1,4,5-trisphosphate (InsP3). A protein family of phosphoinositide-3-kinases is responsible for the production of PtdIns- 3-P, PtdIns-3,4-P2, and PtdIns-3,4,5-P3 [4]. All of the 3-phosphorylated phosphoinositides act as intracellular signaling molecules [5].
The levels of PtdIns-3-P, PtdIns-4-P, PtdIns-3,5-P2 and PtdIns-4,5-P2 are relatively stable at specific membranes where they are distributed under normal cell growth conditions. These phosphoinositides are concentrated in different cell membranes. PtdIns-3-P and PtdIns- 4-P are concentrated at endosomes and Golgi membranes respectively, while PtdIns-3,5-P2 concentrates at late endocytic compartments and PtdIns-4,5-P2 at the plasma membrane. Effector proteins that bind phosphoinositides govern the selective distribution of these phospholipids in different membranes. On the other hand, PtdIns- 3,4-P2, and PtdIns-3,4,5-P3 concentration is more unstable, they are synthesized in response to extracellular stimuli and are involved in cell proliferation and survival [6]. Shenker et al. [7] have previously reported these kinds of responses for PtdIns-3,4,5-P3 and proposed that this phosphoinositide may represent a potent target for developing therapeutic approaches to down regulate mast cell function and, in turn, reduce the severity of mast cell dependent disease.
Cell migration is a dynamic and highly orchestrated cellular process, which is involved in a variety of morphogenic events during organ and tissue development. Studies on disruption of Golgi were accompanied by the inhibition of cell migration and allowed researchers to conclude that Golgi has an active role in cell migration, in association with the centrosome, when its structural integrity is intact. There is increasing evidence for the role of signal transduction in regulating the Golgi during cell migration. GOLPH3 is a Golgi protein that binds PtdIns-4-P and depends on this phosphoinositide for its Golgi localization. Dippold et al. [8] have recently demonstrated that GOLPH3 binds myosin (thus connecting Golgi to F-actin), as well as the need of this linkage for the normal Golgi morphology and trafficking function. GOLPH3 was also shown to regulate the mammalian target of rapamycin (mTOR) signaling, and link PtdIns 4-kinase activity present in this organelle with the regulation of cell growth. Endocytic trafficking of transmembrane receptors has a role in cell migration and is also regulated by GOLPH3 [9]. Piao and Mayinger [10] demonstrated that only a restricted pool of PtdIns-4-P at the trans-Golgi network (TGN) is required for Golgi integrity. In mammalian cells, the stress-activated mitogenactivated protein kinase (MAPK) p38 plays a critical role in transmitting nutrient signals to the phosphoinositide signaling machinery at Golgi and the ER.
Regulation of PtdIns-3-P has a role in early endosomal traffic and autophagy. PtdIns-3-P is synthesized by class III PI 3-kinase Vps34 in yeast and mammals and Vps34 forms specific complexes with co-factors. Phospholipase D1 (PLD1) and mTOR complex 1 (mTORC1) are involved in metabolic signaling of PtdIns-3-P. Several phosphatases belonging to the myotubularin (MTM) and myotubularin-related protein (MTMR) family are able to dephosphorylate PtdIns-3-P. Regulation of PtdIns-3,5-P2 has a function in late endosomal dynamics and in autophagy and its synthesis and turnover are tightly coupled. Different authors’ results suggest that endolysosome localized, mucolipin transient receptor potential (TRPML) channels, are PtdIns-3,5-P2 effectors that control Ca2+- dependent membrane dynamics in response to extracellular stimuli. Both 5-phosphatases and myotubularins dephosphorylate PtdIns-3,5-P2. While the first ones generate PtdIns-3-P, the second enzymes dephosphorylate this molecule 3-position of the inositol headgroup to produce PtdIns [6].
PtdIns-5-P is known for its role in nuclear signaling and membrane dynamics. This molecule can be synthesized by phosphatases via dephosphorylation of PtdIns- 3,5-P2 or PtdIns-4,5-P2, depending on the organism. Recently, mammalian type I and II PtdIns-4-phosphatases have been identified that generate PtdIns-5-P in vitro using PtdIns-4,5-P2 as substrate. PtdIns-5-P is able to bind to the nuclear adaptor denominated inhibitor of growth protein-2 (ING2) that regulates chromatin rearrangement in response to stress. But there is also increasing evidence that PtdIns-5-P may play an important role in regulating membrane dynamics in both endocytic and exocytic processes [6].
Phosphatidylcholine (PC) is the principal source of free fatty acids (FFA) which in turn may act as second messengers or as precursors of bioactive molecules like prostaglandins, thromboxanes and leukotrienes. Polyunsaturated fatty acids (PUFA) derive from the sn-2 glycerol carbon of cell membrane phospholipids and are precursors of a number of eicosanoids. Other FFA such as docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), are precursors of various other lipid mediators. Phospholipase D (PLD) is the enzyme located at the plasma membrane, which is responsible for PC hydrolysis rendering phosphatidic acid (PA) and is activated through external stimulation of appropriate receptors. PA is then metabolized through dephosphorylation to DAG. Another pathway for agonist mediated PC hydrolysis is the activation of phospholipase A2 (PLA2) leading to FFA release and lysophospholipids formation. DAG from this source activates protein kinase C (PKC) in the same way as DAG produced by phosphoinositides hydrolysis and FFA positively regulates this activation [11].
2.2. Nuclear Major Phosphoinositides and Lipids
Certain phosphoinositide species can regulate proteinchromatin and protein-nucleic acid interactions, and specific nuclear target proteins link nuclear signaling lipids to gene expression [12].
It has been demonstrated that nuclear PtdIns-4,5-P2 is a crucial molecule that acts both as a messenger and as a precursor for many additional messengers. There is some evidence suggesting that nuclear phosphoinositides occupy several functionally distinct compartments [13]. Nuclear phosphoinositides and inositol phosphates derived from them have been implicated in cell proliferation, differentiation, apoptosis, gene expression, etc. [14]. Nuclear phosphoinositide signaling depends on PtdIns- 4,5-P2 generation and at least four kinases, which product is this phosphoinositide, are located at the nucleous; however, they also have cytoplasmic functions. The enzymes that do not have a defined nuclear localization sequence, depends on different mechanisms, including one associated to oxidative stress. There is some evidence indicating that the phosphoinositide cycle has two versions within the nucleous, one of them associated with nuclear membranes and the other one separated from them. Phosphatidylinositol phosphate kinase IIβ (PIPK IIβ) is inhibited through MAPK dependent phosphorylation upon cellular stress, thus leading to inhibittion of PtdIns-4,5-P2 formation. Also, cellular stress is responsible for the translocation of type I 4-phosphoinositide phosphatase to the nucleous, which catalyses PtdIns-4,5-P2 degradation. Both mechanisms act together and maintain an increased PtdIns-5-P pool in this organelle. Increased PtdIns-5-P concentration causes the translocation of a nuclear PtdIns-5-P binding protein to a chromatin-enriched fraction, which in turn induces apoptosis through p53 acetylation. On the other hand, there is new evidence demonstrating that PtdIns-5-P also plays a role modulating ubiquitinilation.
Few nuclear proteins that bind phosphoinositides have been identified. One of them is the enzyme star-PAP which is specifically regulated by PtdIns-4,5-P2. StarPAP-dependent genes are localized in mammalian near the nuclear pore, resulting in PtdIns-4,5-P2-stimulated polyadenylation at the envelope. PtdIns-4,5-P2 also plays a role in RNA exportation from the nucleus as it is the precursor of InsP3, and this molecule is in turn the precursor of inositol 1,2,3,4,5,6-hexakisphosphate (InsP6), which is a positive mediator of mRNA export [13].
The PtdIns cycle has been linked to actin dynamics, at the first time in the cytoplasm, were PtdIns-4,5-P2 regulates the activity of many regulatory proteins that control actin polymerization and binding to other proteins, and later in the nucleus. Profilin I, a regulatory component of actin organization in the nucleus, co-localizes with PtdIns-4,5-P2 at nuclear speckles and is regulated by this phosphoinositide. Besides, chromatin remodeling complexes contain β-actin as an integral component. PtdIns- 4,5-P2 modulates chromatin remodeling possibly through regulation of the PtdIns-4,5-P2 actin binding site on the chromatin remodeling protein BRG1 [13].
