Journal of Surface Engineered Materials and Advanced Technology
Vol.4 No.1(2014), Article ID:42052,8 pages DOI:10.4236/jsemat.2014.41004
New Polysiloxane Surfaces Modified with ortho-, meta- or para-Nitrophenyl Receptors for Copper Adsorption*
Smaail Radi1,2*, Nouraddine Basbas1, Said Tighadouini1, Maryse Bacquet3
1LCAE; Faculté des Sciences; Université Med I, Oujda, Morocco; 2Centre de l’Oriental des Sciences et Technologies de l’Eau (COSTE), Université Mohamed I, Oujda, Morocco; 3Université des Sciences et Technologies de Lille, UMET: Unité Matériaux et Transformations UMR8207, Equipe Ingénierie des Systèmes Polymères, Villeneuve d’Ascq, France.
Email: *radi_smaail@yahoo.fr
Copyright © 2014 Smaail Radi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Smaail Radi et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received July 14th, 2013; revised August 12th, 2013; accepted September 10th, 2013
ABSTRACT
Porous SiO2 has been chemically modified with functional ortho-, metaor para-nitrophenyl moieties using the heterogeneous route. This synthetic route involved the reaction of carbaldehyde derivatives with 3-aminopropyl trimethoxysilane prior to immobilization on the support. The new modified surfaces have been characterized by elemental analysis, FT-IR, 13C NMR of the solid state, nitrogen adsorption-desorption isotherm, BET surface area, B.J.H. Pore sizes, thermogravimetry curves (TGA) and scanning electron microscope (SEM). The new materials exhibit good chemical and thermal stability. These products were employed as a Cu(II) adsorbent from aqueous solutions at room temperature using the batch technique. Flame atomic absorption spectrometry was used to determine the Cu(II) concentration in the filtrate after the adsorption process. The results indicate that under the optimum conditions, the maximum adsorption value for Cu(II) was 20.0 mg Cu(II) g−1 modified silica, whereas the adsorption capacity of the unmodified silica was only 1.0 mg Cu(II) g−1 silica. On the basis of these results, it can be concluded that it is possible to modify chemically SiO2 with functional groups and use it as adsorbents for metals in aqueous media.
Keywords: Modified Surfaces; Chemical Synthesis; Characterization; Adsorption; Cu(II)
1. Introduction
The high Cu2+ concentration not only endangers the growth of the aquatic animals and plants, obstructs the self-purification of the water bodies, but also exerts a deleterious effect on the human’s health. Owing to the toxicity of copper, the World Health Organization (WHO) recommended the maximum acceptable concentration of this element in drinking water to be 2.0 mg∙L−1 [1]. Metal separation via adsorption using appropriates surfaces is a very promising technique because of its simplicity and reversibility. Therefore, extensive research effort has been directed towards the development of new surfaces for the removal of heavy metals from water.
Recently, various studies have focused on the removal of this toxic heavy metal ions from water. One of the potential remedies is the use of adsorption technology. Activated carbon and a number of low-cost adsorbents such as rice husk, montmorillonite and natural bentonite have been used for the removal of Cu(II) [2-4]. Nevertheless, some of these materials suffer from inherent problems such as the low removal capacity, low selectivity, long equilibrium time, mechanical and thermal instability.
In recent years, the preparation of porous materials based adsorbents has generated considerable interest due to their unique large specific surface area, regular pore structure and well-modified surface properties [5-7]. Moreover, they can also be regenerated for many times after adsorption saturation [8].
To enhance the adsorption capacity for heavy metals, the organically modified porous silicas have drawn much attention as promising adsorbents. The porous silicas are usually modified by the post-synthesis or one-pot synthesis. In both methods, the organic functional groups are used. Indeed, the aptitude of attached chelate is mainly owed to the presence of donor atoms, such as oxygen, nitrogen and sulfur [9-13].
In continuation of our work in this field [14-25], this paper describes the synthesis and the characterization of new surface materials obtained by grafting functionalized orth-, metaor para-nitrophenyl on porous silica. The adsorption capacities of unmodified silica, and nitrophenyl-modified silicas towards Cu(II) were investigated and the extracted amounts of metals ions were determined by atomic absorption measurements.
