Open Journal of Inorganic Chemistry
Vol.05 No.04(2015), Article ID:60207,9 pages
10.4236/ojic.2015.54012
Crystal Structure and Physicochemical Properties of a New Tris (2-Amoniumbenzamide) Sulfate (C7H9N2O)3HSO4SO4
Saloua Belghith*, Sondes Chmengui, Latifa Ben Hamada
Laboratoire d’Energie et de Matériaux (LabEM), Ecole Supérieure des Sciences et de la Technologie de Hammam Sousse, Tunisia
Email: *saloua.belghith@yahoo.fr
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 23 July 2015; accepted 9 October 2015; published 12 October 2015
ABSTRACT
Physicochemical properties of a new hybrid compound (C7H9N2O)3HSO4SO4 are synthesized in aqueous solution and characterized by various physicochemical studies. This compound crystallizes in the monoclinic space group P21/c and a unit cell with a = 10.3028(2)A˚, b = 12.4995(2)A˚, c = 20.6730(2)A˚, V = 2600.61(7)A˚3, and Z = 8. The structure has been solved using direct method and refined to a reliability R factor of 4.6%. The atomic arrangement of this compound is built up by (HS2O8)3− anionic pairs interconnected with two types
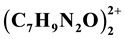 cationic pairs via (N, O)-H...O hydrogen bonds. The characterization of these salts was carried out using X-ray diffraction, IR spectroscopy and thermal analysis.
cationic pairs via (N, O)-H...O hydrogen bonds. The characterization of these salts was carried out using X-ray diffraction, IR spectroscopy and thermal analysis.
Keywords:
Chemical Preparation, Crystal Structure, Thermal Behaviour, Infrared Spectroscopy, 2-Aminobenzamide

1. Introduction
The synthesis of new hybrid materials based sulfate, phosphate and arsenate may contain original physical properties, is one of several research studies in chemistry laboratories in the world, due to its importance in both biological processes; in various industrial applications and technological [1] [2] . The cohesion forces in these hybrid compounds are dominated by electrostatic interactions, Vander Waals contacts, and hydrogen bonds (O-H…O and N-H…O). These hydrogen bonds play an important role in the mechanism of association of molecules that either biological or not. The strong characteristics and orientation of these links are extremely important in obtaining new materials such as proton conductors and frequency doublers. Thus, organic sulfates resulting from the interaction between sulfuric acid and organic molecules in which one of atom, at least, carries a lone pair, owe their stability to hydrogen bonds [3] -[5] . This work reports the chemical preparation, crystal structure and physico-chemical study of a new organic sulfate (C7H9N2O)3HSO4SO4.
2. Experiment
2.1. Chemical Preparation
Crystals of the title compound, (C7H9N2O)3HSO4SO4, were prepared by slow evaporation at room temperature of an aqueous solution of sulfuric acid (98wt% from Fluka) and the organic molecule 2-Aminobenzamide (Sigma-Aldrich ) in a 2:3 molar ratio. The corresponding acid-base chemical reaction can be written as follows:
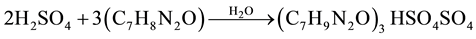
After agitation, the resulting solution was left to slowly evaporate at room temperature until single crystals suitable for X-ray structure analysis formulates and remain stable under normal conditions of temperature and humidity.
2.2. Investigation
The title compound has been studied by various physico-chemical methods: X-ray diffraction, Infrared spectroscopy and Thermal analysis.
2.2.1. X-Ray Structure Determination
X-ray intensity data of the title compound were collected on a Nonius Kappa-CCD diffractometer using monochromated Mo Kα radiation. For the crystal, 90 frames were recorded, each being of 2˚ in
 and 60 s duration. Each frame is doubled to eliminate the uncertain electronic impulses. The first 10 frames were used for indexing reflections using the DENZO package and refined to obtain final cell parameters [6] . Preliminary photographs indicated monoclinic symmetry and systematically absent reflections showed the space group to be P21/c.
and 60 s duration. Each frame is doubled to eliminate the uncertain electronic impulses. The first 10 frames were used for indexing reflections using the DENZO package and refined to obtain final cell parameters [6] . Preliminary photographs indicated monoclinic symmetry and systematically absent reflections showed the space group to be P21/c.
