Advances in Biological Chemistry
Vol. 2 No. 2 (2012) , Article ID: 18971 , 9 pages DOI:10.4236/abc.2012.22013
“Smart fats”, healthy brain and function of lipid-sensing NMDA receptors
![]()
1School of Biomedical Sciences, University of Queensland, Brisbane, Australia
2Molecular Cardiology and Biophysics Division, Victor Chang Cardiac Research Institute and St Vincent’s Clinical School, University of New South Wales, Sydney, Australia
Email: a.kloda@uq.edu.au, B.Martinac@victorchang.edu.au
Received 26 March 2012; revised 19 April 2012; accepted 29 April 2012
Keywords: NMDA receptors; lipid bilayer; membrane phospholipids; fatty acids; synaptic plasticity; dementia; Alzheimer’s Disease
ABSTRACT
NMDA receptor channels play a significant role in learning and memory and their dysfunction can cause neuronal cell death leading to dementia. Research had shown that lipids change the risk for dementia, especially some omega-3 lipids appear to lower Alzheimer’s risk, yet only limited research exists on the modulation of NMDA receptor channels by lipids. Here we review recent literature concerning molecular determinants that influence the NMDA receptor channel gating via membrane lipids and fatty acids with profound significance for understanding how altered NMDA signalling leads to neuronal cell death linked to age-related dementia’s. Future discovery of lipid-like modulators of NMDA receptor function offer the potential for the development of new bioceuticals and affordable nutritional supplements to combat neuronal degeneration as well as to promote well being and healthy aging.
1. INTRODUCTION
The NMDA receptor is an oligomeric cation channel which mediates long-term potentiation, synaptic plasticity and neuro-degeneration via conditional Ca2+ signalling [1-3]. The channel requires coactivation by glutamate and glycine that bind to a ligand binding core [4]. The complexity of NMDA receptors arises from multiple genes encoding different subunits of the channel and alternative splicing of mRNA, which determines the variability in subunit composition, as well as functional heterogeneity. The ubiquitously expressed NR1 subunit is a product of a single gene encoding eight splice variants (Figures 1(a) and (b)) whereas four NR2 subunits (NR2A-NR2D) and two NR3 subunits are encoded by
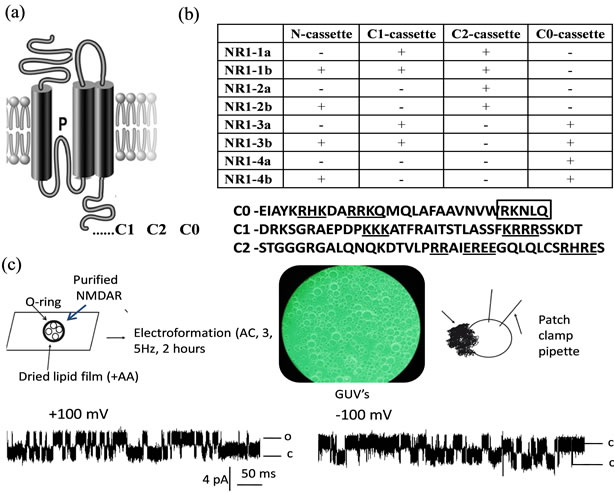
Figure 1. (a) Membrane topology and location of exon cassettes of the NMDA receptor NR1. Alternatively spliced Cterminal exon cassettes are marked as C1, C2, C0; (b) Upper eight splice variant of NMDA receptor NR1 formed by the presence of one N-terminal and three C-terminal cassettes. Below: Boxed are actinin binding residues within C0. Underlined are clusters of charged residues proposed to act as a lipid sensor; (c) Reconstituted NMDA receptor NR1 into Giant Unilammelar Vesicles (GUVs) prepared with Vesicle-Prep-Pro (Nanion technologies) form functional homomeric channel activated by agonists.
distinct genes and their products are differentially distributed throughout the central nervous system [5-7]. The transmembrane topology of NMDA receptor proteins predicts an extracellular N-terminus, followed by three transmembrane domains (TM1 - TM3) and intracellular C-terminus (Figure 1(a)). A cytoplasmic reentrant loop (P) lines the channel pore, whereas an extracellular loop links the TM2 and TM3 domains. In the NMDANR1 subunit the cytoplasmic C-terminus is the subject of alternative splicing (Figure 1(b)) which differentially affects its interaction with intracellular proteins and phospholipids [8].
Several studies suggest that NMDA receptors influence neuronal growth, synaptogenesis and neuronal plasticity as well as fine-tuning of neuronal connections. During development, NMDA receptors are important for neuronal survival, differentiation, and neuronal migration [9,10] as well as for formation and stabilization of synapses and neuronal circuits [11,12]. In the postnatal and adult brain, NMDA receptors act as molecular coincidence detectors of presynaptic and postsynaptic activity facilitated by pre-synaptic release of glutamate and simultaneous depolarization of the postsynaptic membrane that are requirements for the channel gating. Coincidence detection by the NMDA receptor depends on a voltagedependent channel block by extracellular Mg2+. This voltage-controlled Ca2+ influx via the NMDA receptor is thought to be essential for activity-dependent modulations in synaptic strength [1,13]. De-regulation of NMDA receptor function has been associated with excitotoxic cell death where neuronal cells are damaged and killed by excessive stimulation by glutamate and excitotoxins. NMDA receptor-mediated excitotoxicity has been implicated in many important human brain pathologies, ranging from amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinson’s disease, depression, epilepsy, trauma and stroke to schizophrenia [14].