2. Experimental
2.1. Materials and Methods
All solvents and other chemicals employed in this investigation were of analytical grade and used without further purification. Silica gel (E. Merck) with particle size in the range of 70 - 230 mesh and a median pore diameter of 60 Å, was activated before use by heating it at 160˚C for 24 h. The silylating agent 3-aminopropyltrimethoxysilane purchased from Janssen Chimica was used without purification. Solid state 13C NMR spectrum of the product was obtained with a CP MAS CXP 300 MHz spectrometer. Elemental analyses were performed by Microanalysis Central Service (CNRS). FT-IR spectra were recorded with a Perkin-Elmer 1310 Fourier transform infrared Spectrophotometer. X-ray diffraction spectra were performed by UATRS-CNRST using Panalytical X’Pert PRO-Philips diffractometer. Atomic absorption measurements were performed acquired with the aid of a Spectra Varian A.A. 400 Spectrophotometer. A specific area of modified silica was determined by using the BET equation. The nitrogen adsorption-desorption was obtained by means of a Thermoquest Sorpsomatic 1990 analyzer, after the material had been purged in a stream of dry nitrogen. The mass loss determinations were performed in 90:10 oxygen/nitrogen atmospheres on a TGA Q50 V6.7 Build 203 instrument, at a heating rate of 10˚C∙min−1.
2.2. Preparation of Silica-Immobilized Propylamine (SiNH2)
The first stage in the preparation was the reaction between the silylating agent and the silanol groups on the silica surface. Activated silica gel (SiG) (25 g) suspended in 150 mL of dried toluene was refluxed and mechanically stirred under nitrogen atmosphere for 2 h. To this suspension, 10 mL of aminopropyltrimethoxysilane was added dropwise and the mixture was kept under reflux for 24 h. The solid was filtered, washed with toluene and ethanol and was then Soxhlet extracted with a mixture of ethanol and dichloromethane (1:1) for 12 h, to remove the silylating reagent residue. The obtained immobilized silica gel, named SiPr, was dried in vacuum at room temperature.
2.3. Synthesis of Nitrophenyl-Substituted Silicas (Si-o-NO2), (Si-m-NO2) and (Si-p-NO2)
A mixture of 3-aminopropylsilica (SiNH2) (10 g) and ortho-nitroacetophenone, meta-nitroacetophenone, or para-nitroacetophenone, (3 g) in 100 mL of dry diethyl ether was stirred at room temperature for 12 h leading to (Si-o-NO2), (Si-m-NO2) and (Si-p-NO2) respectively. After being filtered, the solid products were Soxhlet and extracted with acetonitrile, methanol and dichloromethane for 12 h. The products were then dried under vacuum at 70˚C over 24 h.
2.4. Batch Experiments
A 100 mg sample of modified silica and 10 mL of an aqueous solution of a copper ion (0.006 g) were shaken for 1 min to 24 h at 25˚C and under various pH. The mixture was then filtered off and the amount of metal ion in the filtrate was determined by atomic absorption spectrophotometry using standard solutions for calibration. Solutions of the metal ions were prepared by dissolution of the nitrate salt. Analyses were performed in duplicate for each sample and only the mean data were reported.
3. RESULTS AND DISCUSSION
3.1. Linker Synthesis
The synthetic route of the new chelating materials can be summarized in Scheme 1. The preparation involves reacting the activated silica gel with 3-aminopropyltrimethoxysilane in toluene to form the amino groups attached to the silica surface (SiG) [26]. These NH2-groups onto the silica surface were then reacted with ortho-, metaor para-nitrobenzaldehyde under gentle conditions (room temperature, atmospheric pressure, 12 h), using anhydrous diethyl ether as solvent to form the new chelating sorbents (Si-o-NO2), (Si-m-NO2) and (Si-p-NO2) respectively.
