Open Journal of Inorganic Chemistry
Vol.3 No.1(2013), Article ID:27483,8 pages DOI:10.4236/ojic.2013.31004
Synthesis, characterisation and microbial studies of [bis(1,10-phenanthroline) (ethylenediamine) copper(II)] diperchlorate and its bromide analogue
![]()
Department of Pure and Applied Chemistry, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
Email: *estherdr@rocketmail.com
Received 17 August 2012; revised 22 September 2012; accepted 10 October 2012
Keywords: Tris-Chelate Complexes; X-Ray Data; Electronic Spectra; Microbial Studies
ABSTRACT
Two new tris-chelate-complexes have been synthesized and characterized with elemental and spectroscopic methods. IR and thermal studies correlate with the structures of the complex in the solid state. The structure of [Cu(en)(phen)2]· was determined with X-ray data using single crystal X-ray diffractometer while the molecular structure of [Cu(en) (phen)2]·2Br−·2Phen·8H2O was deduced from the used characterization methods. [Cu(en)(phen)2]·
was determined with X-ray data using single crystal X-ray diffractometer while the molecular structure of [Cu(en) (phen)2]·2Br−·2Phen·8H2O was deduced from the used characterization methods. [Cu(en)(phen)2]·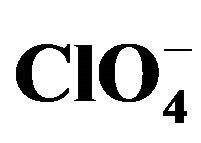 crystallizes as orthorhombic with space group Pbcn. Both complexes have distorted octahedral geometry. Microbial activities of these complexes against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptoccocus pyogeneous, Candida albicans, and Aspergillus niger were also reported.
crystallizes as orthorhombic with space group Pbcn. Both complexes have distorted octahedral geometry. Microbial activities of these complexes against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptoccocus pyogeneous, Candida albicans, and Aspergillus niger were also reported.
1. INTRODUCTION
Copper(II) ions and its metal complexes have continued to attract attention of coordination chemists due to its various interesting structural features, its usefulness as models of the active centers of various metallo-enzymes, magnetic, electronic, catalytic and biological properties [1-3].
In recent times, Copper(II) complexes from various ligand systems such as 1,10-phenanthroline and 1,2-diaminoethane have been synthesized and characterized with the aim of gaining a better understanding of the chemistry of copper and the various ligands in different environments.
We have previously described [4] the synthesis and spectral studied of tris chelate of mixed-ligand complexes of copper(II) ion of nitrate and that of chloride with 1,2-diaminoethane and 1,10-phenanthroline in which the H-bonded network of lattice water molecules and the anions in both compounds are quite different from one another. The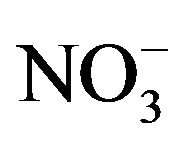 , H2O, H-bonded network has a 1D chain structure while Cl−, H2O network has a 3D structure. The change in the structure is due to the change in the size of anions as Cl− ions allow very close H-bonding because of its smaller size compare to
, H2O, H-bonded network has a 1D chain structure while Cl−, H2O network has a 3D structure. The change in the structure is due to the change in the size of anions as Cl− ions allow very close H-bonding because of its smaller size compare to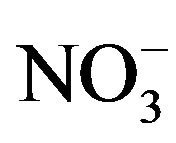 . We are considering the effect of
. We are considering the effect of 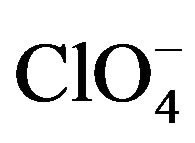 ion upon these supramolecular structures. The activities of these two complexes on micro-organisms such as Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptoccocus pyogeneous, Candida albicans, and Aspergillus niger being the micro-organisms that affect man and plants were also carried out.
ion upon these supramolecular structures. The activities of these two complexes on micro-organisms such as Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptoccocus pyogeneous, Candida albicans, and Aspergillus niger being the micro-organisms that affect man and plants were also carried out.
In this paper, we therefore report the synthesis, characterization, structure, and microbial studies of [Cu(en) (phen)2](ClO4)2-(1) and [Cu(en)(phen)2]2Br·2Phen·8H2O-(2).
2. EXPERIMENTAL
All the chemicals used, were analytical reagent from commercial sample. They were procured from Fluka and Merck. The reagents were used without further purification.