Sphingomyelins are phospholipids different from glycerophospholipids, present in plasma membranes of most cells. They affect the lateral structure of membranes, and regulate cholesterol distribution within membranes. However, they have other functions but many of them are currently unknown. Sphingomyelins and other sphingolipids, in contrast to glycerophospholipids, have important hydrogen bonding properties, conferring them, specific functional properties [15]. Sphingomyelin and the ganglioside GM1 have been reported as major signaling lipids in the nuclear envelope. Other classes of lipidic compounds, as terpenoids and steroids, eicosanoids, and lysophospholipids, act mainly through two mechanisms: first, direct interaction between the bioactive lipid and a specific protein; and second, the formation of lipid microdomains or rafts. However, a new third mechanism has been recently described and it involves lipid second messengers as regulators for the energy and redox balance of differentiating neural stem cells (NSCs) [16].
Genes, nuclear factors and receptors influence on signal pathways. Fatty acids generated from triacylglycerol and phospholipids can significantly affect cell signaling not only due to activation of PKC at the plasma membrane, but also through their effects on transcription factors. Long chain unsaturated FA (C20-22) and their metabolites are effective ligands of nuclear receptors of some transcription factors as peroxisome proliferatoractivated receptor (PPAR) -α, -β/δ, -γ1 and -γ2; liver X receptors (LXR) type α and hepatic nuclear factor (HNF) 4α; sterol regulatory element binding protein (SREBP) -1 and -2 and carbohydrate regulatory element binding protein/Maxlike factor X (ChREBP/MLX) [11].
2.3. Organochlorine and Organophosphate Pesticides Effect
Souza et al. [17] have described the effect of organophosphorous (OP) and organochlorine (OC) pesticides on phosphoinositides metabolism in human placenta, by incubating 32P labelled cell-free homogenates with similar pesticides concentrations to those reported in vivo. The OC Heptachlor (HC) and dichloro-diphenyl-trichloroethane (o-p’ DDT) increased PtdIns, PtdIns-4-P and PtdIns-4,5-P2 phosphorylation while the OP azinphosmethyl (AM) increased PtdIns-4,5-P2 labeling, associated to an activation of PtdIns-4-kinase activity. The strongest effect was seen with o-p’ DDT in nuclear fraction PtdIns-4-kinase activity. Authors proposed that the binding of o-p’ DDT to the estrogen receptor may facilitate the pesticide transport to the nucleus.
3. PTDINS-4,5-P2
Different authors had documented the role of phosphoinositides as lipid mediators that generate second messengers as the result of different agonist stimulations in a variety of cells [18,19]. InsP3 and DAG generation by PtdIns-4,5-P2 hydrolysis, together with the activation of calcium release and PKC, generated by these second messengers, have been the pathways reported to be responsible for this phosphoinositide participation in hormone signaling and exocytosis [20,21]. More recently different authors have demonstrated that phosphoinositides and especially PtdIns-4-P and PtdIns-4,5-P2 are not only precursors of second messengers but also directly interact with many protein effectors and their localisation and/or activity [3,22]. Although muscarinic stimulation of PLC-coupled M1 receptors in neurons leads to PtdIns- 4,5-P2 hydrolysis, InsP3 release and diacylglycerol (DAG) generation, almost none Ca2+ rises were detected as the result of such stimulation. On the contrary, stimulation of PLC-coupled bradykinin B2 or purinergic P2Y receptors produced reliable Ca2+ signals. One Hypothesis postulated by Zaika et al. [22] involves subcellular clustering of certain plasma membrane PLC-linked receptors into microdomains together with endoplasmic reticulum membrane InsP3 receptors, allowing B2 but not M1 receptors, to physically interact with InsP3 receptors. In agreement with this theory, the two proteins have been shown to strongly co-localize, when they were studied under confocal microscopy. On the other hand, several regulators of InsP3 receptors have been recently characterized as modifiers of the efficacy of InsP3 to open its receptor. InsP3 receptor-binding protein (IRBIT) is released together with InsP3 and has been postulated as a possible candidate responsible for tuning the extent of receptorinduced Ca2+ rises [22].
Regulation of PtdIns-4,5-P2 has an important role in vesicular traffic. This phosphoinositide is synthesized by different kinases, depending on the organisms. PtdIns- 4,5-P2 is enriched at the plasma membrane, a localization where it has other different functions from the ones cited above, as the regulation of clathrin-mediated endocytosis and exocytosis of synaptic vesicles and secretory granules. Also, its rapid turnover by lipid phosphatases from the synaptojanin family is important for rapid recycling of endocytic vesicles. Early endosomal trafficking and recycling was also demonstrated to be regulated by the lipid phosphatase OCRL1 [6]. Removal of foreign particles by phagocytosis plays a central role in innate immunity and also mediates the clearance of apoptotic bodies that is essential for tissue remodeling and homeostasis. Mammalian cells express two types of phagocytic receptors and the signal transduction pathways of both of them elicit actin remodeling mediated by GTPases. Different authors demonstrated that PtdIns-3,4,5-P3 is required for these events to take place and that it is formed by phosphorylation of PtdIns-4,5-P2 by class I phosphoinositide 3-kinases [23,24]. Wen et al. [25] have also described novel aspects of neuroexocytosis. Apart from the well known role of PtdIns-4,5-P2 in the mobilization of secretory vesicles to the plasma membrane, other phosphoinositides have also been involved in this pathway. Positive and negative control of neuroexocytosis have been attributed to PtdIns-3-P and PtdIns-3,5-P2 respectively.
Calcium entry into the cells, activated through PLCcoupled receptors is mediated by DAG activation of canonical transient receptor potential proteins (TRCP). Trebak et al. [26] have proposed membrane phosphoinositides playing at least two functions in the regulation of this pathway. They found that depletion of PtdIns-4-P and PtdIns-4,5-P2, caused by inhibition of phosphatidylinositol 4-kinase, activated calcium entry and membrane currents in TRPC5-expressing but not in TRPC3- or TRPC7-expressing cells. Paradoxically, depletion of PtdIns-4,5-P2 with a directed 5-phosphatase strategy inhibited TRPC5. Then, they concluded that their findings indicate complex functions for regulation of TRPC5 by PtdIns-4,5-P2, and proposed distinct functions for phosphoinositide regulation of TRPC5.
3.1. Organochlorine Pesticides Effects
Criswell et al. [27] tested the hypothesis that the OC lindane (or gamma-hexachlorocyclohexane) inhibits gap junctional communication in myometrial myocytes due to the release of phosphoinositide-dependent second messengers. They studied the effect of lindane in cultured rat myometrial smooth muscle cells by monitoring transfer of the fluorescent dye Lucifer yellow. Their results showed a rapid, concentration-dependent, but reversible inhibittion of dye transfer at 4-min exposures, and complete inhibition at 10 μM lindane. They also reported that lindane stimulated the production of the Ca2+-releasing species InsP3 which peaked at 5 min (100 pmol/mg protein) and remained elevated after a 15-min exposure. Ca2+ ionophore 4-br-A23187 also inhibited gap junctional communication, but inhibition was not complete even at concentrations that appeared cytotoxic (70% inhibition at 2 μM A23187). Then they loaded cells with the Ca2+ chelator BAPTA-AM, which blocked the lindane-induced rise in calcium, and repeated dye transfer experiments with lindane in Ca2+-free medium. Their results demonstrated that the inhibition of dye transfer was still complete under these conditions, showing that increased intracellular calcium was not required for lindane-induced inhibition of gap junctional communication. In addition, working with 10 μM lindane was shown to produce a sustained increase in PKC activity and known activators of PKC, 12-O-tetradecanoylphorbol 13-acetate (TPA) and 1,2-dioctanoyl-sn-glycerol, abolished gap junctional communication at nanomolar concentrations. Afterwards they worked with PKC inhibitor staurosporine, which failed to reverse lindane’s inhibitory action, in contrast to depletion of PKC activity through prolonged exposure to TPA, which partially reversed lindane’s effect. The authors suggested that PKC activation was able to potentiate but did not solely mediate lindane’s inhibitory action on gap junctional communication.
3.2. Organophosphate Pesticides Effects
Balduini et al. [28] examined muscarinic receptor number, receptor-stimulated phosphoinositide hydrolysis and m1 mRNA expression in the cerebral cortex and hippocampus of rats treated during postnatal development or in adult age with the organophosphate diisopropyl fluorophosphate. They treated adult animals for 14 days and developing rats from postnatal days 4 - 9 or from postnatal days 4 - 20, and then they were killed on days 10 and 21, respectively, 24 h after the last administration of diisopropyl fluorophosphate. They found that acetylcholinesterase activity and muscarinic receptor number were significantly reduced in all groups of treatment. However, their results showed that muscarinic receptor-stimulated phosphorinositide turnover, was significantly reduced in postnatal days 4 - 20 and adult treated rats but not in the postnatal days 4 - 9 group. Conversely, they reported that m1 mRNA expression was significantly reduced both in the cerebral cortex and hippocampus of postnatal days 4 - 9 treated rats, but not of postnatal days 4 - 20 and adult treated rats. These results lead the authors to report that chronic inhibition of acetylcholinesterase in developing rats resulted in significant alterations in muscarinic neurotransmission and that these alterations may account for some of the long-lasting neurotoxic effects observed after developmental exposure to OP pesticides.