2. SUBUNIT COMPOSITION PRESENTS A CHALLENGE TO STUDY NMDA RECEPTOR’S FUNCTION
The subunit composition of the N-methyl-D-aspartate (NMDA) glutamate receptor affects both its channel activity and its sensitivity to modulation by a wide variety of substances. Despite research in this area, currently the exact subunit/splice variant composition of native NMDA receptor channels is not known although there is some evidence that the functional NMDA receptor forms a heterotetrameric channel composed of two NR1 and two NR2 subunits and multiple NMDA receptor channel isoforms exist resulting from selective splicing of the NR1 transcripts and differential expression of the NR2 subunits [15]. Different physiological studies indicate that endogenous neuronal postsynaptic NMDA receptors are heterooligomeric complexes composed of NR1 and NR2 subunits [5,16,17]. However immunohistological studies revealed the presence of homomeric NMDANR1 receptors in the hippocampus, visual cortex, amygdale, stria terminalis [18-20]. The most commonly studied NMDANR1 splice variant NR1a forms a functional channel when heterologously expressed in oocytes, yeast strains but not certain mammalian cell lines, whereas four modulatory NR2 subunits function only if co-expressed with any of the NR1 subunits in any heterologous expression system [21-25]. However, functional homomeric complexes composed of NR1 subunits have been reported to exist in the central nervous system [26]. Electrophysiological studies of reconstituted NMDA receptor complexes isolated from total brain membranes showed a multi-conductance state behaviour which could be a result of several multimeric subunits of the receptor present in native neuronal membranes [27].
High Ca2+ permeability and its control by voltage-dependent Mg2+ block are defining features of NMDA receptors [28,29]. These features are lost if the principal NR1 subunit carries an asparagine (N) to arginine (R) substitution in the pore forming region of M2 on the NR1 subunit (residue 598). Such substitution not only abolishes Ca2+ permeability and voltage-dependent Mg2+ block [30,31] but also reduces single channel conductance [32] and modulates potentiation and block by polyamines, inhibition by protons and Zn2+, and channel sensitivity to glutamate and glycine [33-36]. Furthermore, mice that express mutant NMDA receptors in position 598 of the NR1 subunit developed dysfunction in the nervous system with phenotypes ranging from reduced life expectancy, growth retardation, impaired nurturing reflex in females to death before weaning [37]. This indicates that the activity-dependent Ca2+ influx through the NMDA receptor channels controlled by NMDANR1 subunit is likely to play an essential role in synaptic transmission in the central nervous system. Neuronal excitotoxicity is thought to be dependent on subunit composition of individual NMDA receptor complexes which greatly affect the electrophysiological and pharmacological properties of the intrinsic receptor channel. There is evidence that native NMDA NR1 proteins have distinct biochemical and functional properties and differ in stability and molecular weight [38,39]. This heterogeneity has a further implication in the pathology of Alzheimer’s disease. Alzheimer’s disease, a major cause of dementia in the elderly, is characterized by selective neuronal degeneration within specific sub-regions of the hippocampus. Biochemical and molecular studies have demonstrated alteration in NMDA receptor subunits within a specific region of the hippocampus during Alzheimer’s disease progression [40].
Despite the physiological significance of NMDA NR1 subunit to channel function the homomeric NMDA NR1 receptors have not been studied thoroughly due to the difficulty to isolate single NMDANR1 currents in neuronal cells. Another difficulty that such studies present is the inability to determine which splice variant of the NMDANR1 receptor form functional channels at synapses. New methods of study of ion channels have emerged recently to address this issue. The extensively researched reconstitution methods of heterologously expressed recombinant proteins into liposomes and giant unilamellar vesicles (GUV) allow study of ion channels in isolated systems without complex cellular interactions [41]. We have successfully purified and reconstituted NMDA receptor channels into liposomes and GUV made of soy bean azolectin and performed single channel recordings using the standard patch-clamp techniques [42]; (Figure 1(c)). Our results suggests that the splice variant, NMDANR1(3b), heterologously expressed and purified from mammalian cell line HEK-293 can form a functional homomeric channel when reconstituted into artificial lipid bilayers made from phospholipids and fatty acids (Figure 1(c)). Taken together current evidence suggests that some endogenous NMDANR1 splice variants may exist as functional homomeric complexes in neuronal cells and therefore play an important role in synaptic transmission.
3. MODULATION OF NMDA RECEPTOR CHANNEL BY LIPID BILAYER
NMDA receptors are regulated by polymodal stimuli. The enhancement of NMDA receptor responses by fatty acids, osmotic forces, membrane phospholipids and membrane stretch [43-45]; Kloda et al., 2007 [42-46] has been previously reported. Since lipids form a matrix for insertion of ion channels, it is not surprising that any changes to cell membrane, induced by either physical or chemical stimuli, could affect gating properties of ion channels which are sensitive to such changes. In fact membrane fluidity is determined by the presence of fatty acids (increase membrane fluidity) and the phospholipids/free cholesterol ratio, as cholesterol increases membrane viscosity [47,48]. Thus a diet based on a high proportion of essential polyunsatured fatty acids would allow a higher incorporation of cholesterol in the membranes to balance their fluidity that would further limit the availability of cholesterol in the blood stream.
The correlation of membrane expansion induced by either mechanical or chemical stimuli with the block of the NMDA receptor channel indicates that the synaptic transmission can be altered if NMDA receptor complexes experience local changes in the lipid environment. Such changes may be caused by either dynamic targeting of “raft” proteins to lipid microdomains and modification of biophysical properties of cell membranes due to e.g. insertion of lipophilic compounds. Lipid rafts are distinct parts of the cell membrane enriched in cholesterol and sphingolipids and could affect the function of NMDA receptor channels directly via changes in biophysical properties of the lipid bilayer, which can occur during “raft” recruitment as well as changes in membrane fluidity due to the distinct composition of raft lipids. These microdomains play an important role in cellular signalling processes [49-51]. Indeed, there is evidence that glutamate signalling via Ca2+ influx is regulated by lipid rafts [14]. Although functional regulation of NMDA receptor channels seems to be associated with complex molecular systems including signalling proteins, it is possible that other aspects of the membrane environment play a significant role in this process. NMDA receptor channels have been shown to be associated with lipid rafts [52] and lipid rafts have been found to play a critical role in the maintenance and function of synapses [53,54]. There is evidence that lipid rafts can modulate the function of NMDA receptor channels [14]. Furthermore, depletion of plasma membrane cholesterol plays a critical role in NMDA-receptor mediated Ca2+ influx in hippocampal cultured neurons and has a dramatic effect on neuronal excitability [54]. Thus excitotoxicity, which in AD is mainly glutamate and NMDA receptor dependent, could occur due to altered membrane fluidity. This in turn could affect the functional properties of NMDA receptors such as Mg2+ block via the bilayer mechanism [42], further indicating that the function of the NMDA receptor as a molecular coincidence detector may not only be controlled by voltage but also by the membrane bilayer deformation forces.