2.1. Synthesis of [Cu(en)(phen)2](ClO4)2 (1)
Cu(ClO4)2∙6H2O (0.370 g, 1 mmol) and 1,2-diaminoethane (0.20 mL, 2.92 mmol) were dissolved in 25 mL water and heated to 80˚C for 10 mins with stirring. To this, 5 mL ethanolic solution of 1,10-phenanthroline (0.198 g, 1.00 mmol) was added and stirred for another 5 mins and the solution was filtered. There was blue precipitate. The precipitate was recrystallized with methanol. Greenish blue block shaped X-ray type crystals were obtained after four days. Yield: 0.3576 g (52.35%). Anal. calcd. for C26H24CuN6Cl2O8:
C 45.73, H, 3.54; N, 12.31, Found: C 45.72; H, 3.50; N, 12.27.
IR (KBr disk, cm−1): 3393, 3312, 2953, 2853, 1622, 1585, 1507, 1094, 843, 728, 422.
2.2. Synthesis of [Cu(en)(phen)2]·2Br−·2(phen)·8H2O (2)
The procedure was similar to that of 1, except for the use of CuBr2, (0.223 g, 1 mmole). Blue crystals in the form of hexagonal shaped were obtained from the filtrate after four days Similar crystals were obtained when the synthesis was carried out with the reagent in ratio 1:1:4. of Cu:en:phen in order to maximize the yield. Yield: 0.1857 g (64.68%). Anal. calcd. for C50H56Br2CuN10O8: C, 52.29; H, 4.91; N, 12.19 Found: C, 52.19; H, 4.88; N, 12.14.
IR (KBr disk, cm−1): 3403, 3226, 3093, 1623, 1585, 1506, 1420, 846, 727, 420.
2.3. Physical Measurements
IR spectra were recorded using KBr discs on a Perkin Elmer, GX-FTIR spectrometer in the range 4000 - 400 cm−1. Elemental analysis for C, H and N was performed on a Perkin-Elmer, Series II, 2400. Electronic spectra measurements were obtained using a Shimadzu UV-3101 PC spectrophotometer. Thermogravimetric analysis was done using Mettler Toledo.
2.4. X-Ray Crystallography
X-ray data were collected on a Bruker SMART APEX CCD X-ray diffractometer, using graphite-monochromated Mo-Ka radiation (λ = 0.71073 Å) at 298 K. Data were reduced using SAINTPLUS [5] and a multi-scan absorption correction using SADABS [6] was performed. The structures were solved by direct method using SHELXS- 97 and full-matrix least-square refinement was carried out using SHELXL-97. [7] All ring hydrogen atoms were assigned on the basis of geometrical considerations and were allowed to ride upon the respective carbon atoms. Drawings were made using Mercury [8].
2.5. Antimicrobial Screening
The antimicrobial screening was performed by paper diffusion method. The activities were expressed in terms of millimeter (mm) by diameter of zone of inhibition. Ampicilline, Chloramphenicol, Ciprofloxacin and Norfloxacin were used as standard drugs for bacteria. Greseofulvin and Nystatin were used for fungi.
2.5.1. Microorganisms Used
The following common standard strains were used for screening of antibacterial and antifungal activities. The strains, listed below, were procured from Institute of Microbial Technology, Chandigarh India. The bacteria used are 1) Staphylococcus aureus MTCC 96; 2) Escherichia coli MTCC 443; 3) Pseudomonas aeruginosa MTCC 1688; and 4) Streptoccocus pyogeneous MTCC 442, while fungi used are 5) Candida albicans MTCC 227; and 6) Aspergillus niger MTCC 282.
2.5.2. Culture Media
Nutrient agar medium was prepared by dissolving 7.0 g of agar powder in 250 mL of distilled water in a conical flask (for bacteria). Potato dextrose agar medium was prepared by dissolving 9.75 g of potato dextrose agar powder in 250 mL of distilled water in a conical flask (for fungi). The conical flask was capped with cotton wool and aluminium foil and then sterilized in an autoclave for 15 minutes at 121˚C.
Using an inoculate wire loop, the micro organisms were collected from slant cultures of all the different micro-organisms and were inoculated separately into fresh sterile nutrient broths for bacteria and the resulting cultures were incubated at 37˚C for 18 hrs and at room temperature to obtain new cultures of bacteria respectively.
2.5.3. Zone of Inhibition
The zone of inhibition of the complexes was carried out by dipping sterile paper disc into a range of metal complexes solutions using sterile forceps. These were then carefully and aseptically laid on the media flooded with cultures of test organisms. The concentrations used were 250, 100, 50, 25 and 5 μg/mL and finally, the Petri plates were incubated for 26 - 30 hrs, at 28˚C ± 2˚C. The zone of inhibition was measured in millimetre. The results are shown in Table 3 for bacteria and Table 4 for the fungi.