Crystal data and experimental parameters used for the intensity data collection are summarized in Table 1. The structure was solved with a direct method, from the SHELXS-97 programs, which allows the location of the SO4 groups. The remaining non-hydrogen atoms were found by the successive difference Fourier maps using the SHELXL-97 programs [7] . The formula structure was drawn by Diamond [8] . In the final least-squares refinement of atomic parameters with isotropic thermal factors of H atoms, R has decreased to 4.6% (Rw = 11.41%) for the title compound.
2.2.2. Thermal Analysis
Setaram TG-DTA92 star system Mettler Toledo thermoanalysers were used to perform thermal treatment on samples of (C7H9N2O)3HSO4SO4. The TG-DTA experiments were carried out with 19.8 mg sample in an open alumina crucible. In this technique, samples were heated in an air atmosphere with heating rates of 5˚C∙min−1.
2.2.3. Infrared Spectroscopy
IR spectrum of the compound was recorded at room temperature with a Biored FTS 6000 FTIR spectrometer over the wave number range of 4000 - 400 cm−1 with a resolution of about 4 cm−1. Thin transparent pellet was made by compacting an intimate mixture obtained by shaking 2 mg of the samples in 100 mg of KBr.
3. Results and Discussion
3.1. Structure Description
The atomic arrangement of the structure of the tris (2-amoniumbenzamide) sulfate (C7H9N2O)3HSO4SO4 was
Table 1. Crystal data and structure refinement.
described by a three-dimensional network of structural units formed by a cluster (HS2O8)3− sulfate and three organic cations (C7H9N2O)+. Figure 1 shows an ORTEP [9] stereoscopic projection of the crystal packing. The mineral skeleton of this compound is formed by basic
 and acid groups
and acid groups
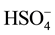 which are interconnected via a hydrogen bond type O(2)-H∙∙∙O(7), and are organized in isolated clusters (HS2O8)3− in the plane (a, c) (Figure 2). The short distance O(2)∙∙∙O(7)= 2.485(3)Å, shows that this hydrogen bond was considered strong. The distance S(1)∙∙∙S(2) being of the order of 4.525(1)Å. Distances and bond angles describing anions (
which are interconnected via a hydrogen bond type O(2)-H∙∙∙O(7), and are organized in isolated clusters (HS2O8)3− in the plane (a, c) (Figure 2). The short distance O(2)∙∙∙O(7)= 2.485(3)Å, shows that this hydrogen bond was considered strong. The distance S(1)∙∙∙S(2) being of the order of 4.525(1)Å. Distances and bond angles describing anions (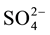 ) and (
) and (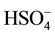 ) are shown in Table 2. S-O distances vary in the range [1.438(3) - 1.506(4)Å]. The review of these distances reveals that the last distance S-O(2) [1.506(4)Å] is the longest, this is due to the location of a proton on
) are shown in Table 2. S-O distances vary in the range [1.438(3) - 1.506(4)Å]. The review of these distances reveals that the last distance S-O(2) [1.506(4)Å] is the longest, this is due to the location of a proton on
Figure 1. ORTEP stereoscopic projection of the atomic arrangement (for clarity, H-bonds are represented by dashed lines). Thermal ellipsoids are given at 50% probability.
Figure 2. Clusters (HS2O8)3− viewed down the crystallographic b axis.