4. MODULATION OF NMDA RECEPTORS VIA MEMBRANE LIPIDS
Evidence shows that NMDA receptor channels are potential targets for membrane phospholipids [8,44]. Lysophospholipids inhibit NMDA-mediated responses further suggesting that the membrane tension and/or curvature are important modulators of the NMDA receptor function [44,42]. In contrast Phosphatidylinositol-4,5-Biphosphate (PIP2), which has been reported to modulate the activity of many other ion channels and transporters [55] is proposed to regulate NMDA receptor activity through interaction with alpha-actinin. A recent study demonstrated that the NMDANR1 subunit forms complexes with actin protein and further, the authors identified sites on the NMDANR1 subunit that sense PIP2 [8].
The C-terminus of the NR1 subunit can be divided into three domains: C0, C1 and C2 (Figures 1(a) and (b)). C-terminal domains of NMDA NR1 subunit are not only targets for alternative splicing [56] but also harbour sites for protein-protein interaction of the NMDA receptor channel with e.g. tubulin, spectrin, calmodulin and others [57,58]; 1997 [59-62]. According to [8] it is a C0 domain of NMDA NR1 subunit that is most likely involved in PIP2 sensing via binding of alpha actinin. The proposed model suggests that alpha-actinin tethers C-terminal regions of the NR 1 subunit of NMDA receptors and PIP2 in the plasma membrane to keep the channel open. Upon cleavage of PIP2 by phospholipase C (PLC) into inositol triphosphate and diacylglycerol, alpha actinin detaches from the membrane resulting in channel closure and suppression of currents through the NMDA receptor channel. Indeed previous studies have suggested that displacement of alpha-actinin by calmodulin is responsible for calcium-dependent inactivation of NMDA receptors [60,63]. On the other hand actin depolymerization has been reported to reduce NMDA channel activity [64], decrease the number of synaptic NMDA receptor clusters [65], and trigger long-term depression of NMDA synaptic responses in the hippocampus [66]. Alpha actinin has been shown to interact with not only proteins including PDZ-type proteins, certain types of kinases and collagen [67-69] but also membrane lipids including diacylglycerol (DG) and palmitic acid (PA) [70], PIP2 [71,72] as well as other negatively charged phospholipids [73-75]. Because PIP2 concentration on the cytosolic leaflet at the plasma membrane undergoes a constant cycle of regeneration and breakdown [55,76], it is conceivable that any perturbation of this pathway is likely to affect the functioning of ion channels that are regulated by phospholipids such as PIP2. These events have physiological significance as PIP2 are cleaved in response to various stimuli including activating G-coupled receptors that couple to phospholipase C such as acetylcholine receptor. Indeed physiological studies showed that blocking PIP2 synthesis or stimulating PIP2 hydrolysis reduces NMDAR-mediated currents in cortical neurons and formation of the NMDA-receptor depended long term depression [77,78]. Furthermore, a reduced level of phosphoinositides in the brain has been linked to symptoms of Alzheimer’s disease [79-81].
Many pharmacological probes such as poly-lysine and spermine show a spectrum of effects on NMDA receptor channels including voltage-dependent block as well as glycine-dependent and glycine-independent enhancement of NMDA-mediated responses [82-84]. These cationic molecules have a high affinity for anionic phospholipids that could modulate NMDA receptor function by interacting with phospholipid sensing domain of the receptor protein. Indeed a cluster of charged residues has been identified within the C-terminus of NMDA NR1 C0 and C1 splice variants (Figure 1(b)) and may act as a phospholipid sensor. A similar cluster of positive charges in the proximal C-terminal domain of TREK-1 is central to the effect of phospholipids on the channel function. This cationic region is required for the interaction between the C-terminal domain of TREK-1 and the plasma membrane lipids [85] and has been shown to inhibit TREK-1 currents [85,86]. It would be of great interest to investigate further the role of C-terminal domains of NMDA receptor splice variants and their possible role in phospholipid sensing.
5. REGULATION OF NMDA RECEPTORS BY FATTY ACIDS—POTENTIAL “BRAIN FOOD”
Modulation of NMDA receptor channels by fatty acids including arachidonic acid and linoleic acid have been reported and could occur either via changes in biophysical properties of the lipid bilayer such as curvature or a direct effect via a binding site [8,42,87,88]. Similar to phospholipids, fatty acids are components of all biological membranes and their presence increases membrane fluidity [47]. Furthermore, the NMDANR1 subunit contains a sequence similar to that of a family of fatty acid binding proteins [88].
Several studies suggest that a positive feedback mechanism may exist between fatty acids and NMDA receptor activation. This is because initial Ca2+ influx through activated NMDA receptor channels stimulates endogenous phospholipase A2 [89] and thus release of more fatty acids from cellular membranes. Liberation of these fatty acids into intracellular fluid further potentiates NMDA receptor responses [90]. Omega-3 and omega-6 fatty acids have been shown to stimulate cell membrane expansion at the growth cones indicating their role in neurogenesis [91], which is thought to be mediated via NMDA receptor channels. This further indicates that the composition of the lipid bilayer is an important modulator of the NMDA receptor function and could play an important role in NMDA receptor mediated signalling, synaptogenesis and excitotoxicity. Recently we have demonstrated that in the liposome system, stretching of the lipid bilayer, as well as the application of arachidonic acid, alleviate Mg2+ block and potentiate currents through recombinant NMDA receptor channels [42]. Similar results have been obtained with the use of linoleic acid [87]. This further indicates that the composition of the lipid bilayer is an important modulator of the NMDA receptor function and could play an important role in NMDA receptor mediated signalling, synaptogenesis and excitotoxicity. It can also provide an explanation as to why the functioning of certain types of NMDANR1 splice variants depends on the cell type they are expressed in. Interestingly omega-3 and omega-6 fatty acids have been shown to stimulate cell membrane expansion at the growth cones indicating their role in neurogenesis [91], which is thought to be mediated via NMDA receptor channels.