3. RESULTS AND DISCUSSION
3.1. Synthesis
The two complexes were prepared by a similar method of synthesis using the reagents in molar ratio of 1:3:1. of Cu2+:en:phen. For the complex 2 it was resynthesized in ratio 1:1:4 to improve its yield. Greenish blue good crystals were isolated from the precipitate of 1 after re-crystallization from methanol while navy blue crystals can be isolated directly from the filtrate of 2 but its structure could not be refined. The complexes are non hygroscopic and thermally stable. They were obtained in fairly good yield and the C, H and N analysis calculated agrees with the experimental value for the two complexes. The anions of the complexes are different in that there are no free phenanthroline and lattice water in 1 as found in 2. This may be due to dissolution of the free phenanthroline and water during recrystallisation of precipitate of 1. The complexes are soluble in methanol, ethanol, DMF, DMSO, acetonitrile and water.
3.2. Infrared Spectra
The two complexes have almost similar IR absorption bands. Broad bands for ν (NH2) stretching vibration in 1,2-diaminoethane appeared at 3393 cm−1, 3312 cm−1 for complex 1. Both asymmetric and symmetric ν (C-H) bands in 1,2-diaminoethane could be seen at 2953 cm−1 and 2853 cm−1. NH2 deformation bands appeared at 1585 cm−1, while aromatic ring stretching vibration for phenanthroline occurs at 1507 cm−1. ClO4 band is observed at 1094 cm−1. Complex 2 also has similar bands as found in 1, ν (O-H), from uncoordinated water and ν (NH2) stretching band from 1,2-diaminoethane could be seen at 3403 cm−1 and 3226 cm−1 respectively while ν (C-H) aromatic stretching vibrational band could be seen at 3093 cm−1, aromatic ring stretching occurred at 1506 cm−1 for the coordinated and at 1623 cm−1 for the uncoordinated phenanthroline rings. NH2 deformation band is found at 1585 cm−1 [9]. The IR result shows that the two complexes coordinated via the N atoms from the 1,2- diaminoethane and from the N atoms of the aromatic rings. Molecules of water also present in complex 2 with free phenanthroline molecules. This is also confirmed from the C, H, N analysis. Cu-N band could not be observed for phenanthroline as its absorb at relatively low frequency [9]. The ν (Cu-N) from 1,2-diaminoethane for complexes 1 and 2 are 422 and 420 cm−1 respectively.
3.3. Electronic Spectra
The electronic absorption spectra of both complexes were measured in methanol solution. [Cu(en)(phen)2] (ClO4)2 absorb at 15.3 ´ 103 cm−1 (652 nm), while [Cu(en)(phen)2]Br2·2phen·8H2O absorb at 15.3 ´ 103 cm−1 (646 nm). (Figures 1(a) and (b)). All the four d-d transitions of the rhombic distorted octahedral copper(II) chromophore may be placed within the broad envelop of the observed band. The energy band in this position for the two complexes is typically expected for a rhombic distorted octahedral configuration and may be assigned to 2Eg → 2T2g transition [10].