oxygen O(2) of the anion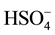 , this characteristic is in line with those observed in the protonated oxoanions [10] -[12] , the mean value of S-O distances and angles O-S-O are: 1.466(3)Å, 109.46(19), 1.473(3)Å, 109.38(20) respectively for the tetrahedra S(1)O4 and S(2)O4. These values are also in good agreement with those observed for similar anionic groups [13] [14] . The oxygen atoms O(1) and O(3) of the tetrahedron are doubly protonated, the HSO4 have the longest distances [1.456(3), 1.466(3)Å], while O(4) engaged in a single hydrogen bond it is a S-O distance smaller [1.438(3)Å]. The interaction of the sulfuric acid with the organic molecule (C7H8N2O) leads to the protonation of nitrogen grafted on the benzene ring and the formation of three cations (C7H9N2O)+ crystallographically independent. Respectively denoted: A{C(1) C(7)}, B{C(8) C(14)} et C{C(15) C(21). Figure 3 shows a projection along the a axis of the atomic arrangement in (C7H9N2O)3HSO4SO4. Main geometrical characteristics of these cations are summarized in Table 3; these organic species are no local symmetry in the structure. Note that the two cations A and C are associated with two hydrogen bonds N(1)-H(1N1)∙∙∙O(10)
, this characteristic is in line with those observed in the protonated oxoanions [10] -[12] , the mean value of S-O distances and angles O-S-O are: 1.466(3)Å, 109.46(19), 1.473(3)Å, 109.38(20) respectively for the tetrahedra S(1)O4 and S(2)O4. These values are also in good agreement with those observed for similar anionic groups [13] [14] . The oxygen atoms O(1) and O(3) of the tetrahedron are doubly protonated, the HSO4 have the longest distances [1.456(3), 1.466(3)Å], while O(4) engaged in a single hydrogen bond it is a S-O distance smaller [1.438(3)Å]. The interaction of the sulfuric acid with the organic molecule (C7H8N2O) leads to the protonation of nitrogen grafted on the benzene ring and the formation of three cations (C7H9N2O)+ crystallographically independent. Respectively denoted: A{C(1) C(7)}, B{C(8) C(14)} et C{C(15) C(21). Figure 3 shows a projection along the a axis of the atomic arrangement in (C7H9N2O)3HSO4SO4. Main geometrical characteristics of these cations are summarized in Table 3; these organic species are no local symmetry in the structure. Note that the two cations A and C are associated with two hydrogen bonds N(1)-H(1N1)∙∙∙O(10)
Table 2. Main interatomic distances (Å) and bond angles (°) in the SO4 and HSO4 tetrahedra of (C7H9 N2O)3HSO4SO4.
Figure 3. Projection along the a axis of the atomic arrangement in (C7H9N2O)3HSO4SO4.
Table 3. Main interatomic distances (Å) and bond angles (°) in the organic groups of (C7H9N2O)3HSO4SO4.
and N(5)-H(2N5)∙∙∙O(11) to form a first type of dimmer located in planes z = (2n + 1)/4. A second type of dimmer formed by two B cations that associate through hydrogen bond N(3)-H(2N3)・・・O(9) is situated around the center of inversion (0, 0, 0) (Figure 4, Figure 5).
The work demonstrating the important role of hydrogen bonds, the stability of the structure reveals two types of connections: O-H∙∙∙O and N-H∙∙∙O, the structure studied in this work, contains a single hydrogen bond of first type and the second type seventeen. The only link O(2)-H(O2)∙∙∙O(7) considered high [O(2)-H(O2)∙∙∙O(7) = 2.485(3)Å] [14] , brings the two anionic species as a cluster (HS2O8)3−. it is noted, among the seventeen hydrogen bonds of the N-H∙∙∙O, six strong for which the distance N∙∙∙O range from 2.621(1)Å to 2.756(1)Å [14] and eleven moderately low [N∙∙∙O > 2.76Å] [15] . The second type of hydrogen bonds connecting the various clusters to generate the three-dimensional network structure.
The characteristics of the different hydrogen bonds are given in Table 4. As a result, the two types of hydrogen bonds, O-H∙∙∙O and N-H-O, contribute to the cohesion in the network of the present crystal structure.
3.2. Thermal Behavior
The thermal study was conducted using a thermoanalyzer type Setaram TG-ATD92. The thermogram (TG- DTA) of Figure 6 is registered under an air atmosphere using a mass of 19.8 mg sample placed in a platinum crucible and heated from ambient to 400˚C.
The TG curve shows no mass loss in the area, room temperature 200˚C. However it shows a significant loss from 200˚C up. The DTA curve shows two endothermic peaks less intense at 98˚C and 110˚C which is attributed to two transitions likely stage since at this temperature was noticed no mass loss. Note that the observed thermal phenomena in differential thermal analysis are many and varied. The majority of these peaks are endothermic such as melting, evaporation, sublimation, dehydration. The remaining peaks are exothermic such as adsorption, crystallization and decomposition; however, the last two phenomena can also be endothermic. The DTA curve shows a succession of exothermic and endothermic peaks between 200˚C and 400˚C can be explained by the decomposition of the molecule. The endothermic peak observed at 199˚C is attributed to the melting of the anhydrous compound.
Figure 4. Dimmer A-C.
Figure 5. Dimmer B-B.