3.2. Materials Characterization
3.2.1. Elemental Analysis
Elemental analyses (Table 1) of carbon and nitrogen (not present in the starting activated silica gel SiG) of aminopropyl-silica (SiNH2) make it possible to characterize the modification on the silica gel surface. The microanalysis
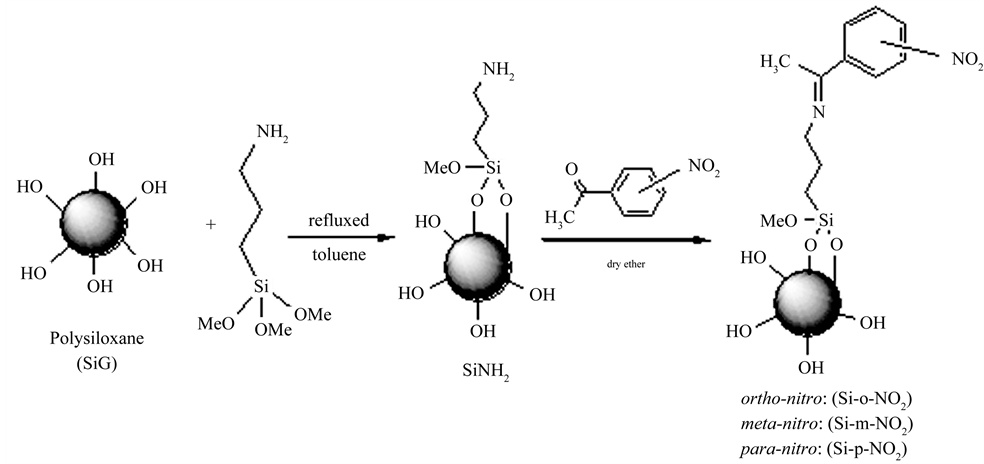
Scheme 1. The synthesis route of modified silicas.
Table 1. Adsorption and texture parameters of the initial (SiG) and of the modified samples (SiNH2), (Si-o-NO2), (Si-m-NO2) and (Si-p-NO2).
suggests that two methoxy groups were substituted by silanol and the -NH2 content of SiNH2 was 1.14 mmol/g. The final materials (Si-o-NO2), (Si-m-NO2) and (Si-pNO2) showed also an increase in the percentages of C and N attributed to the nitrophenyl fraction immobilized on the silica gel surface.
3.2.2. FT-IR Characterization
The modified silica gel was also confirmed by FT–IR analysis. As shown in Figure 1, a characteristic feature of the 3-aminopropylsilica (SiNH2) when compared with the native silica (SiG) was the appearance of a ν(NH2) around 1580 cm−1 and a ν(C-H) weak bands at 2700 cm−1 corresponding to the carbon chain of the pendant group attached to the inorganic silica matrix. On the spectrum of the final materials as Si-m-NO2, we note the disappearance of the absorption band at 1580 cm−1 which testifies the reactivity of the primary amine (-NH2) and the appearance of new characteristic bands around 1554 cm−1 resulted from C = N vibrations. The final materials reveal also the appearance of new characteristic band around 1540 and 1350 cm−1 due to N-O groups. These re-sults showed that the nitrophenyl units had been grafted onto the surface of silica gel after modification.
3.2.3. 13C NMR Characterization
The presence of the organic spacer arm on the inorganic polysiloxane was asserted by 13C solid state NMR spectroscopy (Figure 2). Three well-formed peaks, at 9.4, 25.0 and 42.6 ppm were attributed to the propyl carbon, Si-CH2, -CH2- and N-CH2, respectively. The signal at 53.1 ppm was assigned to unsubstituted methoxy group –OCH3 as confirmed by microanalysis. For the (Si-oNO2), (Si-m-NO2) and (Si-p-NO2), the spectra reveal other signals at 100 - 160 ppm corresponding to specific carbons of nitrophenyl units.