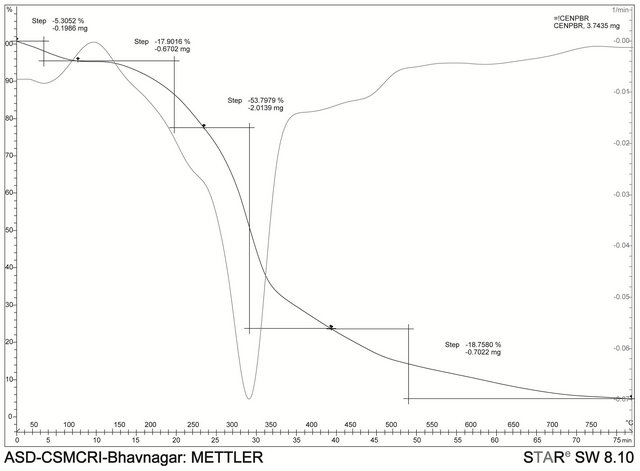
3.4. Thermal Gravimetric Analysis
Thermal gravimetric studies and differential thermal analysis were carried out on the complexes in the temperature range of 50˚C to 800˚C at a heating rate of 10˚C/ min. The measurements were done primarily to determine the composition of materials and to predict their thermal stability and also to determine extent of weight loss or gain due to decomposition, oxidation or dehydration. The thermal analysis shows that there are two broad endothermic peaks in the differential thermal analysis curve of the bromide analogue which is typical of dehydration reaction [11], whereas the perchlorate analogue showed three sharp endothermic peaks which shows changes in the crystalline or fusion process in the complex. The two
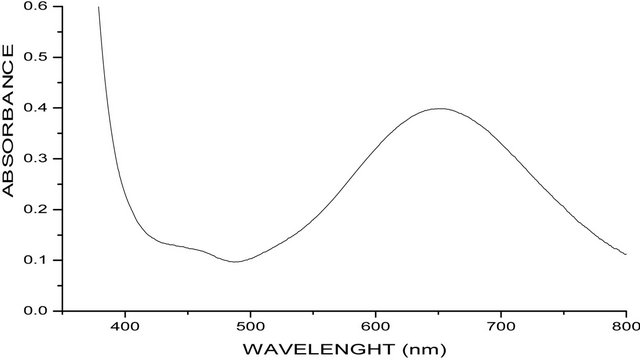 (a)
(a)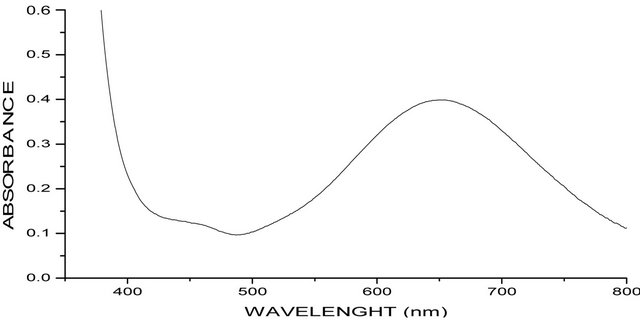 (b)
(b)
Figure 1. (a) UV-Visible spectra of [Cu(en)(phen)2]2phen·2Br·8H2O; (b) UV-Visible spectra of [Cu(en)(phen)2]·2CIO4)−.
complexes showed no weight loss up to 170˚C indicating the absence of uncoordinated water molecules in the complexes. The bromo complex decomposes in two endothermic peaks the first step occurs from 55˚C - 300˚C and this corresponds to the loss of the eight H2O molecules and second from 300˚C - 500˚C represent the decomposition of the organic part. The perchlorate decomposes with two peaks the first from 150˚C - 250˚C then to 350˚C. The remaining organic part decomposes with the peak occurring from 350˚C - 650˚C.