Sun et al. [29] studied the effects of the OP paraoxon on the salivary gland. They examined the effects of exposure to low concentrations of the paraoxon on inositol InsP3 formation and Ca2+ mobilization in response to acetylcholine (Ach) or ATP in the human parotid cellline HSY. Their results showed that exposure to 0.1 and 1 nM, but not 10 nM, paraoxon for 24 hr significantly elevated the basal cytosolic free Ca2+ ([Ca2+](i)) through this cation release from the InsP3-sensitive store and that this increase was abolished by the muscarinic receptor antagonist atropine. The authors suggested that paraoxon increases the sensitivity of InsP3 receptors, as the pesticide inhibited InsP3 formation but did not alter mChR expression. In addition, Ca2+ release elicited by InsP3 in streptolysin O toxin-permeabilized cells was significantly larger in cells pre-exposed to 0.1 nM paraoxon. They also reported that paraoxon exposure induced a concentration-dependent reduction in the total capacity of intracellular Ca2+ stores, whereas the capacity of the InsP3- sensitive Ca2+ store was not altered by paraoxon, as judged by discharging of the InsP3-sensitive Ca2+ store with thapsigargin (TG). Their results also showed that Ca2+ influx stimulated by Ach or ATP was also enhanced by 0.1 nM, but not by 1 and 10 nM paraoxon, but, on the other hand, Ca2+ influx activated by TG was enhanced by exposure to all concentrations of paraoxon, indicating that paraoxon modulates the Ca2+ entry pathway. The authors then concluded that low concentrations of paraoxon interact with elements of the PtdIns pathway, enhancing Ca2+ release and influx mechanisms.
Zhang et al. [30] treated neonatal (7-day), juvenile (21-day) and adult (90-day) rats with either peanut oil or chlorpyrifos (CPF) at 0.3× or 1× the maximum tolerated dosage (MTD: 45, 127 and 279 mg/kg for 7-day, 21-day and 90-day rats, respectively), to evaluate the effect of this OP on phosphoinositide hydrolysis and cAMP formation. They selected different neurochemical end-points including acetylcholinesterase (AChE) activity, muscarinic receptor ([3H]quinuclidinyl benzilate (QNB), and [3H]oxotremorine) binding, phosphoinositide hydrolysis, and cAMP formation in cortex were evaluated at 4 h, 24 h, or 96 h after treatment. Their results showed maximal degrees of cholinesterase (ChE) inhibition, with varying times to peak inhibition among these age groups (24 h in neonates and juveniles, 96 h in adults). Total muscarinic receptor (QNB) binding was reduced in all three age groups with 1x MTD exposure, at both 24 h and 96 h in neonates and juveniles, but only at 96 h in adults. Oxotremorine binding was also reduced at 96 h after MTD exposure in all three age groups. Their results also showed that basal cAMP formation significantly increased by MTD exposure in all three age groups 4 h after exposure, and at 4 h, 24 h, and 96 h after exposure in juveniles and Forskolin/Mn2+-stimulated cAMP formation was increased in neonates and juveniles at 96 h, and in juveniles also at 24 h, but was significantly decreased in adults at 96 h after MTD exposure. In contrast, neither basal nor carbachol-stimulated inositol phosphates accumulation was affected in any age group or at any time point following CPF exposure. The authors concluded that the PtdIns pathway is unaffected by CPF under their assay conditions, but cortical cAMP signaling pathway may be particularly sensitive to CPF exposure in neonatal, juvenile, and adult rats, possibly due to a direct interaction between CPF (or its oxon) and mAChRs or other components of the adenylyl cyclase cascade.
4. PHOSPHOINOSITIDE AND PHOSPHATIDYLCHOLINE SPECIFIC PHOSPHOLIPASES C
Phosphoinositide-specific phospholipase isozymes have been identified, and they are divided into six groups: PLCbeta, -gamma, -delta, -epsilon, -zeta and -eta. PLC isozymes contain the X and Y domains that are responseble for catalytic activity. Other domains include the PH domain, the C2 domain and EF hand motifs and are involved in various biological functions of PLC isozymes as signaling proteins. PLC isozymes distribution is tissue and organ specific and each PLC isozyme bears a unique function in the modulation of physiological responses [31].
Almost all known signaling proteins, consist of one or more conserved structural domains attached to an active site that, in some way, modulate their catalytic activity. PLCs can be found at all stages in evolution. The simplest bacterial PLCs consist solely on the catalytic lipase domain which requires Ca2+ for activity. As species progressed PLCs became larger and more complex, when regulatory domains were added. Mammalian PLCs isoforms differ in structural organization, regulation, activation and tissue distribution. PLC-δs can be found in all tissue types, require Ca2+ for their activity and are inactive at basal levels of Ca2+. The increase in intracellular Ca2+ resulting from the activation of other PLCs activates PLC-δ, thus locating this enzyme downstream of other PLCs in signaling pathways. Different protein regulators change the level of Ca2+ required for PLC-δ activation. PLC-βs are the major effectors of the Gαq family of heterotrimeric G proteins. This family is coupled to receptors that bind ligands such as angiotensin II, catecholamines, endothelin 1 and prostaglandin F2α, etc. There are four known family members of PLC-β that differ in their response to G proteins. All of them are strongly activated by GTPbound Gαq. PLC-β2 and PLC- β3 are also activated by Gβγ subunits [32].
There have been identified several protein regulators for PLC-δ1 but none of them appear to significantly control its cellular activity. Some regulators lower the level of Ca2+ needed for PLC-δ1 activation. PLC-β2 and PLC- β3 are PLC-δ1 inhibitors. It was shown that PLC-δ1 and PLC-β2 associate on membrane surfaces and that this association inhibits the activity of PLC-δ1, but not PLC- β2. Gβγ subunits released upon cell stimulation displace PLC-δ1 bound to PLC-β2. Inhibition of PLC-δ1 may prevent spurious or premature activation of this enzyme, thus better controlling cellular Ca2+ levels. The main cellular activators of PLC-β enzymes are Ras-like GTPase of the Rho family and heterotrimeric G proteins. Rac1, 2, 3, and to a lesser extent Cdc42Hs, were shown to activate PLC-β2 and β3 by binding to their PH domains, probably by recruiting PLC-βs to the membrane surface. On the other hand, heterotrimeric G proteins activate PLC-βs without promoting its binding to membranes and also without affecting the calcium dependence of its activity. The strength of activation of PLC-β2 and -β3, at least by Gβγ subunits, seems to be directly proportional to the strength of association between the proteins [32].
Several signal transduction pathways involve the hydrolysis of phosphatidylcholine (PC) instead of the generation of second messengers from PtdIns-4,5-P2. Phosphatidylcholine specific phospholipase C (PC-PLC) has been studied by several authors and different functions have been attributed to this enzyme according to its tissue or cell localization. Suzuki et al. [33] have recently evaluated the Involvement of PC-PLC in thromboxane A2 receptor-mediated extracellular Ca²+ influx in rat aorta. They concluded that DAG produced by PC-PLC seems to activate two types of cation channels independently of PKC, which in turn leads to VDCC-dependent and independent Ca2+ influx, thereby eliciting contraction. Strielkov et al. [34] investigated the role of PC-PLC in hypoxic pulmonary vasoconstriction (HPV) and concluded that PC-PLC plays an important role in sustained HPV possibly through the activation of PKCindependent mechanism, which may be coupled with phosphocholine release. Xia et al. [35] evaluated the involvement of PC-PLC in osteoclastogenesis induced by TNF-α through upregulating InsP3R1 expression and their results lead them to suggest that TNF-α promotes RANKL-induced osteoclastogenesis, at least partially, through PC-PLC/InsP3R1/NFATc1 pathway.
Some of the results cited above on PC-PLC role in different tissues as well as other recent investigation results encouraged the authors to suggest new therapeutic approaches for different diseases [36-39].