One physiological consequence of the effect of fatty acids on the block and permeability of NMDA receptors could be its interference with LTP. Indeed, there is evidence that DHA is crucial for induction of LTP and the inhibition of phospholipase A2 (to block arachidonic acid release) prevents induction of LTP in the hippocampal CA1 region [92]. Interestingly, the dietary intake of DHA enhanced the learning ability and improved spatial cognition of rodents [93,95] further indicating the importance of fatty acids in learning and memory. Indeed a deficiency in DHA is associated with a loss of learning ability and visual acuity in several mammalian species [93,95]. Moreover, epidemiological studies suggest that patients with Alzheimer’s disease show extremely low levels of DHA in their brains [96]. Fatty fish are the major source of DHA and reduced fish or DHA intake increases risk for Alzheimer’s disease [97]. The DHA depletion aggravates cognitive deficits in mouse whereas DHA supplementation is neuroprotective and slows down pathogenesis of Alzheimer’s disease [98].
Several epidemiological studies identified low dietary intake of omega-3 polyunsaturated fatty acids as a candidate risk factor for Alzheimer’s disease. Omega-3 (present in linseed oil, nuts, soya beans, wheat and cold water fish) and omega-6 (present in maize, sunflower and sesame oil) are two types of essential polyunsaturated fatty acids (PUFA) that cannot be synthetised by the organism and have to be obtained from diet. Brain membranes have a very high content of essential polyunsatured fatty acids and omega-3 and omega-6 are necessary for the differentiation and functioning of cultured brain cells [99]. Furthermore, a reduction in both omega- 3 and omega-6 types of fatty acids (decosahexaenoic and arachidonic acids respectively) is observed during ageing [99]. Dietary lack of essential polyunsatured fatty acids (in particular omega-3) has been reported to influence cerebral development, affect learning efficiency and memorizing tasks, as well as prevent neuropsychiatric disorders, particularly depression and dementia, including Alzheimer's disease [47,99] all of which are NMDA receptor mediated. From the historical perspective, an increase in consumption of animal tissue rich in polyunsaturated fatty acids such as bone marrow (another source of PUFA) and diet including marine resources during human evolution is believed to mark the origins of modern humans and the appearance of complex cognition [100].
6. EPIDEMIOLOGICAL STUDY LINK TO DEMENTIA
There is a growing body of evidence that disturbances of NMDA receptor based neurotransmission may underlie the pathogenesis and cognitive deficits of Alzheimer’s dementia [101,102]. In Australia the prevalence of dementia seems to increase continuously especially among people from low socio-economic backgrounds including Aboriginal people. For example recent study [103] found that 27% of Aboriginal Australians over the age of 65 years living in the Kimberley region have dementia. The high prevalence is at odds with what has consistently been found in other populations with low literacy, smoking and a western diet. Aboriginal people used to be hunter-gatherers whose daily diet varied according to the type of food available in the particular location and season. Their diet included variety of plants, nuts, reptiles and extensive range of marine animals all of them rich in essential fatty acids. Since the European arrival, the traditional Aboriginal diet shifted towards processed western foods which are very low in essential fatty acids but high in fat and cholesterol. Thus it is possible that diet and increased prevalence of smoking which causes lipid peroxidation—a marker of aging brain, are a potential causal factor for the high rates of non-specified dementia reported in certain population groups.
7. CONCLUSIONS
The biological and nutritional aspects of polyunsaturated fatty acids and dietary fish oils have long been recognized. The omega-3 linolenic and omega-6-linoleic acids are plant derived dietary supplements which are essential for all mammals. These fatty acids play an important role in human health including brain development and neuronal regeneration [104,105]. Many dietary supplements of essential fatty acids are freely available and can be taken safely in high doses and thus are suitable for primary prevention of neurodegenerative disorders. Therefore, a better understanding of the role of phospholipids and fatty acids and their action on different NMDA receptor subunits could aid in developing novel lipophilic therapeutic agents and nutritional strategies for better treatment and prevention of these important medical conditions.
Further study towards understanding cellular processes involved in regulation of NMDA receptors by lipid bilayer and cytoskeletal proteins and protein-lipid interaction that affects channel gating will shed new light on the molecular mechanism of NMDA-receptor mediated plasticity that underlies memory formation. Better understanding of how memories are formed could lead to new treatments for age related dementias such as Alzheimer’s disease.
8. ACKNOWLEDGEMENTS
Supported by ARC grant DP0771341 from Australian research Council, Australia.
![]()
![]()
REFERENCES
- Bliss, T.V. and Collingridge, G.L. (1993) A synaptic model of memory: Long-term potentiation in the hippocampus. Nature, 361, 31-39 doi:10.1038/361031a0
- Castellano, C., Cestari, V. and Ciamei, A. (2001) NMDA receptors in learning and memory processes. Current Drug Targets, 2, 273-283. doi:10.2174/1389450013348515
- Cull-Candy, S., Brickley, S. and Farrant, M. (2001) NMDA receptor subunits: Diversity, development and disease. Current Opinion in Neurobiology, 11, 327-335. doi:10.1016/S0959-4388(00)00215-4
- Furukawa, H. and Gouaux, E. (2003) Mechanisms of activation, inhibition and specificity: Crystal structures of the NMDA receptor NR1 ligand-binding core. The EMBO Journal, 22, 2873-2885. doi:10.1093/emboj/cdg303
- Dingledine, R., Borges, K., Bowie, D. and Traynelis, S.F. (1999) The glutamate receptor ion channels. Pharmacological Reviews, 51, 7-61.