3.5. Structure of [Cu(en)(phen)2](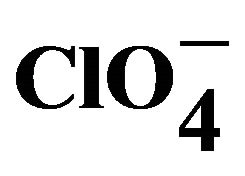 ght=39.9 />)2 (1)
ght=39.9 />)2 (1)
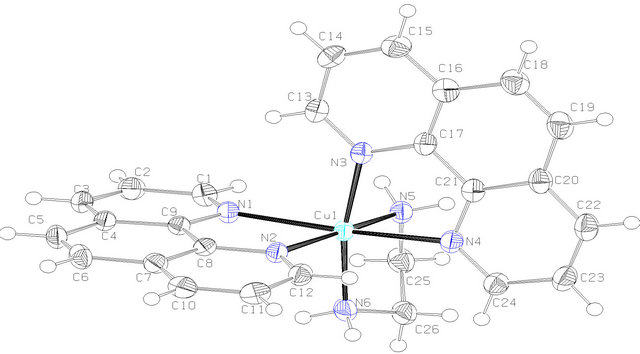
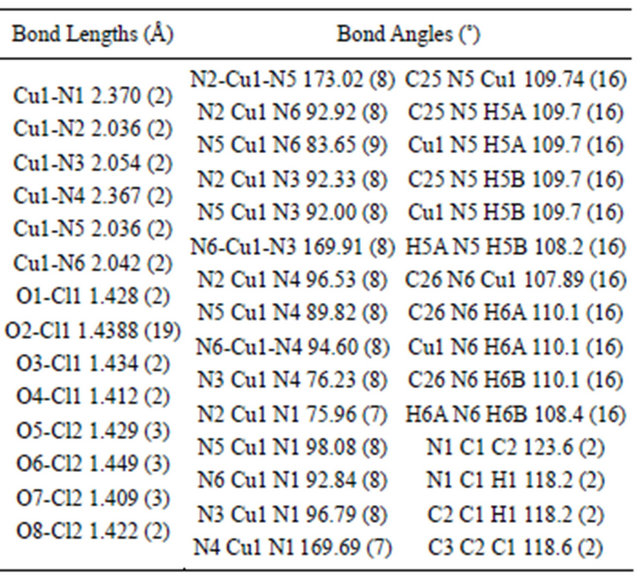
The molecular structure of complex 1 was established by single crystal X-ray study. The crystal system in 1 is orthorhombic with space group Pbcn (Table 1). The coordination sphere of copper consists of one 1,2-diaminoethane and two phenanthroline molecules with distorted octahedral geometry. The equatorial plane of hexa coordinated copper consists of both nitrogen atoms of 1,2- diaminoethane molecule and nitrogen from the two phenanthroline molecules (Figure 2(a)). Two perchlorate anions also present in the lattice, there are no water molecules. The equatorial distances are Cu1-N1: 2.370 (2), Cu1-N2: 2.036 (2) Å and Cu1-N4: 2.367 (2) Å and Cu1-N5: 2.036 (2) Å, while the axial distances are Cu1- N3: 2.054 (2) Å and Cu1-N6: 2.042 (2) Å compared to the bond distances of its nitrate and chloride ions analogy
Table 1. Crystallographic data for [Cu(en)(phen)2]·(ClO4)2 (1).
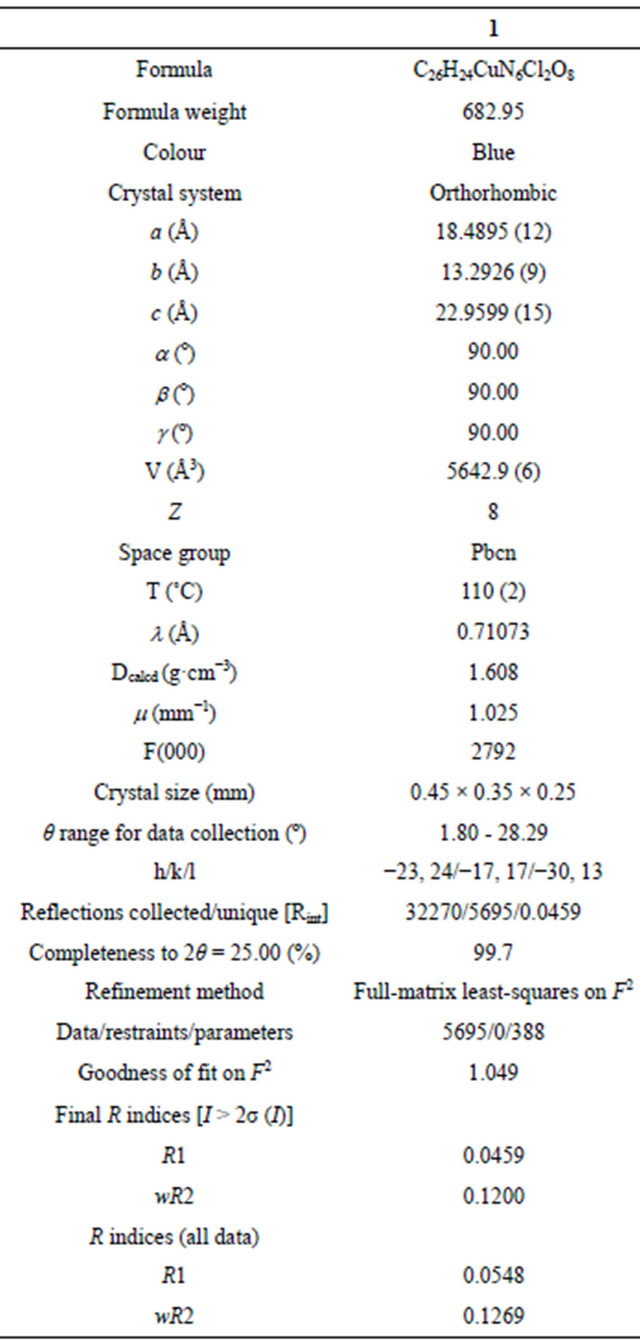
[4], which have Cu-N1: 2.390 (2), Cu-N2 2.0608 (17), Cu-N3: 2.0210 (18) Å and 2.406 (3), 2.071 (3), 2.028 (3) Å respectively. Selected geometrical parameters are presented in Table 2. The packing diagram for 1 showing hydrogen bonding interactions as viewed along the b-axis is shown in Figure 2(b). There is H-bonding interaction between the hydrogen of N5 from 1,2-diaminoethane and from O2 and O6 from perchlorate ions [N-H-O2 = 2.936 Å, N-H-O6: 3.064] Å.