3.2.4. TGA Analysis and Thermal Stability
The thermogravimetric curves reflect the thermal stability of these new products. The quantity decomposed in each stage confirms the amount of the compounds grafted (Figure 3). Native silica gel (SiG) presents two losses attributed to physisorbed water molecules released and to the condensation of silanol groups bonded to the surface. Different from silica, the 3-aminopropyl-silica (SiNH2) presents an additional weight loss, after the drainage of physically adsorbed water, mainly attributed to the organic arm. The final materials (Si-o-NO2), (Si-m-NO2) and (Si-p-NO2) showed also an increase of mass loss
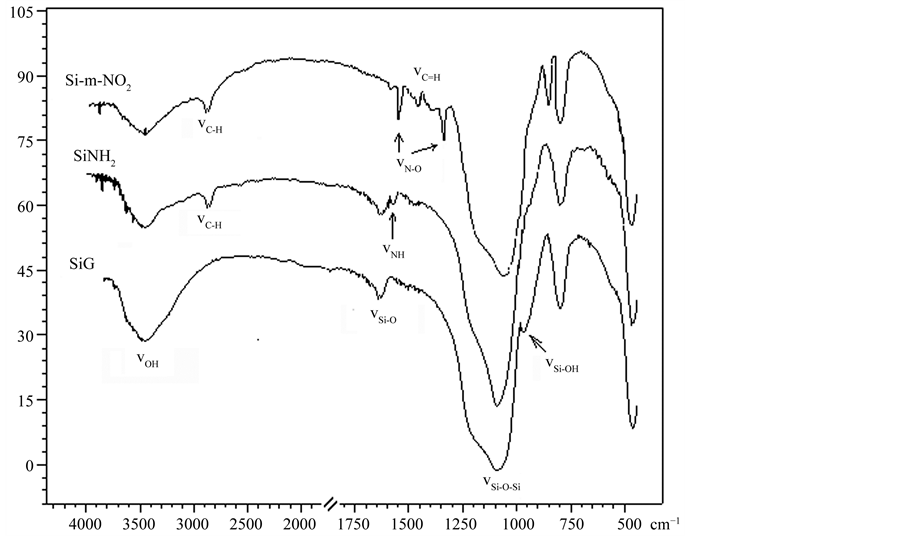
Figure 1. FT-IR spectra of silica gel (SiG) and of the modified samples (SiNH2) and (Si-m-NO2).
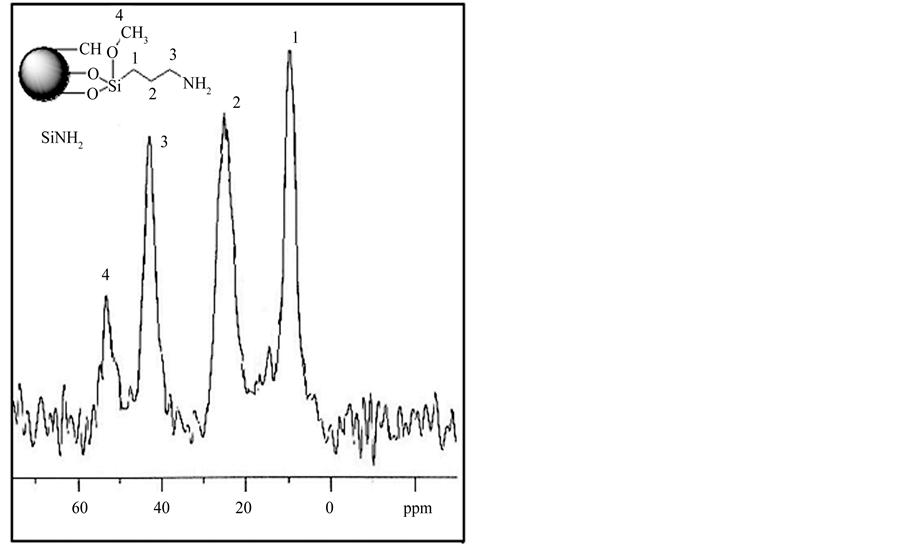
Figure 2. 13C NMR spectra of the modified sample (SiNH2).
allotted to the decomposition of the nitrophenyl fraction immobilized on the surface of silica gel, together with the condensation of the remaining silanol groups. The pronounced increase in mass loss reflects the higher amount of the anchored organic groups with an order of para > meta > ortho because of the steric effects.
