Open Journal of Pediatrics
Vol. 3 No. 3 (2013) , Article ID: 35872 , 2 pages DOI:10.4236/ojped.2013.33032
Potentially life-threatening intravenous acetaminophen overdose in a 3-month-old (40 weeks’ post-menstrual age), 2.3 kg baby girl
![]()
1Department of Anaesthesia, Royal Aberdeen Children’s Hospital, Aberdeen, UK
2Department of Medicine & Therapeutics, University of Aberdeen, Aberdeen, UK
3Intensive Care Medicine, Auckland Children’s Hospital, Auckland, New Zealand
Email: shane.campbell@nhs.net
Copyright © 2013 Shane Campbell et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 4 June 2013; revised 6 July 2013; accepted 14 July 2013
Keywords: Neonate; Acetaminophen Overdose; No Sequelae; PK Models; Drug Error
ABSTRACT
A case is presented of a serious, potentially life-threatening intravenous acetaminophen overdose in a 3- month-old (40 weeks’ post menstrual age), 2.3 kg baby girl. The neonate was scheduled for urgent laser therapy for retinopathy of prematurity. Instead of an intended intravenous Hartmann’s solution bolus of 10 ml∙kg−1, the neonate received a 17 ml bolus of correctly labelled intravenous 1% acetaminophen. The National Poisons Bureau was immediately contacted for advice and in the absence of data suggested a treatment with N-acetylcysteine for a 24-hour period. Baseline blood samples for clotting, liver function, urea and electrolytes, full blood count and plasma acetaminophen concentration were taken 30 min, 8.25 h, 12.5 h, 18.5 h and 120 h after the overdose. Acetaminophen concentration was 78 mg∙L−1 at 30 min, but it was undetectable at any other time. Using a recent and complete PK-PD dataset we are able to show that the measured plasma acetaminophen concentration fits well on PK estimates for acetaminophen in this neonate. The non-detectable (low) plasma acetaminophen concentration at >8 h is also consistent with this model, especially if clearance is slightly increased in the premature nursery graduate. Medical errors are rarely the fault of an individual and they are often due to a combination of factors. Contributing factors, in this case, are described under the following headings: Catalyst event, system fault, loss of situational awareness, and human error.
1. CASE REPORT
A 3-month-old (40 weeks’ post menstrual age), 2.3 kg neonate was scheduled for urgent laser therapy for retinopathy of prematurity as an add-on case, following the completion of an elective operating room (OR) list. The OR was prepared and setup, medication doses were manually calculated and prepared by the anesthesia resident prior to patient arrival.
Just prior to the arrival of the patient the fire alarm for the OR complex was raised and the patient was sent back to the in-patient surgical ward and the OR evacuated. It had been at this point that a calculation error was made in the preparation of intravenous acetaminophen with a 75 mg∙kg−1 instead of 7.5 mg∙kg−1 dose being prepared in a 20 ml syringe.
A short time later, the patient was allocated an adjacent OR for treatment. Peri-induction, instead of an intended intravenous Hartmann’s solution bolus of 10 ml∙kg−1, the neonate received a 17 ml bolus of correctly labelled intravenous 1% acetaminophen. The error was instantly recognised and the correct content of the syringe and initial dose calculation verified.
The National Poisons Bureau was immediately contacted for advice and in the absence of data suggested a treatment with N-acetylcysteine for a 24-hour period. N-acetylcysteine was commenced 45 minutes after the initial overdose of acetaminophen and the patient admitted to a high-dependency unit for observation.
Baseline blood samples for clotting, liver function, urea and electrolytes, full blood count and plasma acetaminophen concentration were taken 30 min, 8.25 h, 12.5 h, 18.5 h and 120 h after the overdose. Acetaminophen concentration was 78 mg∙L−1 at 30min but was undetectable at any other time. All other parameters were within normal limits throughout. There were no clinical sequelae.
Until recently, only limited information of the pharmacokinetics (PK) and pharmacodynamics (PD) in the neonate were available and universally known [1]. Using this recent and most complete PK-PD dataset we are able to show that the measured plasma acetaminophen concentration fits well on PK estimates for acetaminophen in this neonate (Figure 1). The non-detectable (low) plasma acetaminophen concentrations at >8 h are also consistent with this model, especially if clearance is slightly increased in the premature nursery graduate.
Several publications in the literature report the acetaminophen PK in neonates, albeit mostly for non-intravenous routes of administration. The volume of distribution of acetaminophen at birth is 174% in that of older children [2] and the CYP2E1 is immature in neonates and unable to produce the toxic metabolite N-acetyl-pbenzoquinone-imine (NAPQI). Although theoretically possible, hepatotoxicity is, therefore, rarely seen clinically even in large overdoses e.g. 446 mg∙kg−1 [3,4].