3.6. Biological Activities
The antibacterial and antifungi activities of the com-
 (a)
(a)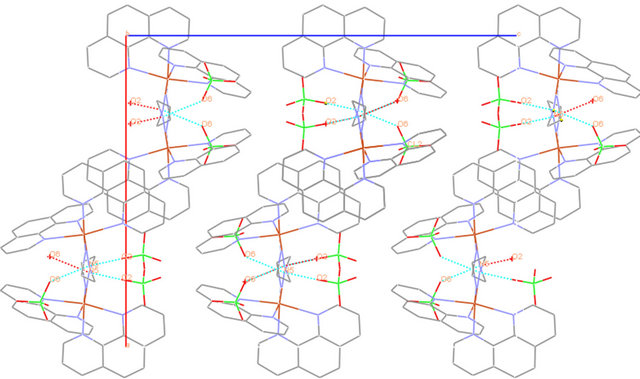 (b)
(b)
Figure 2. (a) ORTEP diagram depicting the cationic part of the copper complex with atom numbering scheme (50% probability factor for the thermal ellipsoids); (b) Packing structure of complex 1 showing the H-bonding between diaminoethane-H and O-from perchlorate as viewed along the b-axis.
Table 2. Bond lengths and bond angles for [Cu(en)(phen)2] (ClO4)2.
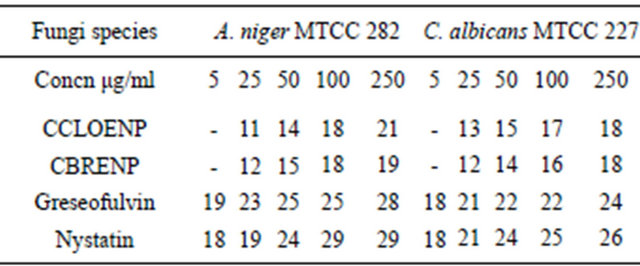
plexes can easily be observed from Tables 3 and 4 and from the bar chart as seen in Figures 3-8. The data were obtained for the bacteria and fungi which cause diseases in man and plants with that of the standard drugs normally used for these micro-organisms. From the results it can be observed that at higher concentrations the perchlorate analog have higher zone of inhibitions than their bromide counterpart when tested against the following bacteria: E. coli, P. aeruginosa, S. aureus and P. pyogenes compared to the zone of inhibitions observed with known antibiotics like Ampicillin and Chloralphenicol (Figures 3-6). The zones of inhibition are however lower when the values are compared to other antibiotics like Ciproflaxin and Norfloxacin. In general the results indicate that the complexes have potential and promising activity. Further investigations may however be required to illustrate the mechanisms by which the complexes exhibit its antibacterial effect [12].
Similarly, the perchlorate complex was more potent than the bromide complex as shown by the results of the zone of inhibition observed when tested with A. niger and C. albicans (Figures 7 and 8). Though the zones of inhibitions observed for standard antifungal drugs like Greseofulvin and Nystatin are higher than the ones observed for the complexes they are significant enough to indicate some antifungal properties inherent in the complexes.
Table 3. Zone of inhibition of complexes and the standard drugs against the bacteria (mm).
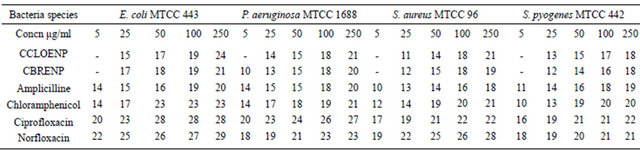
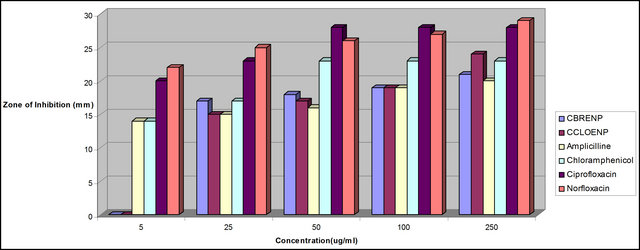
Figure 3. Zone of inhibition of E. coli against the concentration of the standard drugs and the complexes.