Table 4. Bond lengths (Å) and angles (˚) in the Hydrogen-bonding schemea.
aSymmetry operators: i) −x, y − 1/2, −z + 1/2; ii) −x, y + 1/2, −z + 1/2; iii) x − 1, y, z; iv) x, −y + 3/2, z + 1/2; v) −x, −y + 1, −z + 1; vi) x − 1, −y + 3/2, z − 1/2; vii) −x + 1, y − 1/2, −z + 1/2.
Figure 6. TG-DTA thermo grams of (C7H9N2O)3HSO4SO4.
Figure 7. IR Spectrum of (C7H9N2O)3HSO4SO4.
3.3. IR Absorption Spectroscopy
The literature study, conducted over several sulfates [16] [17] shows that specific frequencies of vibration of the free ion SO4, in its ideal Td symmetry are ν1 = 981 cm−1, ν2 = 451 cm−1, ν3 = 1104 cm−1 and ν4 = 614 cm−1 [18] . Frequently encountered in structures, SO4 tetrahedron are often distorted have low symmetry sites. The lifting of degeneracy and activity of inactive modes, in the ideal symmetry, multiply the number of bands in the infrared spectrum. The IR spectrum of compound (C7H9N2O)3HSO4SO4 is reported in Figure 7.
An attempt to assign frequencies to different stretching vibrations and deformation of the organic cation is performed based on previous work [19] [20] . Bands observed in the region 2562 - 3375 cm−1 are assigned to symmetric and asymmetric vibrations of valence ν(NH3), ν(NH2), ν(CH) and ν(OH). The bands between 1538 - 1681 cm−1 are attributed to deformation vibrations of bonds (NH3) and (NH2) as well as vibrations of valences ν(C=C) and ν(CN). Vibration symmetrical and asymmetrical deformation δs(CH) and δas(CH), occur in the area from 1276 to 1499 cm−1. The deformation vibrations rocking type: ρ(NH3), ρ(NH2) and ρ(CH) appear in the 721 - 965 cm−1 region. The twists τ(NH3) and τ(NH2) appear in 514 - 560 cm−1 bands. Finally, the bands 1926 - 2369 cm−1 domains are assigned to overtones and combination bands. The frequency bands in the region 410 - 484 cm−1 are attributed to the symmetric deformation vibration of δs(SO4). The asymmetric deformation symmetry δas(SO4) was observed in the area 602 - 671 cm−1. While that connected to the symmetry of valence SO4 group is presented by the band 992 cm−1. Bands observed in the 992 - 1186 cm−1, on asymmetrical valence vibration νs(SO4)δas(SO4) region.
3.4. Supplementary Material
Crystallographic data for the structural analysis have been deposited at the Cambridge Crystallographic Data Centre, CCDC No 1000722. Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge, CB2 IEZ, UK (fax: +44-1226-336033; e-mail: deposit@ccdc.cam).
Acknowledgements
The authors gratefully acknowledge financial support from the Ministry of Higher Education, Scientific Research and Technology of Tunisia.
Cite this paper
SalouaBelghith,SondesChmengui,Latifa BenHamada, (2015) Crystal Structure and Physicochemical Properties of a New Tris (2-Amoniumbenzamide) Sulfate (C7H9N2O)3HSO4SO4. Open Journal of Inorganic Chemistry,05,112-121. doi: 10.4236/ojic.2015.54012
References
- 1. Finn, R.C., Zubieta, J. and Haushalter, R.C. (2003) Crystal Chemistry of Organically Templated Vanadium Phosphates and Organophosphonates. Progress in Inorganic Chemistry, 51, 451.
- 2. Koo, B., Ollette, W., Burkholder, E.M., Golub, V., O’connor, C.J. and Zubieta, J. (2004) Hydrothermal Synthesis and Structural Characterization of Organically-Templated Copper Vanadium Phosphates: The Two-Dimensional [{Cu2-(bisterpy)}V3O5(HPO4)2(PO4)] (1) and the Three-Dimensional[{Cu2(bisterpy)}V2O5(HPO4)2] (2) (bisterpy=2,2′:4′,4″: 2″,2'''-quarterpyridyl, 6′,6″-dipyridine). Solid State Sciences, 6, 461-468.
http://dx.doi.org/10.1016/j.solidstatesciences.2004.01.001 - 3. Bataille, T. and Louer, D. (2002) Two New Diamine Templated Lanthanum Sulfates, La2(H2O)2(C4H12N2)(SO4)4 and La2(H2O)2(C2H10N2)3(SO4)6·4H2O, with 3D and 2D CRYSTAL STRUCTURES. Journal of Materials Chemistry, 12, 3487-3493.