- Kohr, G. (2006) NMDA receptor function: Subunit composition versus spatial distribution. Cell and Tissue Research, 326, 439-446. doi:10.1007/s00441-006-0273-6
- Paoletti, P. and Neyton, J. (2007) NMDA receptor subunits: Function and pharmacology. Current Opinion in Neurobiology, 7, 39-47. doi:10.1016/j.coph.2006.08.011
- Michailidis, I.E., Helton, T.D., Petrou, V.I., Mirshahi, T., Ehlers, M.D. and Logothetis, D.E. (2007) Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin. The Journal of Neuroscience, 27, 5523-5532. doi:10.1523/JNEUROSCI.4378-06.2007
- Brewer, G.J. and Cotman, C.W. (1989) NMDA receptor regulation of neuronal morphology in cultured hippocampal neurons. Neuroscience Letters, 99, 268-273. doi:10.1016/0304-3940(89)90458-8
- Komuro, H. and Rakic, P. (1993) Modulation of neuronal migration by NMDA receptors. Science, 260, 95-97. doi:10.1126/science.8096653
- Constantine-Paton, M., Cline, H.T. and Debski, E. (1990) Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annual Review of Neuroscience, 13, 129-154. doi:10.1146/annurev.ne.13.030190.001021
- Fox, K. and Daw, N.W. (1993) Do NMDA receptors have a critical function in visual cortical plasticity? Trends in Neurosciences, 16, 116-122. doi:10.1016/0166-2236(93)90136-A
- Malenka, R.C. and Nicoll, R.A. (1993) NMDA-receptor-dependent synaptic plasticity: Multiple forms and mechanisms. Trends in Neurosciences, 16, 521-527. doi:10.1016/0166-2236(93)90197-T
- Schrattenholz, A. and Soskic, V. (2006) NMDA receptors are not alone: Dynamic regulation of NMDA receptor structure and function by neuregulins and transient cholesterol-rich membrane domains leads to disease-specific nuances of glutamate-signalling. Current Topics in Medicinal Chemistry, 6, 663-686. doi:10.2174/156802606776894519
- Sugihara, H., Moriyoshi, K., Ishii, T., Masu, M. and Nakanishi, S. (1992) Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochemical and Biophysical Research Communications, 185, 826-832. doi:10.1016/0006-291X(92)91701-Q
- Hollmann, M. and Heinemann, S. (1994) Cloned glutamate receptors. Annual Review of Neuroscience, 17, 31- 108. doi:10.1146/annurev.ne.17.030194.000335
- Moriyoshi, K., Masu, M., Ishii, T., Shigemoto, R., Mizuno, N. and Nakanishi, S. (1991) Molecular cloning and characterization of the rat NMDA receptor. Nature, 354, 31-37. doi:10.1038/354031a0
- Siegel, S.J., Brose, N., Janssen, W.G., Gasic, G.P., Jahn, R., Heinemann, S.F. and Morrison, J.H. (1994) Regional, cellular, and ultrastructural distribution of N-methyl-Daspartate receptor subunit 1 in monkey hippocampus. Proceedings of the National Academy of Sciences USA, 91, 564-568. doi:10.1073/pnas.91.2.564
- Aoki, C., Venkatesan, C., Go, C.G., Mong, J.A. and Dawson, T.M. (1994) Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. The Journal of Neuroscience, 14, 5202-5222.
- Gracy, K.N. and Pickel, V.M. (1995) Comparative ultrastructural localization of the NMDAR1 glutamate receptor in the rat basolateral amygdala and bed nucleus of the stria terminalis. The Journal of Comparative Neurology, 362, 71-85.
- Kutsuwada, T., Kashiwabuchi, N., Mori, H., Sakimura, K., Kushiya, E., Araki, K., Meguro, H., Masaki, H., Kumanishi, T., Arakawa, M. and Mishina, M. (1992) Molecular diversity of the NMDA receptor channel. Nature, 358, 36-41. doi:10.1038/358036a0
- Meguro, H., Mori, H., Araki, K., Kushiya, E., Kutsuwada, T., Yamazaki, M., Kumanishi, T., Arakawa, M., Sakimura, K. and Mishina, M. (1992) Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature, 357, 70-74. doi:10.1038/357070a0
- Monyer, H., Sprengel, R., Schoepfer, R., Herb, A., Higuchi, M., Lomeli, H., Burnashev, N., Sakmann, B. and Seeburg, P.H. (1992) Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science, 256, 1217-1221. doi:10.1126/science.256.5060.1217
- Ishii, T., Moriyoshi, K., Sugihara, H., Sakurada, K., Kadotani, H., Yokoi, M., Akazawa, C., Shigemoto, R., Mizuno, N., Masu, M. and Nakanishi, S. (1993) Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. The Journal of Biological Chemistry, 268, 2836-2843.
- Becker, J., Li, Z. and Noe, C.R. (1998) Molecular and pharmacological characterization of recombinant rat/mice N-methyl-D-aspartate receptor subtypes in the yeast Saccharomyces cerevisiae. European Journal of Biochemistry, 256, 427-435. doi:10.1046/j.1432-1327.1998.2560427.x
- Wang, J.K.T. and Thukral, V. (1996) Presynaptic NMDA receptors display physiological characteristics of homomeric complexes of NR1 subunits that contain the exon 5 insert in the N-terminal domain. Journal of Neurochemistry, 66, 865-868. doi:10.1046/j.1471-4159.1996.66020865.x
- Aistrup, G.L., Szentirmay, M., Kumar, K.N., Babcock, K.K., Schowen, R.L. and Michaelis, E.K. (1996) Ion channel properties of a protein complex with characteristics of a glutamate/N-methyl-D-aspartate receptor. FEBS Letters, 394, 141-148. doi:10.1016/0014-5793(96)00938-6
- Mayer, M.L., Westbrook, G.L., Guthrie, P.B. (1984) Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurons. Nature, 309, 261-263. doi:10.1038/309261a0
- Nowak, L., Bregestovsky, P., Ascher, P., Herbet, A. and Prochiantz, A. (1984) Magnesium gates glutamate-activated channels in mouse central neurons. Nature, 307, 462-465. doi:10.1038/307462a0
- Burnashev, N., Monyer, H., Seeburg, P.H. and Sakmann, B. (1992) Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron, 8, 189-198. doi:10.1016/0896-6273(92)90120-3
- Kloda, A. and Adams, D.J. (2006) Mutations within the selectivity filter of the NMDA receptor-channel influence voltage dependent block by 5-hydroxytryptamine. British Journal of Pharmacology, 149, 163-169. doi:10.1038/sj.bjp.0706849
- BEhe, P., Stern, D.J.A., Wyllie, M., Nassar, R., Schoepfer, D. and Colquhoun, D. (1995) Determination of NMDA NR1 subunit copy number in recombinant NMDA receptors. Proceedings of the Royal Society of London Series B-Biological Sciences, 262, 205-213. doi:10.1098/rspb.1995.0197
- Kashiwagi, K., Pahk, A.J., Masuko, T., Igarashi, K. and Williams, K. (1997) Block and modulation of N-methylD-aspartate receptors by polyamines and protons: Role of amino acid residues in the transmembrane and pore-forming regions of NR1 and NR2 subunits. Molecular Pharmacology, 52, 701-713.