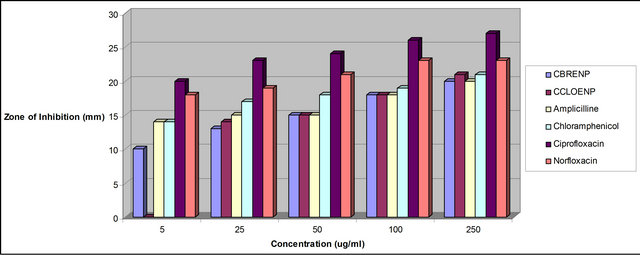
Figure 4. Zone of inhibition of P. aeruginosa against the concentration of the standard drugs and the complexes.
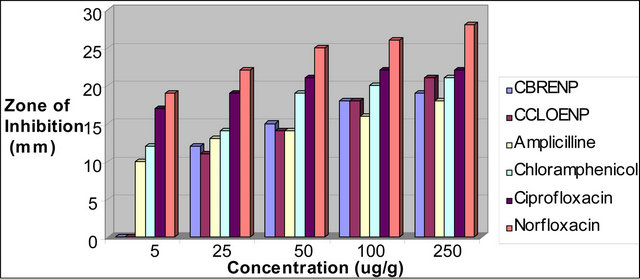
Figure 5. Zone of inhibition of S. aureus against the concentration of the standard drugs and the complexes.
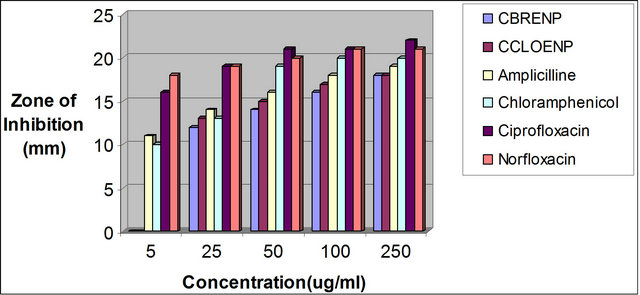
Figure 6. Zone of inhibition of S. ptogenes against the concentration of the standard drugs and the complexes.
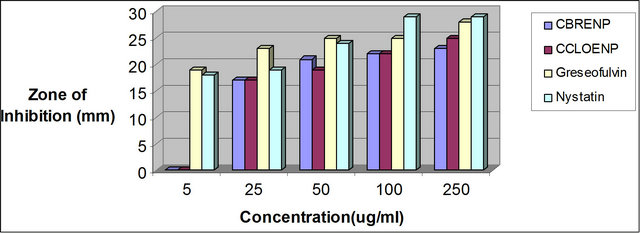
Figure 7. Zone of inhibition of A. niger against the standard drugs and the complexes.
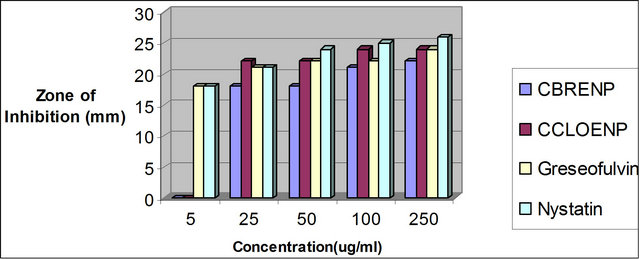
Figure 8. Zone of inhibition of C. albican against the concentration of the standard drugs and the complexes.
Table 4. Zone of inhibition of the complexes and the standard against the fungi (mm).
4. CONCLUSION
Two new mixed ligand complexes of copper(II) with two chelating ligands have been synthesized and characterized. Both complexes 1 and 2 have the same [Cu(en) (phen)2]2+ cation, but differ in anionic part, though complex 1 has no water molecule, hydrogen bonding exists between the H in 1,2-diaminoethane and O from chlorate ion which form network within the lattice. The two complexes exhibit antimicrobial activities which are comparable to the known standard drugs used for some of the micro-organisms considered.