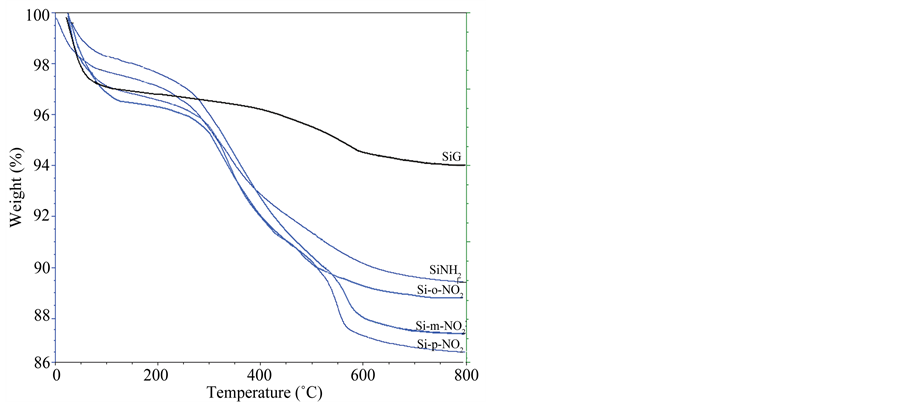
Figure 3. Thermogravimetric curves of silica gel (SiG) and of the modified samples (SiNH2), (Si-o-NO2), (Si-m-NO2) and (Si-p-NO2).
3.2.5. Surface Properties
To gain insight into the porosity changes of the porous polysiloxane induced by the introduction of notrophenyl groups, we measured the surface area and pore volumes of modified silicas (SiNH2), (Si-o-NO2), (Si-m-NO2) and (Si-p-NO2) with nitrogen adsorption-desorption isotherms (BET) (Figure 4) and Barrett-Joyner-Halenda (BJH) pore diameters methods [27,28]. The porous polysiloxane (SiG) has a BET (Brunauer-Emmett-Teller) surface area of 305 m2/g and a pore volume of 0.77 cm3/g.
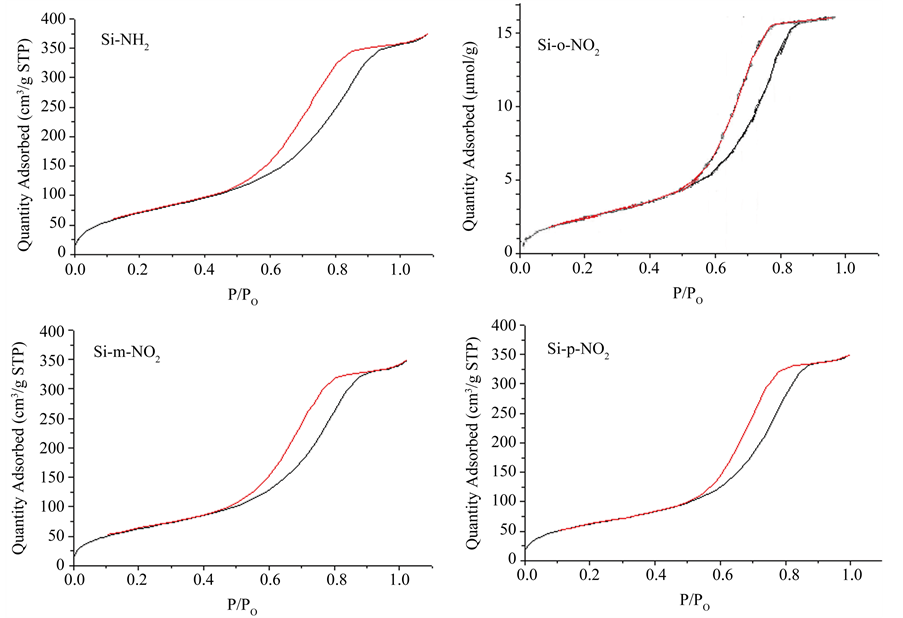
Figure 4. Nitrogen adsorption–desorption isotherm plots of porous materials: (SiNH2), (Si-o-NO2), (Si-m-NO2) and (Si-pNO2).
On the other hand, we observed that SiNH2 has a decrease BET surface area as additional groups’ immobilization takes place to give 241 m2/g and a pore volume of 0.67 cm3/g. Moreover, the (Si-o-NO2), (Si-m-NO2) and (Si-p-NO2) have a decrease surface area and BJH pore diameters. The decreased parameters are attributable to the grafted nitrophenyl units on the porous polysiloxane (Table 1). Moreover, the nitrogen adsorption-desorption isotherm for silica derivatives, shown in Figure 4, are type IV according to the IUPAC classification and display a pronounced hysteresis for partial pressures 1 > P/P0 > 0.4 which is the direct evidence of the presence of mesopores. The hysteresis loops is Type H2 indicating that there is a uniform pore diameter distribution.