- Schneggenburger, R. and Ascher, P. (1997) Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron, 18, 167-177.
- Traynelis, S.F., Burgess, M.F., Zheng, F., Lyuboslavsky, P. and Powers, J.L. (1998) Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. The Journal of Neuroscience, 18, 6163-6175.
- Zheng, X., Zhang, L., Wang, A.P., Araneda, R.C., Lin, Y., Zukin, R.S. and Bennett, M.V.L. (1999) Mutation of structural determinants lining the N-methyl-D-aspartate receptor channel differentially affects phencyclidine block and spermine potentiation and block. Neuroscience, 93, 125-134. doi:10.1016/S0306-4522(99)00154-2
- Single, F.N., Rozov, A., Burnashev, N., Zimmermann, F., Hanley, D.F., Forrest, D., Curran, T., Jensen, V., Hvalby, O., Sprengel, R. and Seeburg, P.H. (2000) Dysfunctions in mice by NMDA receptor point mutations NR1(N598Q) and NR1(N598R). The Journal of Neuroscience, 20, 2558-2566.
- Hall, R. A. and Soderling, T.R. (1997) Differential surface expression and phosphorylation of the N-methyl-Daspartate receptor subunits NR1 and NR2 in cultured hippocampal neurons. The Journal of Biological Chemistry, 272, 4135-4140. doi:10.1074/jbc.272.7.4135
- Huh, K.H. and Wenthold, R.J. (1999) Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. The Journal of Biological Chemistry, 274, 151-157. doi:10.1074/jbc.274.1.151
- Mishizen-Eberz, A.J., Rissman, R.A., Carter, T.L., Iconomovic, M.D. Wolfe, B.B. and Armstrong, D.M. (2004) Biochemical and molecular studies of NMDA receptor subunits NR1/2A/2B in hippocampal subregions throughout progression of Alzheimer’s disease pathology. Neurobiology of Disease, 15, 80-92. doi:10.1016/j.nbd.2003.09.016
- Martinac, B., Rodhe, P.R., Battle, A.R., Petrov, E., Pal, P., Foo, A.F.W., Vasquez, V., Huynh, T. and Kloda, A. (2010) Studying mechanosensitive ion channels using liposomes. In: Weissig, V., Ed., Methods in Molecular Biology-Liposomes, Humana Press, London, 31-53.
- Kloda, A., Lua, L., Hall, R., Adams, D.J. and Martinac, B. (2007) Liposome reconstitution and modulation of recombinant N-methyl-D-aspartate receptor channels by membrane stretch. PNAS, 104, 1540-1545. doi:10.1073/pnas.0609649104
- Paoletti, P. and Ascher, P. (1994) Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron, 13, 645-655. doi:10.1016/0896-6273(94)90032-9
- Casado, M. and Ascher, P. (1998) Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: Common features with mechanosensitivity. Journal of Physiology, 513, 317-330. doi:10.1111/j.1469-7793.1998.317bb.x
- Johnson, J.-W. and Ascher, P. (1990) Voltage-dependent block by intracellular Mg2+ of N-methyl-D-aspartate-activated channels. Biophysical Journal, 57, 1085-1090. doi:10.1016/S0006-3495(90)82626-6
- Singh, P., Doshi, S., Spaethling, J.M., Hockenberry, A.J., Patel, T.P., Geddes-Klein, D.M., Lynch, D.R. and Meaney, D.F. (2012) N-methyl-D-aspartate receptor mechanosensitivity is governed by C terminus of NR2B subunit. The Journal of Biological Chemistry, 287, 4348-4359. doi:10.1074/jbc.M111.253740
- Colin, A., Reggers, J., Castronovo, V. and Ansseau, M. (2003) Lipids, depression and suicide. Encephale, 29, 49- 58.
- Bastiaanse, E.M., Höld, K.M. and Van der Laarse, A.T. (1997) The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovascular Research, 33, 272-283. doi:10.1016/S0008-6363(96)00193-9
- Ikonen, E. (2001) Roles of lipid rafts in membrane transport. Current Opinion in Cell Biology, 13, 470-477. doi:10.1016/S0955-0674(00)00238-6
- Simons, K. and Toomre, D. (2000) Lipid rafts and signal transduction. Nature Reviews Molecular Cell Biology, 1, 31-39. doi:10.1038/35036052
- Brown, D.A. and London, E. (1998) Functions of lipid rafts in biological membranes. Annual Review of Cell and Development Biology, 14, 111-136. doi:10.1146/annurev.cellbio.14.1.111
- Becher, A. White, J.H. and McIlhinney, R.A. (2001) The gamma-aminobutyric acid receptor B, but not the metabotropic glutamate receptor type-1, associates with lipid rafts in the rat cerebellum. The Journal of Neuroscience, 79, 787-795. doi:10.1046/j.1471-4159.2001.00614.x
- Hering, H., Lin, C.C. and Sheng, M. (2003) Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. The Journal of Neuroscience, 23, 3262-3271.
- Frank, C., Giammarioli, A.M., Pepponi, R., Fiorentini, C. and Rufini, S. (2004) Cholesterol perturbing agents inhibit NMDA-dependent calcium influx in rat hippocampal primary culture. FEBS Letters, 566, 25-29. doi:10.1016/j.febslet.2004.03.113
- Suh, B.C. and Hille, B. (2005) Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Current Opinion in Neurobiology, 15, 370-378. doi:10.1016/j.conb.2005.05.005
- Zukin, R.S. and Bennett, M.V. (1995) Alternatively spliced isoforms of the NMDARI receptor subunit. Trends in Neurosciences, 18, 306-313. doi:10.1016/0166-2236(95)93920-S
- Wechsler, A. and Teichberg, V.I. (1998) Brain spectrin binding to the NMDA receptor is regulated by phosphorylation, calcium and calmodulin. The EMBO Journal, 17, 3931-3939. doi:10.1093/emboj/17.14.3931
- Ehlers, M.D., Zhang, S., Bernhadt, J.P. and Huganir, R.L. (1996) Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell, 84, 745- 755. doi:10.1016/S0092-8674(00)81052-1
- Wyszynski, M., Lin, J., Rao, A., Nigh, E., Beggs, A.H., Craig, A.M. and Sheng, M. (1997) Competitive binding of alpha-actinin and calmodulin to the NMDA receptor. Nature, 385, 439-442. doi:10.1038/385439a0
- Krupp, J.J., Vissel, B., Thomas, C.G., Heinemann, S.F. and Westbrook, G.L. (1999) Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+- dependent inactivation of NMDA receptors. The Journal of Neuroscience, 19, 1165-1178.