Although specific data on the likely toxicity of intravenous paracetamol overdose in the neonate are sparse, it is known that the clearance of acetaminophen is reduced in older infants, which in overdose may mean that any treatment with N-acetylcysteine may have to be extended until acetaminophen levels are below toxic concentrations [5].
A study of the developmental pharmacokinetics in premature neonates through to infancy has suggested the following age-appropriate dosing to maintain a mean steady state target concentration >10 mg∙L−1 at trough –25 mg∙kg−1∙d−1 in premature neonates at 30 weeks’ post-conceptual age, 45 mg∙kg−1∙d−1 at 34 weeks’, 60 mg∙kg−1∙d−1 at term and 90 mg∙kg−1∙d−1 at 6 months of age [6]. The potential for hepatotoxicity may exist in some patients if it’s used for longer than 2 - 3 days. In the presented patient, the lack of changes in liver function
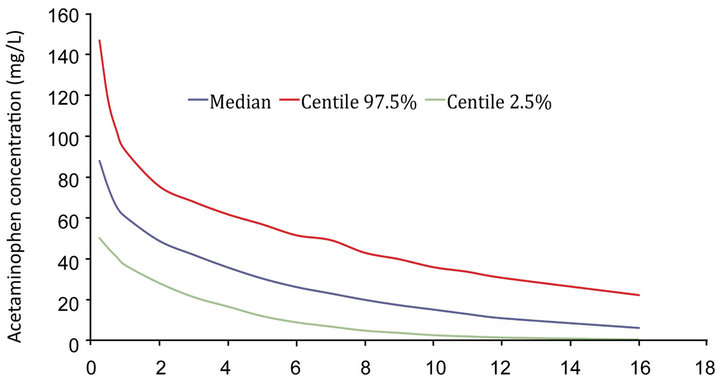
Figure 1. Simulated acetaminophen plasma concentration in this 3 months old, 2.3 kg, 40 weeks post menstrual age baby girl based on the PK dataset [1].
and clotting could be attributed to her likely immaturityof oxidative clearance pathways.
Medical errors are rarely the fault of an individual and they are often due to a combination of factors. Contributing factors, in this case, can be described under the following headings: Catalyst event, system fault, loss of situational awareness, of course human error. Safety barriers, which could potentially trap and mitigate such medical error, would need to be multifaceted in the approach and acted synergistically. Area of interest would come from technology, proficiency, standard operating procedures, and judgment.
The final barrier of patients’ harm, with respect to this child i.e. the early recognition of the error, was not breached. Had the error been recognized much later, or not recognized at all, it can only be speculated that the clinical outcome could have been very different.
REFERENCES
- Allegaert, K., Palmer, G.M. and Anderson, B.J. (2011) The pharmacokinetics of intravenous paracetamol in neonates: Size matters most. Archives of Disease in Childhood, 96, 575-580. doi:10.1136/adc.2010.204552
- Anderson, B.J., Woollard, G.A. and Holford, N.H.G. (2000) A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. British Journal of Clinical Pharmacology, 50, 125-134. doi:10.1046/j.1365-2125.2000.00231.x
- Allegaert, K., Anderson, B.J., Naulears, G., de Hoon, J., Debeer, A., Devlieger, H. and Tibboel, D. (2004) Intravenous paracetamol (propacetamol) pharmacokinetics in premature neonates. European Journal of Clinical Pharmacology, 60, 191-197. doi:10.1007/s00228-004-0756-x
- Porta, R., Sánchez, L., Nicolás, M., Garcia, C. and Martinez, M. (2012) Lack of toxicity after paracetamol overdose in an extremely preterm neonate. European Journal of Clinical Pharmacology, 68, 901-902. doi:10.1007/s00228-011-1165-6
- Anderson, B.J., Pons, G., Autret-Leca, E., Allegaert, K. and Boccard, E. (2005) Paediatric intravenous paracetamol (propacetamol) pharmacokinetics; a population analysis. Pediatric Anesthesia, 15, 282-292. doi:10.1111/j.1460-9592.2005.01455.x
- Anderson, B.J., van Lingen, R.A., Hansen, T.G., Lin, Y.-C. and Holford, N.H.G. (2002) Acetaminophen developmental pharmacokinetics in premature neonates and infants: A pooled population analysis. Anesthesiology, 96, 1336-1345. doi:10.1097/00000542-200206000-00012