4.1. Organochlorine Pesticides Effects
Moya de Juri et al. [40] evaluated the ability of the OC heptachlor (HC) to interfere with platelet phosphoinositides metabolism and related signaling events stimulated by thrombin. They found that HC 1 and 10 μM increased PKC activity and PtdIns-4,5-P2 and PA phosphorylation but 100 μM HC increased PtdIns-4,5-P2 phosphorylation and reduced serine/threonine kinases activity. The authors proposed that signal transduction steps downstream PLC were unphysiologically activated by heptachlor and facilitated by the increase in PtdIns-4,5-P2, the substrate for PLC activity, thus producing an accumulation of phosphatidic acid. The elevated level of this compound itself or the transient increase in DAG produced may cause calcium mobilization and the activation of PKC. Besides the alterations they described in phosphoinositide and phospholipid phosphorylation, they could not detect any change in the aggregation properties of the platelets.
Lu et al. [41] studied the effect of lindane on Ca2+ mobilization in Madin-Darby canine kidney cells, through examination by fluorimetry using fura-2 as a Ca2+ indicator. They found that lindane was able to trigger Ca2+ influx and Ca2+ release. In Ca2+-free medium, 0.15 mM lindane increased [Ca2+]i after pretreatment with carbonylcyanide m-chlorophenylhydrazone (CCCP, 2 μM), a mitochondrial uncoupler, and two endoplasmic reticulum Ca2+ pump inhibitors, thapsigargin and cyclopiazonic acid. Conversely, pretreatment with lindane abolished CCCPand thapsigargin-induced Ca2+ release. These results suggested that 0.15 mM lindane released Ca2+ from the endoplasmic reticulum, mitochondria and other stores. However, Lindane (0.15 mM)-induced Ca2+ release was not reduced by inhibiting PLC with 2 μM U73122, thus also indicating that a mechanism similar to the activation of PLC, but different from this one, as the responsible for the OC effect.
Hansen and Matsumura [42] evaluated the effects of the OC liver tumor promoter heptachlor epoxide (HE; 0, 0.1, 1, 10, and 50 μM) on several cellular tumor promoter-sensitive parameters in mouse 1c1c7 hepatoma cells. They studied the levels of Ca2+ in the endoplasmic reticulum (ER) store, connexin43 (Cx43), PLCgamma(1), nPKCvarepsilon, and AP-1 DNA binding in the nucleus. Their results showed that particulate PLCgamma(1) and AP-1 DNA binding were found to be the most sensitive parameters affected by HE on both dose and temporal bases. The authors suggested then that the tyrosine kinase growth factor receptor pathway is the probable critical pathway for HE-induce tumor promotion with the critical target most likely being upstream of PLC gamma (1) and AP-1.
4.2. Organophosphate Pesticides Effects
Katz and Marquis [43] studied the effect of paraoxon on muscarinic receptor-mediated phosphoinositide hydrolysis in the SK-N-SH neuroblastoma cell line. They reported that at 0.1 nM (no effect concentration for the classical muscarinic receptor agonist carbachol), paraoxon caused a time-dependent increase in the accumulation of inositol phosphates. In contrast, carbachol-induced increases in phosphoinositide hydrolysis at higher concentrations (1 mM) were markedly larger (five-fold) than the increases induced by paraoxon. The authors also found that only 50% of the maximal increase in the accumulation of inositol phosphates due to paraoxon treatment was antagonized by saturating concentrations of muscarinic receptor antagonists, while the response to carbachol was completely antagonized. Additional studies consisting on treatment of the cells with pertussis toxin blocked 75% of the stimulatory effects of carbachol, but inhibited only 38% of the paraoxon-induced response. Neomycin (a PLC inhibitor), rendered completely blockage of both the paraoxon and carbachol-induced stimulation of phosphoinositide hydrolysis. The results allowed the authors to suggest that paraoxon can modulate signal transduction by indirect activation of muscarinic receptors as well as by acting at a distal site along the pathway.
An association between changes in PtdIns kinase activity, the turnover rate of phosphatidylinositols and alterations in erythrocyte morphology induced by phosmetoxon 300 nM were proposed by Magnarelli de Potás and Pechén de D’ Angelo [44]. They described that the lipid that rendered the highest 32P phosphate incorporation was PtdIns, followed by PtdIns-4-P and PtdIns-4,5-P2 and that 150 and 300 nM phosmetoxon activate PtdIns kinase. The authors did not find any change in PI-PLC activity and suggested an association between changes in PtdIns kinase activity, the phosphorylation cycle of phosphatidylinositols and alterations in erythrocyte morphology induced by the pesticide.
5. DAG GENERATION FROM PHOSPHATIDIC ACID
5.1. Enzymes Involved
DAG is a ubiquitous lipid that plays different roles, as being the precursor molecule in the synthetic pathway of other lipids or a second messenger transient generated in a variety of cells. Phosphatidic acid (PA) phosphatases represent a family of enzymes called lipins, which are conserved from yeasts to humans, their activity is regulated by phosphorylation and dephosphorylation and they produce DAG though PA hydrolysis [45]. Lipins family members (lipin 1 and 2) interact in vivo in different organs of mammalian and maintain lipid (triacylglycerol and phospholipids) homeostasis [46]. Lipins and their regulators play roles in nuclear envelope organization, gene expression and the maintenance of lipid homeostasis [47]. Valdearcos et al. [48] described the subcellular localization of lipin-1 in macrophages´ lipid droplets and the lipin-mediated regulation of the activity of a phospholipase A. Domart et al. [49] have described two conserved functions for DAG: a structural role in organelle morphology and a role related to membrane fusion, both in mammalian and echinoderm. Disassembly of the nuclear lamina, a key step during open mitosis in higher eukaryotes, is triggered by different kinases, including PKC. DAG activates PKC and is also responsible for the lamin B1 phosphorylation-dependent disassembly. This finding provides evidence for a lipid-mediated mitotic signaling event, in which lipins are responsible for the activation of a PKC-dependent pathway during mitotic lamin disassembly [50].
The activation of numerous G-protein-coupled-receptors and tyrosine kinase receptors allows DAG generation from distinct phospholipid hydrolysis, which in turn activates not only PKC but also protein kinase D (PKD). Kunkel and Newton [51] have described basally elevated DAG levels at Golgi membranes (probably attributed to phosphatidic acid phosphatase and sphyngomielin synthase activities). They also reported Golgilocalized DAG levels increase as the consequence of Ca2+ rise coupled to G-protein-coupled-receptors signaling from the plasma membrane and concluded that Ca2+ is a key second messenger of that pathway, that render both PKC and PKD activation.
5.2. Organophosphate Pesticides Effects
Chlorpyrifos oxon (CPO) activates extracellular signalregulated kinase (ERK 44/42) in Chinese hamster ovary (CHOK1) cells but the mechanism is not defined. Bomser et al. [52] performed an evaluation to test the hypothesis that DAG is the secondary messenger responsible for CPO-induced ERK 44/42 activation. As it is known that DAG is sequentially hydrolyzed by DAG lipase and monoacylglycerol (MAG) lipase, both of which are OP sensitive, the authors postulated that the inhibition of these enzymes might therefore lead to the accumulation of DAG and MAG, being only DAG a secondary messenger. Their experiments showed that treatment of CHOK1 cells with CPO significantly inhibited DAG/MAG lipase activity and elevated cellular DAG levels. Additional pretreatment studies of CHOK1 cells with CPO or a carbamate known to be a DAG lipase inhibitor, followed by treatment with a cell-permeable DAG (1,2-dihexanoyl-sn-glycerol), resulted in synergistic activation of ERK 44/42. CPO-potentiated DAGinduced ERK 44/42 activation was both time and concentration dependent and was blocked by inhibitors of PKC and mitogen-activated protein kinase, suggesting that these enzymes are important in CPO/DAG cellular signaling. The results described above as well as the fact that the activation by a stable DAG analogue (phorbol ester) was not altered by CPO, allowed the authors to suggest that DAG metabolism is the probable target for CPO-potentiated DAG-induced ERK 44/42 activation and that these observations support the hypothesis that CPO potentiates DAG signaling in CHOK1 cells by inhibiting a CPO-sensitive DAG lipase, thereby providing a potential mechanism of toxicity not associated with acetylcholinesterase inhibition.
6. OOCYTES´PHOSPHOINOSITIDES SIGNALING DURING REPRODUCTION
Ramos et al. [53] demonstrated the presence of intracellular calcium storages, as well as their localization and their migration during Bufo arenarum oocytes maturation. They also observed that these storages were located near the cortical region in mature oocytes and that they were ready for release during fertilization. Both the intracellular calcium role during cortical granule exocytosis taking place during fertilization and calcium waves generated in response to InsP3 stimuli elicited by sperm union to the oocyte, have been extensively studied [54-56].