- Van Rossum, D., Kuhse, J. and Betz, H. (1999) Dynamic interaction between soluble tubulin and C-terminal domains of N-methyl-D-aspartate receptor subunits. The Journal of Neuroscience, 72, 962-973. doi:10.1046/j.1471-4159.1999.0720962.x
- Leonard, A.S., Bayer, K.U., Merrill, M.A., Lim, I.A., Shea, M.A., Schulman, H. and Hell, J.W. (2002) Regulation of calcium/calmodulin-dependent protein kinase II docking to N-methyl-D-aspartate receptors by calcium/calmodulin and alpha-actinin. The Journal of Biological Chemistry, 277, 48441-48448. doi:10.1074/jbc.M205164200
- Zhang, S., Ehlers, M.D., Bernhardt, J.P., Su, C.T. and Huganir, R.L. (1998) Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors. Neuron, 21, 443-453. doi:10.1016/S0896-6273(00)80553-X
- Rosenmund, C. and Westbrook, G.L. (1993) Calcium induced actin depolarization reduces NMDA channel activity. Neuron, 10, 805-814. doi:10.1016/0896-6273(93)90197-Y
- Allison, D.W., Gelfand, V.I., Spector, I. and Craig, A.M. (1998) Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: Differential attachment of NMDA versus AMPA receptors. The Journal of Neuroscience, 18, 2423-2436.
- Morishita, W., Marie, H. and Malenka, R.C. (2005) Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nature Neuroscience, 8, 1043-1050. doi:10.1038/nn1506
- Bauer, K., Kratzer, M., Otte, M., de Quintana, K.L., Hagmann, J., Arnold, G.J., Eckerskorn, C., Lottspeich, F. and Siess, W. (2000) Human CLP36, a PDZ-domain and LIM-domain protein, binds to alpha-actinin-1 and associates with actin filaments and stress fibers in activated platelets and endothelial cells. Blood, 96, 4236-4245.
- Gonzalez, A.M., Otey, C., Edlund, M. and Jones, J.C. (2001) Interactions of a hemidesmosome component and actinin family members. Journal of Cell Science, 114, 4197-4206.
- Feng, S.J., Reséndiz, J.C., Christodoulides, N., Lu, X., Arboleda, D., Berndt, M.C. and Kroll, M.H. (2002) Pathological shear stress stimulates the tyrosine phosphorylation of alpha-actinin associated with the glycolprotein Ib-IX complex. Biochemistry, 41, 1100-1108. doi:10.1021/bi0156005
- Burn, P., Rotman, A., Meyer, R.K. and Burger, M.M. (1985) Diacylglycerol in large alpha-actinin/actin complexes and in the cytoskeleton of activated platelets. Nature, 314, 469-472. doi:10.1038/314469a0
- Fukami, K., Furuhashi, K., Inagaki, M., Endo, T., Hatano, S. and Takenawa, T. (1992) Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature, 359, 150-152. doi:10.1038/359150a0
- Fukami, K., Sawada, N., Endo, T. and Takenawa, T. (1996) Identification of a phosphatidylinositol 4,5-bis-phosphatebinding site in chicken skeletal muscle alpha-actinin. The Journal of Biological Chemistry, 271, 2646-2650. doi:10.1074/jbc.271.5.2646
- Han, X., Li, G. and Lin, K. (1997) Interactions between smooth muscle alpha-actinin and lipid bilayers. Biochemistry, 36, 10364-10371. doi:10.1021/bi962929v
- Fritz, M., Zimmermann, R.M., Bämann, M. and Gaub, H.E. (1993) Actin binding to lipid-inserted alpha-actinin. Biophysical Journal, 65, 1878-1885. doi:10.1016/S0006-3495(93)81252-9
- Niggli, V. and Gimona, M. (1993) Evidence for a ternary interaction between alpha-actinin, (meta)vinculin and acidic-phospholipid bilayers. European Journal of Biochemistry, 213, 1009-1015. doi:10.1111/j.1432-1033.1993.tb17848.x
- Toker, A. (1998) The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Current Opinion in Cell Biology, 10, 254-261. doi:10.1016/S0955-0674(98)80148-8
- Horne, E.A. and Dell’Acqua, M.L. (2007) Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent longterm depression. The Journal of Neuroscience, 27, 3523- 3534. doi:10.1523/JNEUROSCI.4340-06.2007
- Mandal, M. and Yan, Z. (2009) Phosphatidylinositol (4,5)-bisphosphate regulation of N-methyl-D-aspartate receptor channels in cortical neurons. Molecular Pharmacology, 76, 1349-1359. doi:10.1124/mol.109.058701
- Stokes, C.E. and Hawthorne, J.N. (1987) Reduced phosphoinositide concentrations in anterior temporal cortex of Alzheimer-diseased brains. The Journal of Neuroscience, 48, 1018-1021. doi:10.1111/j.1471-4159.1987.tb05619.x
- Landman, N., Jeong, S.Y., Shin, S.Y., Voronov, S.V., Serban, G., Kang, M.S., Park, M.K., Di Paolo, G., Chung, S. and Kim, T.W. (2006) Presenilin mutations linked to familial Alzheimer’s disease cause an imbalance in phosphatidylinositol 4,5-bisphosphate metabolism. Pharmacy Association of Nova Scotia, 103, 19524-19529. doi:10.1073/pnas.0604954103
- Berman, D.E., Dall’Armi, C., Voronov, S.V., McIntire, L.B., Zhang, H., Moore, A.Z., Staniszewski, A., Arancio, O., Kim, T.W. and Di Paolo, G. (2008) Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism. Nature Neuroscience, 11, 547- 554. doi:10.1038/nn.2100
- McBain, C.J. and Mayer, M.L. (1994) N-methyl-D-aspartic acid receptor structure and function. Physiological Reviews, 74, 723-760.