5. ACKNOWLEDGEMENTS
Onawumi O. O. thanks TWAS (The Academy of Science For Developing World) and CSIR (Council of Scientific and Industrial Research), New Delhi for the award of postdoctoral fellowship. Ladoke Akintola University of Technology, Nigeria is gratefully acknowledged for study leave. Dr. Parimal Paul is highly acknowledged for being a good host at CSMCRI, Bharvnagar, Gujurat. India. Thanks to Vinod K. Agarwal for I.R analysis, E. Suresh for determined the crystal structure. A. S. Onawumi is highly appreciated for his support. Microcare Laboratory at Unapani Road, Surat, India is gratefully acknowledged for conducting microbial experiments. Thanks to Mr. H. Brahmbhatt for technical assistance.
REFERENCES
- Lemoine, P., Viossat, B., Morgant, G., Greenaway, F.T., Tomas, A., Dung, N.H. and Sorenson, J.R.J. (2002) Synthesis, crystal structure, EPR properties, and anticonvulsant activities of binuclear and mononuclear 1,10-phenanthroline and salicylate ternary copper(II) complexes. Journal of Inorganic Biochemistry, 89, 18-28. doi:10.1016/S0162-0134(01)00324-5
- Devereux, M., Shea, D.O., Kellet, A., McCann, M., Walsh, M., Egan, D., Deggan, C., Kedziora, K., Rosair, G. and Muller-Bunz, H. (2007) Synthesis, X-ray crystal structures and biomimetic and anticancer activities of novel copper(II)benzoate complexes incorporating 2-(4’- thiazolyl)benzimidazole (thiabendazole), 2-(2-pyridyl)benzimidazole and 1,10-phenanthroline as chelating nitrogen donor ligands. Journal of Inorganic Biochemistry, 10, 881-892. doi:10.1016/j.jinorgbio.2007.02.002
- Lave-Cambot, A., Cantnuel, M., Leydet, Y., Jonususkas, G., Bassani, D.M. and McClenaghan, N.D. (2008) Improving the photophysical properties of copper(I) bis (phenanthroline) complexes. Coordination Chemistry Reviews, 252, 2572-2584.
- Onawumi, O.O.E., OFaboya, O.P., Odunola, O.A., Prasad, T.K. and Rajasekharan, M.V. (2011) Mixed ligand trischelate of copper(II) with N,N-donor ligands—Synthesis, structure and spectra of [Cu(en)(phen)2]X2·2phen·8H2O (X=Cl–,
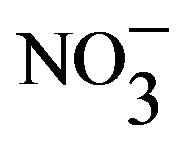 ). Polyhedron, 30, 725-729. doi:10.1016/j.poly.2010.12.004
). Polyhedron, 30, 725-729. doi:10.1016/j.poly.2010.12.004 - (2003) SAINTPLUS: Bruker AXS Inc., Madison.
- Sheldrick, G.M. (1996) SADABS: Program for empirical absorption correction. Universityof Göttingen, Göttingen.
- Sheldrick, G.M. (1997) SHELX-97: Programs for crystal structure analysis. University of Göttingen, Göttingen.
- Macrae, C.F., Edgington, P.R., McCabe, P., Pidcock, E., Shields, G.P., Taylor, R., Towler, M. and van de Streek, J. (2006) Mercury: Visualization and analysis of crystal structures. Journal of Applied Crystallography, 39, 453. doi:10.1107/S002188980600731X
- Nakamoto K. (1963) Infrared and Raman spectra of inorganic and coordination compounds. John Willey and Sons, New York, 155.
- Lever, A.B.P. (1984) Inorganic electronic spectroscopy. Elsevier, Amsterdam.
- Kaur H. (2010) Instrumental methods of chemical analysis. 6th Edition, Pragati Prakashan Publishers, Meerut, 991.
- Prakesh, A. and Singh, M.P.G.K. (2011) Synthesis spectroscopy and biological studies of nickel(II) complexes with tertradentate schiff bases having N2O2 donor group. Journal of Developmental Biology and Tissue Engineering, 3, 13-19.
APPENDIX
CCDC 870713 contain the supplementary crystallographic data for compound 1. These data can be obtained free of charge via
http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223- 336-033; or e-mail: deposit@ccdc.cam.ac.uk.

Thermogravimetric analysis of [Cu(en)(phen)2](ClO4)2.
NOTES
*Corresponding author.