PtdIns-4,5-P2 hydrolysis is catalized by PLCγ in sea urchin eggs [57], starfish eggs [58], Chaetopterus [59], and Xenopus eggs, which is probably activated through tyrosine residues phosphorylation, mediated by a SRC kinase family [60]. This hydrolysis leads to InsP3 and DAG generation.
Halet et al. [61] have demonstrated that cortical granule exocytosis, occurring in recently fertilized mouse oocytes, elicits PtdIns-4,5-P2 resynthesis.
Ito et al. [62] reported that the InsP3 receptor R1 is implicated in the release of calcium from intracellular storages. Wakai et al. [63] have localized this receptor in the endoplasmic reticulum. Lee et al. [64] and Ito et al. [62] described MAPK as regulators of both mitotic spindle organization and InsP3 receptor cortical redistribution.
Other PLC, called PLCzeta (PLCζ, has been described as the spermatic factor responsable for calcium release in the recently fertilized oocyte. Igarashi et al. [65] deribed the first evidences in mouse fertilization for the joint activity of both oocyte’s PLCβ1 and sperm PLCζ Also, Kashir et al. [66] described a failure in the fertilition of human oocytes, due to diminished expression and activity of PLCζ.
The most recently findings on the involvement of PtdIns-4,5-P2 during fertilization have described the biphasic rise of this lipid mediator together with InsP3 formation and the regulation of actin-binding proteins. Chun et al. [67], working on starfish eggs, have studied the roles of PtdIns-4,5-P2 at fertilization by using fluorescently tagged pleckstrin homology (PH) domain of PLC-δ1, which has specific binding affinity to PtdIns- 4,5-P2, in combination with Ca2+ and F-actin imaging techniques and transmission electron microscopy. They have reported that PtdIns-4,5-P2 increased at the plasma membrane in two phases. The first increase was quickly followed by a decrease about 40 seconds after sperm-egg contact and these changes took place only after the Ca2+ wave had already initiated and propagated. Their results have shown that fertilized eggs then displayed a prolonged increase of PtdIns-4,5-P2 that was accompanied by numerous spikes in the perivitelline space during the elevation of the fertilization envelope (FE) and also that these spikes, protruding from the plasma membrane, were filled with microfilaments.
Other lipid mediators, different from phosphoinositides, were also reported as molecules responsible for the generation of second messengers during fertilization. PLD catalyzed PC hydrolysis rendered intracellular rise of choline in different cells, including recently fertilized oocytes. Stith et al. [68] described the intracellular rise in choline, DAG and InsP3 in recently fertilized Xenopus oocytes. They reported that the increase in InsP3 formation was 280-fold smaller than the DAG increase, that PC did not rise in those cells and that artificial elevation of intracellular calcium ([Ca2+]i) increased DAG levels but prevention of the [Ca2+]i increase after fertilization did not block DAG production. They concluded that sperm stimulate production of DAG and choline through [Ca2+]i-independent and [Ca2+]i-dependent paths.
Morrill and Kostellow [69] reported a cascade of phospholipid mediators’ response as the consequence of Xenopus oocytes’ stimulation with progesterone, leading to DAG production from multiple sources. They described that membrane-receptor binding of progesterone caused the successive activation of: 1) N-methyltransferases, which converted phosphatidylethanolamine (PE) to PC; 2) an exchange reaction between PC and ceramide to form sphingomyelin (SM) and 1,2-diacylglycerol (DAG); 3) PLD/phosphatidate phosphohydrolase, releasing a second DAG transient; and 4) PI-PLC, generating inositol trisphosphate and a third DAG transient. They also reported that diglyceride kinase converted newly formed DAG species to phosphatidic acid within minutes, thus turning off the successive DAG signals and that an early transient fall in intracellular ceramide was followed by a sustained rise in intracellular ceramide lasting 3 - 4 h. They proposed ceramide as an important molecule in later cyclin-dependent steps.
6.1. Organochlorine Pesticides Effects
Dieldrin effects on phospholipid and phosphoinositide metabolism were studied in Bufo arenarum oocytes [70]. Oocytes were incubated during 20 hours in Ringer solution or in Dieldrin 4 mg/L prepared in Ringer solution. 32P phospholipid and phosphoinositide 2 hours incorporation studies were performed. The results showed that Dieldrin pretreated oocytes incorporated significantly lower 32P radioactivity in phospholipids than did control ones, while no differences between the groups were found for phosphoinositides labeling. Also Dieldrin shifted 32P incorporation towards PtdIns and PA in contrast to the preferentially labeling of PE and PC found in control oocytes. Carbachol stimulation of control and Dieldrin treated oocytes rendered an active turnover of both PtdIns-4-P and PtdIns-4,5-P2 only in control ones. Fonovich de Schroeder and Pechén de D’Angelo [71] then studied both phosphoinositides hydrolysis and DAG formation in Dieldrin treated and control oocytes prelabelled with both 3H (with 3H-glycerol) through in vivo injection to the female) and 32P by in vitro incubation of the oocytes. PtdIns-4,5-P2 rendered lower t = 0 32P radioactivity in treated than in control oocytes, while PtdIns and PtdIns-4-P showed no difference between groups. Carbachol stimulation of both groups rendered a decrease in 32P PtdIns-4,5-P2 in control oocytes and a rapid increase in its phosphoinositide labeling in Dieldrin treated ones. Simultaneously, 3H-DAG increased only in control cells, in agreement with PtdIns-4,5-P2 probable hydrolysis. PtdIns kinase activity was also evaluated in vivo and in vitro (using oocyte membranes as substrate). Dieldrin effect was studied at different concentrations and no effect of the pesticide could be observed. Fonovich de Schroeder and Pechén de D’Angelo [72] also evaluated in vitro the effects of Dieldrin on the activity of a PC-PLC, using enriched plasma membrane fraction obtained from Bufo arenarum oocytes as the substrate and quantifying the final concentration of phosphorus in chloroform:methanol (2:1) extracts (phospholipidic phosphorus). In addition, PC, PE and SPH phosphorus were also quantified after these phospholipids separation by thin layer chromatography. The enzyme preferentially degraded PC and PE in control tubes. The pesticide not only increased the enzyme activity but also made it possible to the enzyme to degrade SPH instead of PE. Fonovich de Schroeder and Pechén de D’Angelo [73] then performed a new assay to study the effects of Dieldrin on the glycerol moiety of phospholipids and acylglycerols and in fatty acids exchange from these molecules. 3Hglycerol and 3H-palmitic acid prelabelled oocytes were exposed to Dieldrin or not (control ones), cholorom: methanol (2:1) lipid extracts were prepared, phospholipids, acylglycerols and free fatty acids were separated by thin layer chromatography and radioactivity was finally quantified for each group of compounds. All the individual phospholipids and lysophospholipids rendered lower radioactivities in Dieldrin treated oocytes, while no differences were found for acylglycerols (MAG, 1,2-DAG, 1,3-diacylglycerol (1,3-DAG) and triacylglycerol. Dieldrin treated oocytes also showed an increased free fatty acids fraction as well as increased turnover of palmitic acid between phospholipids and acylglycerols.