- Johnson, T.D. (1996) Modulation of channel function by polyamines. Trends in Pharmacological Sciences, 17, 22- 27. doi:10.1016/0165-6147(96)81566-5
- Williams, K. (1997) Modulation and block of ion channels: A new biology of polyamines. Cell Signaling, 9, 1- 13. doi:10.1016/S0898-6568(96)00089-7
- Chemin, J., Patel, A., Delmas, P., Sachs, F., Lazdunski, M. and Honoré, E. (2007) Regulation of the mechano-gated K2P channel TREK-1 by membrane phospholipids. Current Top in Membranes, 59, 155-170. doi:10.1016/S1063-5823(06)59007-6
- Lopes, C.M., Rohacs, T., Czirjak, G., Balla, T., Enyedi, P. and Logothetis, D.E. (2005) PiP2-hydrolysis underlies agonist-induced inhibition and regulates voltage-gating of 2-P domain K+ channels. The Journal of Physiology, 564, 117-129. doi:10.1113/jphysiol.2004.081935
- Parnas, M., Katz, B., Lev, S., Tzarfaty, V., Dadon, D., Gordon-Shaag, A., Metzner, R.Y. and Minke, B. (2009) Membrane lipid modulations remove divalent open channel block from TRP-like and NMDA channels. The Journal of Neuroscience, 29, 2371-2383. doi:10.1523/JNEUROSCI.4280-08.2009
- Petrou, S,, Ordway, R.W., Singer, J.J. and Walsh, J.V. Jr. (1993) A putative fatty acid-binding domain of the NMDA receptor. Trends in Biochemical Sciences, 18, 41-42
- Dumuis, A., Sebben, M., Fagni, L., Prezeau, L., Manzoni, O., Cragoe, E.J. and Bockaert, J. (1993) Stimulation by glutamate receptors of arachidonic acid release depends on the Na+/Ca2+ exchanger in neuronal cells. Molecular Pharmacology, 43, 976-981.
- Nishikawa, M., Kimura, S. and Akaike, N. (1994) Facilitatory effect of decosahexaenoic acid on N-methyl-Daspartate response in pyramidal neurons of rat cerebral cortex. The Journal of Physiology, 475, 83-93.
- Darios, F. and Davletov, B. (2006) Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin. Nature, 440, 813-817. doi:10.1038/nature04598
- Okada, D., Yamagishi, S. and Sugiyama, H. (1989) Differential effects of phospholipase inhibitors in long-term potentiation in the rat hippocampal mossy fiber synapses and Schaffer/commissural synapses. Neuroscience Letters, 100, 141-146.
- Fujimoto, K., Yao, K., Miyazawa, T., Hirano, H., Nishikawa, M., Kimura, S., Maruyama, K. and Nonaka, M. (1989) Health effects of fish and fish oils. ARTS biomedical Publishers & Distributors, Newfoundland, 275-284.
- Tanabe, Y., Hashimoto, M., Sugioka, K., Maruyama, M., Fuji, Y. Hagiwara, R., Hara, T., Hossain, S.M. and Shido, O. (2004) Improvement of spatial cognition with dietary docosahexaenoic acid is associated with an increase in Fos expression in rat CA1 hippocampus. Clinical and Experimental Pharmacology & Physiology, 31, 700-703.
- Neuringer, M., Connor, W.E., Lin, D.S., Barstad, L. and Luck, S. (1986) Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Pharmacy Association of Nova Scotia, 83, 4021- 4025. doi:10.1073/pnas.83.11.4021
- Soderberg, M., Edlunk, C., Kristensson, K. and Dallner, G. (1991) Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids, 26, 421-425.
- Maclean, C.H., Issa, A.M., Newberry, S.J., Mojica, W.A., Morton, S.C. and Garland, R.H., et al. (2005) Effects of omega-3 fatty acids on cognitive function with aging, dementia, and neurological diseases. Evidence Report— Technology Assessement (Summary), 114, 1-3.
- Calon, F., Lim, G.P., Yang, F., Morihara, T., Teter, B., Ubeda, O., et al. (2004) Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron, 43, 633-645. doi:10.1016/j.neuron.2004.08.013
- Bourre, J.M. (2004) Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. The Journal of Nutrition Health and Aging, 8, 163-174.
- Marean, C.W. (2010) Pinnacle point cave 13B (Western Cape Province, South Africa) in context: The cape floral kingdom, shellfish, and modern human origins. Journal of Human Evolution, 59, 425-443. doi:10.1016/j.jhevol.2010.07.011
- Santos, S.F., Pierrot, N. and Octave, J.N. (2010) Network excitability dysfunction in Alzheimer’s disease: Insights from in vitro and in vivo models. Reviews in the Neurosciences, 21, 153-171. doi:10.1515/REVNEURO.2010.21.3.153
- Decker, H., Jürgensen, S., Adrover, M.F., Brito-Moreira, J., Bomfim, T.R., Klein, W.L., Epstein, A.L., De Felice, F.G., Jerusalinsky, D. and Ferreira, S.T. (2010) N-methylD-aspartate receptors are required for synaptic targeting of Alzheimer’s toxic amyloid-β peptide oligomers. Journal of Neurochemistry, 115, 1520-1529. doi:10.1111/j.1471-4159.2010.07058.x
- Henderson, S. and Broe, G.A. (2010) Dementia in Aboriginal Australians. Australian and New Zealand Journal of Psychiatry, 44, 869-4871. doi:10.3109/00048674.2010.514858
- Marszalek, J.R. and Lodish, H.F. (2005) Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: Breastmilk and fish are good for you. Annual Reviews of Cell and Development Biology, 21, 633-657. doi:10.1146/annurev.cellbio.21.122303.120624
- Wainwright, P.E. (2002) Dietary essential fatty acids and brain function: A developmental perspective on mechanisms. Proceedings of the Nutrition Society, 61, 61-69. doi:10.1079/PNS2001130