3.2.6. Scanning Electron Micrographs
Some representative SEM micrographs of the original and modified materials are shown in Figure 5. They display an increased rough and porous nature, indicating that the materials present a potential to be employed as an adsorbent for metal ion uptake.
3.2.7. Chemical Stability
Finally, the chemical stability of the newly synthesized materials was examined in various acidic and buffer solutions (pH 1 - 7). Samples were mixed with different concentrations and stirred at room temperature during 24 h. No change in the material structure was observed. The high stability exhibited by the attached organofunctional group is presumably due to the length of the pendant group, which binds the nitrophenyl groups to the silica surface. It has been shown that when the length of the hydrocarbon bridge was more than two methylene groups, the rupture of Si-C bond did not occur in a mineral acid medium, due to the length of the chain; longer chains can no longer have a functional handle that can undergo beta-elimination of the Si cation [29,30].
3.3. Solid-Liquid Retention of Cu(II)
The preliminary adsorption properties of the above modified materials (Si-o-NO2), (Si-m-NO2) and (Si-p-NO2) towards copper was evaluated by the batch method. The samples (0.1 g) were equilibrated by shaking with 10 mL of a solution containing 0.006 g of Cu(II) (90 μmol) for different time intervals (5, 10, 15, 30 min and 1, 2, 3, 4, 5, 6 and 24 h). The concentration of metal ion was determined by means of atomic absorption measurements. The amount of metal ion adsorbed by the synthesized materials from aqueous solution was calculated using the following equations [31]:
QM = (C0 – Ce) × V/W
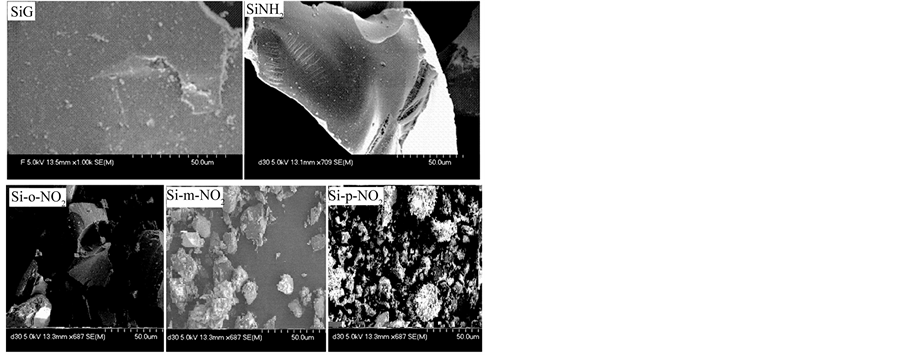
Figure 5. SEM photographs of silica gel (SiG) and of the modified samples (SiNH2), (Si-o-NO2), (Si-m-NO2) and (Sip-NO2).
QW = QM × M where QM is the amount of the metal ion on the adsorbent (mmol∙g−1), QW is the amount of the metal ion on the adsorbent (mg∙g−1), V is the volume of the aqueous solution (l), W is the weight of the adsorbent (g), C0 the initial concentration of metal ion (mmol∙l−1), Ce the equilibrium metal ion concentration in solution (mmol∙l−1) and M the atomic weight for metals (g∙mol−1). Analyses were performed in duplicate for each sample and only the mean data were reported.
From Figure 6, a two-stage kinetic behavior was evident: a very rapid initial adsorption over a few minutes, followed by a constant adsorption according to time. Indeed, the kinetics of adsorption that describes the metal ion uptake’s rate governing the contact time of the sorption reaction is one of the important characteristics that define the efficiency of sorption.
The higher adsorption of Cu(II) by Si-o-NO2 suggests that the two active donor atoms of nitro groups and nitrogen imines are so oriented that their accessibility is not difficult and consequently, fast interaction with the free metal ions present in solution is feasible. Indeed, the two donor atoms act as a convergent chelating bidentate donor. The term convergent refers to the donor atoms coordinating to the same metal centre leading thus to a stable chelates.