7. CONCLUSION
Phosphoinositide, phospholipid, acylglycerols and fatty acids different roles have been recently reported by several authors in a wide range of organisms and cells. Signal transduction pathways, intracellular membrane trafficking, exoand endocytosis, nuclear events, cytoskeleton remodeling, cell growth, cell polarity, etc., are some of the newly discovered cellular sites of action of these hydrophobic molecules. However, little information is available nowadays about the interference that widely distributed xenobiotics, also consisting of hydrophobic molecules like OP and OC can produce them. Although pesticides permitted in most countries do not include these old categories, there is evidence confirming that they are still in use (illegally) in some developing countries. Besides, they accumulate in living organisms due to their high hydrophobicity and they are also very persistent in different environmental compartments. The evidence presented here demonstrates that some pesticides from both organophosphate and organochlorine groups produce different effects on phospholipid and phosphoinositide phosphorylation and hydrolysis, as well as on acylglycerols and fatty acid metabolisms. Figures 1 and 2 show the molecular targets of organochlorine and
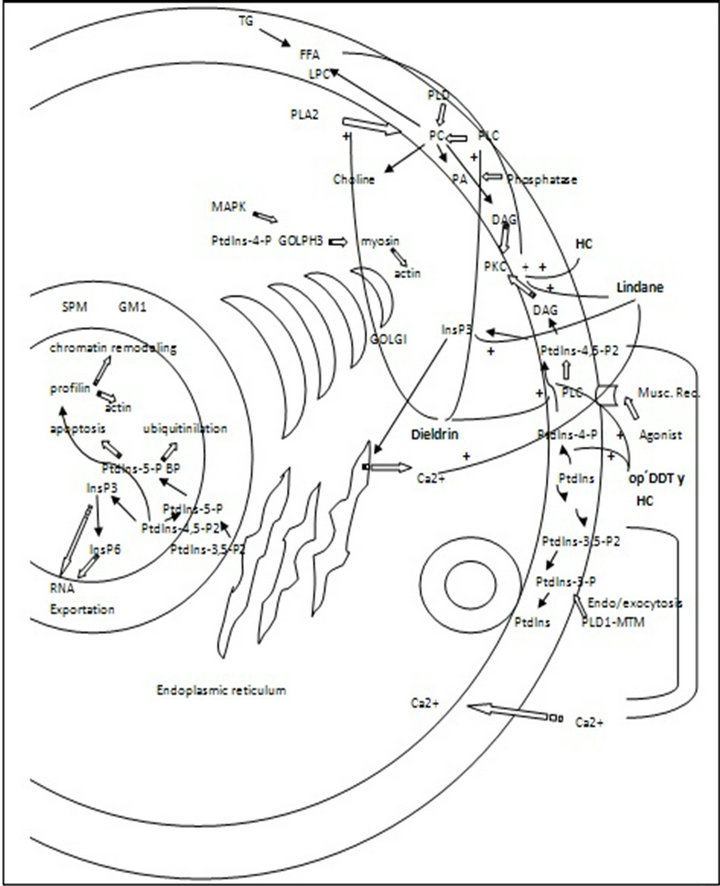
Figure 1. Plasma membrane and nuclear phospholipid and phosphoinositide pathways. Organochlorine pesticides effect. PC: phosphatydilcholine, SPM: sphyngomielin, LPC: lysophosphatydilcholine, GM1: ganclioside GM1, FFA: free fatty acids, PLC: phospholipase C, PLD: phospholipase D, PLA2: phospholipase A2, DAG: diacylglycerol. TG: triacylglycerol, HC: heptachloro.
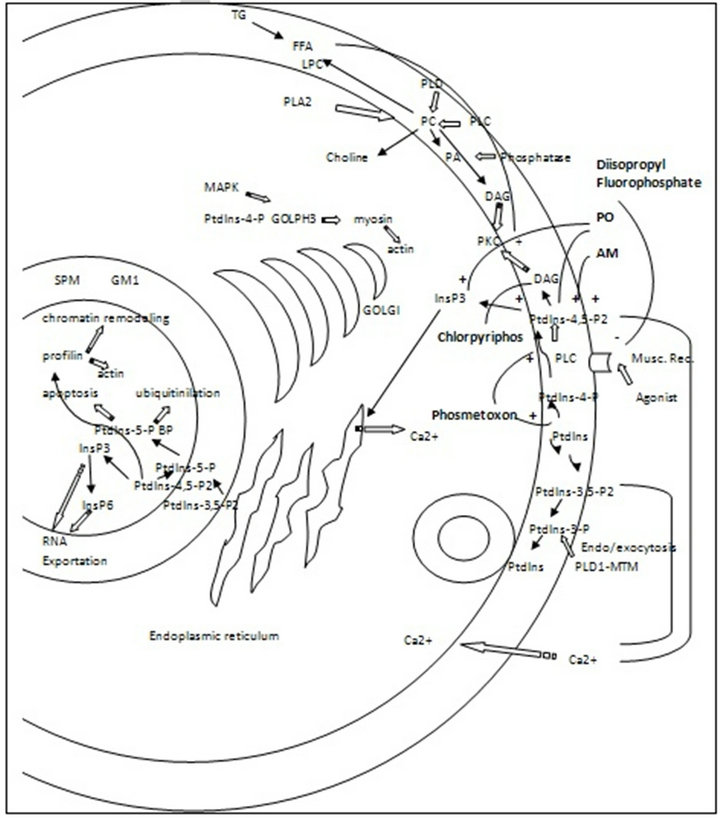
Figure 2. Plasma membrane and nuclear phospholipid and phosphoinositide pathways. Organophosphate pesticides effect. PC: phosphatydilcholine, SPM: sphyngomielin, LPC: lysophosphatydilcholine, GM1: ganclioside GM1, FFA: free fatty acids, PLC: phospholipase C, PLD: phospholipase D, PLA2: phospholipase A2, DAG: diacylglycerol. TG: triacylglycerol, PO: paraoxon, AM: azynphos methyl.
organophosphate pesticides reported in different eukaryotic cells. The mechanisms of action of the majority of the studied pesticides on these pathways remain to be elucidated and should probably constitute very useful tools to better understand their toxicity.
8. FUTURE CHALLENGES
Only few mechanisms of action of individual hydrophobic xenobiotic compounds, including pesticides, are commonly considered as the responsible ones for their toxicity. However, other effects, and especially those that take place on cell membranes, should probably also account for important effects also related to cell damage and death. Phospholipid and phosphoinositide phosphorylation pathways and hydrolysis had not been considered until the last decades as mechanisms involved in signaling processes. Pesticides use rises continuously in accordance to the continuous increase on food demand all over the world. Environmental pollutant residues and particularly pesticide ones can be frequently found as food contaminants and can therefore be responsible for animal and human toxicity. New studies on pesticide effects on phospholipid and phosphoinositide mediated signaling processes must certainly contribute to better understand their toxicity and help decision-making institutions to actualize legislations on their use and their residue limits in food.
9. ACKNOWLEDGEMENTS
The present work was supported by the Universidad Nacional de San Martin and the Universidad Nacional del Comahue.
REFERENCES
- Koch, M. and Holt, M. (2012) Coupling exoand endocytosis: An essential role for PIP2 at the synapse. Biochim Biophys Acta, 1821, 1114-1132.
- Krahn, M.P. and Wodarz, A. (2012) Phosphoinositide lipids and cell polarity: Linking the plasma membrane to the cytocortex. Essays in Biochemistry, 53, 15-27. doi:10.1042/bse0530015
- Delage, E., Puyaubert, J., Zachowski, A. and Ruelland, E. (2013) Signal transduction pathways involving phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5- bisphosphate: Convergences and divergences among eukaryotic kingdoms. Progress in Lipid Research, 52, 1-14. doi:10.1016/j.plipres.2012.08.003
- Kim, Y.J., Jahan, N. and Bahk, Y.Y. (2013) Biochemistry and structure of phosphoinositide phosphatases. BMB Reports, 46, 1-8. doi:10.5483/BMBRep.2013.46.1.261
- Stephens, L., Mc Gregor, A. and Hawkins, P. (2000) Phosphoinositide3-kinases, regulation by cell surface receptors and function of 3-phosphorylated phospholipids in “Biology of Phosphoinositides”. Oxford University Press, Oxford, 32-108.
- Mayinger, P. (2012) Phosphoinositides and vesicular membrane traffic. Biochim Biophys Acta, 1821, 1104-1113. doi:10.1016/j.bbalip.2012.01.002
- Shenker, B.J., Ali, H., Boesze-Battaglia, K. (2011) PIP3 regulation as promising targeted therapy of mast-cellmediated diseases. Current Pharmaceutical Design, 17, 3815-3822. doi:10.2174/138161211798357926
- Dippold, H.C., Ng, M.M., Farber-Katz, S.E., Lee, S.K., Kerr, M.L., Peterman, M.C., Sim, R., Wiharto, P.A., Galbraith, K.A., Madhavarapu, S., Fuchs, G.J., Meerloo, T., Farquhar, M.G., Zhou, H. and Field, S.J. (2009) GOLPH3 bridges phosphatidylinositol-4-phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell, 139, 337-351. doi:10.1016/j.cell.2009.07.052
- Millarte, V. and Farhan, H. (2012) The Golgi in cell migration: Regulation by signal transduction and its implications for cancer cell metastasis. Scientific World Journal, 2012, 498278. doi:10.1100/2012/498278
- Piao, H. and Mayinger, P. (2012) Growth and metabolic control of lipid signalling at the Golgi. Biochemical Society Transactions, 40, 205-209. doi:10.1042/BST20110637
- Kremmyda, L.S., Tvrzicka, E., Stankova, B. and Zak, A. (2011) Fatty acids as biocompounds: Their role in human metabolism, health and disease: a review. part 2: Fatty acid physiological roles and applications in human health and disease. Biomedical Papers, 155, 195-218. doi:10.5507/bp.2011.052
- Viiri, K., Mäki, M. and Lohi, O. (2012) Phosphoinositides as regulators of protein-chromatin interactions. Science Signaling, 5, e19. doi:10.1126/scisignal.2002917
- Barlow, C.A., Laishram, R.S. and Anderson, R.A. (2010). Nuclear phosphoinositides: A signaling enigma wrapped in a compartmental conundrum. Trends in Cell Biology, 20, 25-35.