- 4. Bataille, T. and Louer, D. (2004) New Linear and Layered Amine-Templated Lanthanum Sulfates. Journal of Materials Chemistry, 177, 1235-1243.
http://dx.doi.org/10.1016/j.jssc.2003.10.031 - 5. Xing, Y., Shi, Z., Li, G.H. and Pang, W.Q. (2003) Hydrothermal Synthesis And Structure of [C2N2H10][La2(H2O)4(SO4)4]·2H2O, A New Organically Templated Rare Earth Sulfate with a Layer Structure. Dalton Transactions, 940-943.
- 6. Otwinowski, Z. and Minor, W. (1997) In Methods in Enzymology. Academic Press, New York.
- 7. Sheldrick, G.M. (1997) SHELX-97. University of Göttingen, Göttingen.
- 8. Brandenburg, K. (1998) Diamond Version 2.0 Impact GbR, Bonn.
- 9. Farrugia, L.J. (1997) ORTEP-3 for Windows—A Version of ORTEP-III with a Graphical User Interface (GUI). Journal of Applied Crystallography, 30, 565-568.
http://dx.doi.org/10.1107/S0021889897003117 - 10. Ferraris, G and Ivaldi, G. (1984) X-OH and O-H…O Bond Lengths in Protonated Oxoanions. Acta Crystallographica, B40, 1.
http://dx.doi.org/10.1107/s0108768184001671 - 11. Xu, Y.M., Gao, S. and Ng, S.W. (2009) Bis(4-Hydroxy-Pyridinium) Sulfate Monohydrate. Acta Crystallographica, E65, o3146.
http://dx.doi.org/10.1107/S1600536809048521 - 12. Xu, Y.M., Gao, S. and Ng, S.W. (2009) Tris(4-Hydroxypyridinium) Hydrogen Sulfate-Sulfate Monohydrate. Acta Crystallographica, E65, o3147.
http://dx.doi.org/10.1107/s1600536809048545 - 13. Mostad, A. and Natarajan, S. (1996) Crystal Structure of an Adduct of Sarcosine with Sulfuric Acid (at 140 K). Crystal Research and Technology, 31, 295-300.
http://dx.doi.org/10.1002/crat.2170310306 - 14. Abadi, B.E.A., Moss, D.S. and Palmer, R.A. (1984) Molecular Structure of an Ancient Folk Medicine: Berberine Hydrogen Sulfate. Journal of Crystallographic and Spectroscopic Research, 14, 269-281.
http://dx.doi.org/10.1007/BF01161164 - 15. Brown, I.D. (1976) On the Geometry of O-H…O Hydrogen Bonds. Acta Crystallographica, A32, 24-31.
http://dx.doi.org/10.1107/s0567739476000041 - 16. Nakamoto, K. (1986) IR and Ra Spectra of Inorg. and Coord. Comp. Wiley-Interscience, New York.
- 17. Landolt, H.H. and Börnstein, R. (1951) Physikalish-Chemische Tabellen. Vol. 2, Band. Springer-Verlag, Berlin.
- 18. Hertzberg, G. (1966) Infrared and Raman Spectra of Polyatomic Molecules. Van Nostrand, New York.
- 19. Guerfel, T., Chtioui, A., Gharbi, A. and Jouini, A. (2006) Crystal Structure, Thermal Analysis and IR Spectrometric Investigation of Bis (2-Amino-6-Methyl) Pyridinium Sulfate. Crystal Research and Technology, 41, 416-422.
http://dx.doi.org/10.1002/crat.200510596 - 20. Dahl, T. (1993) Crystal Structure of N(6), N(6)-Dimethyladaninium Hemisulfate Hydrate. The Tautomerism of N(6)- Substituted Adeninium Ions Elucidated by Molecular-Packing Analysis. Acta Chemica Scandinavica, 47, 38-42.
http://dx.doi.org/10.3891/acta.chem.scand.47-0038
NOTES
*Corresponding author.