- Irvine, R.F. (2006) Nuclear inositide signalling—Expansion, structures and clarification. Biochimica et Biophysica Acta, 1761, 505-508.
- Slotte, P.J. (2013) Molecular properties of various structurally defined sphingomyelins—Correlation of structure with function. Progress in Lipid Research, 52, 2, 206- 219. doi:10.1016/j.plipres.2012.12.001
- Bieberich, E. (2012) It’s a lipid’s world: Bioactive lipid metabolism and signaling in neural stem cell differentiation. Neurochemical Research, 37, 1208-1229. doi:10.1007/s11064-011-0698-5
- Souza, M.S., Magnarelli de Potas, G. and Pechén de D’ Angelo, A.M. (2004) Organophosphorous and organochlorine pesticides affect human placental phosphoinositides metabolism and PI-4 kinase activity. Journal of Biochemical and Molecular Toxicology, 18, 30-36. doi:10.1002/jbt.20003
- Downes, C.P. and Macphee, C.H. (1990) Myo-inositol metabolites as cellular signals. European Journal of Biochemistry, 193, 1-18. doi:10.1111/j.1432-1033.1990.tb19297.x
- Catt, K.J., Hunyady, L. and Balla, T. (1991) Second messengers derived from inositol lipids. Journal of Bioenergetics and Biomembranes, 23, 7-27.
- Tse, A. and Tse, F.W. (1998) α-adrenergic stimulation of cytosolic Ca2+ oscillations and exocytosis in identified rat corticotrophs. Journal of Physiology, 512, 385-393. doi:10.1111/j.1469-7793.1998.385be.x
- Kim, M.H., Choi, B.H., Jung, S.R., Sernka, T.J., Kim, S., Kim, K.T., Hille, B., Nguyen, T.D. and Koh, D.S. (2008) Protease-activated receptor-2 increases exocytosis via multiple signal transduction pathways in pancreatic duct epithelial cells. Journal of Biological Chemistry, 283, 18711-18720. doi:10.1074/jbc.M801655200
- Zaika, O., Zhang, J. and Shapiro, M.S. (2011) Combined phosphoinositide and Ca2+ signals mediating receptor specificity toward neuronal Ca2+ channels. Journal of Biological Chemistry, 286, 830-841. doi:10.1074/jbc.M110.166033
- Bohdanowicz, M., Cosío, G., Backer, J.M. and Grinstein, S. (2010) Class I and class III phosphoinositide 3-kinases are required for actin polymerization that propels phagosomes. Journal of Cell Biology, 191, 999-1012.
- Bohdanowicz, M. and Grinstein, S. (2013) Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiological Reviews, 93, 69-106.
- Wen, P.J., Osborne, S.L. and Meunier, F.A. (2012) Phosphoinositides in neuroexocytosis and neuronal diseases. Current Topics in Microbiology and Immunology, 362, 87-98.
- Trebak, M., Lemonnier, L., DeHaven, W.I., Wedel, B.J., Bird, G.S. and Putney Jr., J.W. (2009) Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. European Journal of Physiology, 457, 757-769. doi:10.1007/s00424-008-0550-1
- Criswell, K.A., Loch-Caruso, R. and Stuenkel, E.L. (1995) Lindane inhibition of gap junctional communication in myometrial myocytes is partially dependent on phosphoinositide-generated second messengers. Toxicology and Applied Pharmacology, 130, 280-293. doi:10.1006/taap.1995.1033
- Balduini, W., Cimino, M., Renò, F., Marini, P., Princivalle, A. and Cattabeni, F. (1993) Effects of postnatal or adult chronic acetylcholinesterase inhibition on muscarinic receptors, phosphoinositide turnover and m1 mRNA expression. European Journal of Pharmacology, 248, 281-288.
- Sun, X., Liu, X.B., Martinez, J.R. and Zhang, G.H. (2000) Effects of low concentrations of paraoxon on Ca2+ mobilization in a human parotid salivary cell-line HSY. Archives of Oral Biology, 45, 621-638. doi:10.1016/S0003-9969(00)00043-1
- Zhang, H., Liu, J. and Pope, C.N. (2002) Age-related effects of chlorpyrifos on muscarinic receptor-mediated signaling in rat cortex. Archives of Toxicology, 75, 676-684. doi:10.1007/s00204-001-0309-3
- Suh, P.G., Park, J.I., Manzoli, L., Cocco, L., Peak, J.C., Katan, M., Fukami, K., Kataoka, T., Yun, S. and Ryu, S.H. (2008) Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Report, 41, 415-434. doi:10.5483/BMBRep.2008.41.6.415
- Drin, G. and Scarlata, S. (2007) Stimulation of phospholipase Cbeta by membrane interactions, interdomain movement, and G protein binding—How many ways can you activate an enzyme? Cell Signal, 19, 1383-1392. doi:10.1016/j.cellsig.2007.04.006
- Suzuki, K., Saito, S.Y. and Ishikawa, T. (2012) Involvement of phosphatidylcholine-specific phospholipase C in thromboxane A2 receptor-mediated extracellular Ca2+ influx in rat aorta. European Journal of Pharmacology, 677, 123-130. doi:10.1016/j.ejphar.2011.12.005
- Strielkov, I.V., Kizub, I.V., Khromov, A.S. and Soloviev, A.I. (2013) Evidence for the role of phosphatidylcholinespecific phospholipase C in sustained hypoxic pulmonary vasoconstriction. Vascular Pharmacology, 58, 292-298. doi:10.1016/j.vph.2013.02.002
- Xia, L., Zhang, D., Wang, C., Wei, F. and Hu, Y. (2012). PC-PLC is involved in osteoclastogenesis induced by TNF-α through upregulating IP3R1 expression. FEBS Letters, 586, 3341-3348. doi:10.1016/j.febslet.2012.07.015
- Ernsberger, P., Friedman, J.E. and Koletsky, R.J. (1997) The I1-imidazoline receptor: From binding site to therapeutic target in cardiovascular disease. Journal of hypertension. Supplement, 15, S9-S23. doi:10.1097/00004872-199715011-00002
- Li, H., Zhang, L., Yin, D., Zhang, Y. and Miao, J. (2010) Targeting phosphatidylcholine-specific phospholipase C for atherogenesis therapy. Trends in Cardiovascular Medicine, 20, 172-176. doi:10.1016/j.tcm.2011.02.002
- Shao, J., Sun, C., Su, L., Zhao, J., Zhang, S. and Miao, J. (2012) Phosphatidylcholine-specific phospholipase C/ heat shock protein 70 (Hsp70)/transcription factor B-cell translocation gene 2 signaling in rat bone marrow stromal cell differentiation to cholinergic neuron-like cells. The International Journal of Biochemistry & Cell Biology, 44, 2253-2260. doi:10.1016/j.biocel.2012.09.013
- Abalsamo, L., Spadaro, F., Bozzuto, G., Paris, L., Cecchetti, S., Lugini, L., Iorio, E., Molinari, A., Ramoni, C. and Podo, F. (2012) Inhibition of phosphatidylcholinespecific phospholipase C results in loss of mesenchymal traits in metastatic breast cancer cells. Breast Cancer Research, 14, R50. doi:10.1186/bcr3151
- Moya de Juri, M.G., Magnarelli De Potas, G. and Pechen de D’Angelo, A.M. (2002) Alteration of thrombine-signaling mechanism by heptachlor in human platelets. Journal of Biochemical and Molecular Toxicology, 16, 189- 196. doi:10.1002/jbt.10037
- Lu, C.H, Lee, K.C., Chen, Y.C., Cheng, J.S., Yu, M.S., Chen, W.C. and Jan, C.R. (2000) Lindane (gamma-hexachlorocyclohexane) induces internal Ca2+ release and capacitative Ca2+ entry in Madin-Darby canine kidney cells. Pharmacology Toxicology, 87, 149-155. doi:10.1034/j.1600-0773.2000.d01-65.x
- Hansen, M.E. and Matsumura, F. (2001) Effects of heptachlor epoxide on components of various signal transduction pathways important in tumor promotion in mouse hepatoma cells. Determination of the most sensitive tumor promoter related effect induced by heptachlor epoxide. Toxicology, 160, 139-153. doi:10.1016/S0300-483X(00)00445-5
- Katz, L.S. and Marquis, J.K. (1992) Organophosphateinduced alterations in muscarinic receptor binding and phosphoinositide hydrolysis in the human SK-N-SH cell line. Neurotoxicology, 13, 365-78.
- Magnarelli de Potás, G.M. and Pechén de D’Angelo, A.M. (1993) Phosphoinositide phosphorylation and shape changes produced by phosmet-oxon in human erythrocytes. Comparative Biochemistry and Physiology Part C, 106, 561-566.
- Pascual, F. and Carman, G.M. (2013) Phosphatidate phosphatase, a key regulator of lipid homeostasis. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1831, 514-522. doi:10.1016/j.bbalip.2012.08.006
- Dwyer, J.R., Donkor, J., Zhang, P., Csaki, L.S., Vergnes, L., Lee, J.M., Dewald, J., Brindley, D.N., Atti, E., Tetradis, S., Yoshinaga, Y., De Jong, P.J., Fong, L.G., Young, S.G. and Reue, K. (2012) Mouse lipin-1 and lipin-2 cooperate to maintain glycerolipid homeostasis in liver and aging cerebellum. Proceedings of the National Academy of Sciences of the United States of America, 109, E2486- E2495. doi:10.1073/pnas.1205221109
- Siniossoglou, S. (2013) Phospholipid metabolism and nuclear function: Roles of the lipin family of phosphatidic acid phosphatases. Biochimica et Biophysica Acta (BBA)- Molecular and Cell Biology of Lipids, 1831, 575-581. doi:10.1016/j.bbalip.2012.09.014
- Valdearcos, M., Esquinas, E., Meana, C., Gil-de-Gómez, L., Guijas, C., Balsinde, J. and Balboa, M. A. (2011) Subcellular localization and role of lipin-1 in human macrophages. Journal of Immunology, 186, 6004-6013. doi:10.4049/jimmunol.1003279
- Domart, M.C., Hobday, T.M., Peddie, C.J., Chung, G.H., Wang, A., Yeh, K., Jethwa, N., Zhang, Q., Wakelam, M.J., Woscholski, R., Byrne, R.D., Collinson, L.M., Poccia, D.L. and Larijani, B. (2012) Acute manipulation of diacylglycerol reveals roles in nuclear envelope assembly & endoplasmic reticulum morphology. PLoS One, 7, e51150. doi:10.1371/journal.pone.0051150
- Mall, M., Walter, T., Gorjánácz, M., Davidson, I.F., Nga Ly-Hartig, T.B., Ellenberg, J. and Mattaj, I.W. (2012) Mitotic lamin disassembly is triggered by lipid-mediated signaling. The Journal of Cell Biology, 198, 981-990. doi:10.1083/jcb.201205103
- Kunkel, M.T. and Newton, A.C. (2010) Calcium transduces plasma membrane receptor signals to produce diacylglycerol at Golgi membranes. The Journal of Biological Chemistry, 285, 22748-22752. doi:10.1074/jbc.C110.123133
- Bomser, J.A., Quistad, G.B. and Casida, J.E. (2002) Chlorpyrifos oxon potentiates diacylglycerol-induced extracellular signal-regulated kinase (ERK 44/42) activation, possibly by diacylglycerol lipase inhibition. Toxicology and Applied Pharmacology, 178, 29-36. doi:10.1006/taap.2001.9324
- Ramos, I., Cisint, S.B., Crespo, C.A., Medina, M.F. and Fernández, S.N. (2009) Subcellular localization of calcium and Ca-ATPase activity during nuclear maturation in Bufo arenarum oocytes. Zygote, 17, 253-260. doi:10.1017/S0967199409005334
- Stith, B.J., Goalstone, M., Silva, S. and Jaynes, C. (1993) Inositol 1,4,5-trisphosphate mass changes from fertilization through first cleavage in Xenopus laevis. Molecular Biology of the Cell, 4, 435-443.
- Ciapa, B., Borg, B. and Whitaker, M. (1992) Polyphospho-inositide metabolism during the fertilization wave in sea urchin eggs. Development, 115, 187-195.
- Santella, L., Lim, D. and Moccia, F. (2004) Calcium and fertilization: The beginning of life. Trends in Biochemical Sciences, 29, 400-408. doi:10.1016/j.tibs.2004.06.009
- Shearer, J., De Nadai, C., Emily-Fenouil, F., Gache, C., Whitaker, M. and Ciapa, B. (1999) Role of phospholipase Cgamma at fertilization and during mitosis in sea urchin eggs and embryos. Development, 126, 2273-2284.
- Runft, L.L., Carroll, D.J., Gillett, J., Giusti, A.F., O’Neill, F.J. and Foltz, K.R. (2004) Identification of a starfish egg PLC-gamma that regulates Ca2+ release at fertilization. Developmental Biology, 269, 220-236. doi:10.1016/j.ydbio.2004.01.031
- Yin, X. and Eckberg, W.R. (2009) Characterization of phosphor-lipases C beta and gamma and their possible roles in Chaetopterus egg activation. Molecular Reproduction and Development, 76, 460-470. doi:10.1002/mrd.20961
- Tokmakov, A.A., Sato, K.I., Iwasaki, T. and Fukami, Y. (2002) Src kinase induces calcium release in Xenopus egg extracts via PLCgamma and IP3-dependent mechanism. Cell Calcium, 32, 11-20. doi:10.1016/S0143-4160(02)00078-7
- Halet, G., Tunwell, R., Balla, T., Swann, K. and Carroll, J. (2002) The dynamics of plasma membrane PtdIns(4,5)P2 at fertilization of mouse eggs. Journal of Cell Science, 115, 2139-2149.
- Ito, J., Yoon, S.Y., Lee, B., Vanderheyden, V., Vermassen, E., Wojcikiewicz, R., Alfandari, D., De Smedt, H., Parys, J.B. and Fissore, R.A. (2008.) Inositol 1,4,5-trisphosphate receptor 1, a widespread Ca2+ channel, is a novel substrate of polo-like kinase 1 in eggs. Developmental Biology, 320, 402-413. doi:10.1016/j.ydbio.2008.05.548
- Wakai, T., Vanderheyden, V., Yoon, S.Y., Cheon, B., Zhang, N., Parys, J.B. and Fissore, R.A. (2012) Regulation of inositol 1,4,5-trisphosphate receptor function during mouse oocyte maturation. Journal of Cellular Physiology, 227, 705-717. doi:10.1002/jcp.22778
- Lee, B., Vermassen, E., Yoon, S.Y., Vanderheyden, V., Ito, J., Alfandari, D., De Smedt, H., Parys, J.B. and Fissore R.A. (2006) Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: A role for the MAP kinase pathway. Development, 133, 4355-4365. doi:10.1242/dev.02624
- Igarashi, H., Knott, J.G., Schultz, R.M. and Williams, C.J. (2007) Alterations of PLCbeta1 in mouse eggs change calcium oscillatory behavior following fertilization. Developmental Biology, 312, 321-330. doi:10.1016/j.ydbio.2007.09.028
- Kashir, J., Heindryckx, B., Jones, C., De Sutter, P., Parrington, J. and Coward, K. (2010) Oocyte activation, phospholipase C zeta and human infertility. Human Reproduction Update, 16, 690-703. doi:10.1093/humupd/dmq018
- Chun, J.T., Puppo, A., Vasilev, F., Gragnaniello, G., Garante, E. and Santella, L. (2010) The biphasic increase of PIP2 in the fertilized eggs of starfish: New roles in actin polymerization and Ca2+ signaling. PLoS One, 5, e14100. doi:10.1371/journal.pone.0014100
- Stith, B.J., Woronoff, K., Espinoza, R. and Smart, T. (1997) Sn-1,2-diacylglycerol and choline increase after fertilization in Xenopus laevis. Molecular Biology of the Cell, 8, 755-765.
- Morrill, G.A. and Kostellow, A.B. (1999) Progesterone induces meiotic division in the amphibian oocyte by releasing lipid second messengers from the plasma membrane. Steroids, 64, 157-167. doi:10.1016/S0039-128X(98)00093-2
- Fonovich de Schroeder, T.M. and Pechén de D’Angelo, A.M. (1991) Dieldrin effects on phospholipid and phosphoinositide metabolism in Bufo arenarum oocytes. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 98, 287-292. doi:10.1016/0742-8413(91)90207-A
- Fonovich de Schroeder, T.M. and Pechén de D’Angelo, A.M. (1995a) Dieldrin modifies the hydrolysis of PIP2 and decreases the fertilization rate in Bufo arenarum oocytes. Comparative Biochemistry and Physiology-Part C, 112, 61-67.
- Fonovich de Schroeder, T.M. and Pechén de D’Angelo, A.M. (1995b) The effect of dieldrin on Clostridium perfringens phosphatidylcholine phospholipase C activity. Pesticide Biochemistry and Physiology, 51, 170-177. doi:10.1006/pest.1995.1017