4. CONCLUSIONS
In conclusion, porous polysiloxane with high specific surface and adjustable pore has gained renewed interest as a class of products presenting some particular characteristics when modified with selected organic groups. It is not surprising, therefore, to note that the chemistry of the interior surface of silica plays a dominant role in its chemical and physical behavior. It is this property that
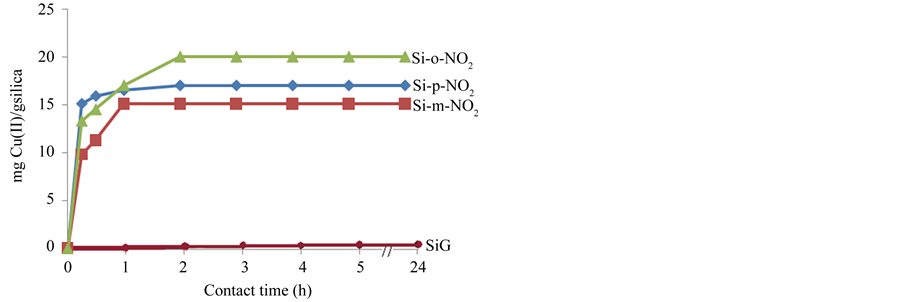
Figure 6. Adsorption kinetics of Cu(II) on modified samples (Si-o-NO2), (Si-m-NO2) and (Si-p-NO2).
makes silica gel an attractive material for use as adsorbents.
Although unmodified porous silica shows no specific interactions with Cu(II), organo-functionalization may be used to make them suitable for such metal ions complexation. Indeed, porous silica-immobilized ligand systems showed strong complexing properties in the presence of some heavy metals.
Acknowledgement
The authors gratefully acknowledge Bertrand REVEL, Research Engineer in the common center of NMR measurements of the University of Lille 1, for his assistance in recording 13C NMR spectra. We would also like to thank Ahmed MRABTI, technician at COSTE (Mohamed the 1st University) for atomic absorption measurements.
[2] World Health Organization, “Guidelines for DrinkingWater Quality: Recommendations-Addendum,” Vol. 1, 3rd Edition, 2008.
[3] K. K. Wong, C. K. Lee, K. S. Low and M. J. Haron, “Removal of Cu and Pb from Electroplating Wastewater Using Tartaric Acid Modified Rice Husk,” Process Biochemistry, Vol. 39, No. 4, 2003, pp. 437-445. http://dx.doi.org/10.1016/S0032-9592(03)00094-3
[4] K. G. Bhattacharyya and S. S. Gupta, “Adsorptive Accumulation of Cd(II), Co(II), Cu(II), Pb(II), and Ni(II) from Water on Montmorillonite: Influence of Acid Activation,” Journal of Colloid and Interface Science, Vol. 301, No. 2, 310, 2007, pp. 411-424. http://dx.doi.org/10.1016/j.jcis.2007.01.080
[5] S. Kubilay, R. Gürkan, A. Savran and T. Sahan, “Removal of Cu(II), Zn(II) and Co(II) Ions from Aqueous Solutions by Adsorption onto Natural Bentonite,” Adsorption, Vol. 13, No. 1, 2007, pp. 41-51. http://dx.doi.org/10.1007/s10450-007-9003-y
[6] D. P. Quintanilla, I. Hierro, M. Fajardo and I. Sierra, “Adsorption of Cadmium(II) from Aqueous Media onto a Mesoporous Silica Chemically Modified with 2-Mercaptopyrimidine,” Journal of Materials Chemistry, Vol. 16, 2006, pp. 1757-1764. http://dx.doi.org/10.1039/b518157g
[7] G. Dubois, C. Reyé, R. J. P. Corriu and C. Chuit, “Organic-Inorganic Hybrid Materials. Preparation and Properties of Dibenzo-18-Crown-6 Ether-Bridged Polysilsesquioxanes,” Journal of Materials Chemistry, Vol. 10, 2000, pp. 1091-1098. http://dx.doi.org/10.1039/a909201c
[8] D. P. Quintanilla, A. Sánchez, I. Hierro, M. Fajardo and I. Sierra, “Preparation, Characterization, and Zn2+ Adsorption Behavior of Chemically Modified MCM-41 with 5-Mercapto-1-Methyltetrazole,” Journal of Colloid and Interface Science, Vol. 313, No. 2, 2007, pp. 551-562. http://dx.doi.org/10.1016/j.jcis.2007.04.063
[9] Y. K. Lu and X. P. Yan, “An Imprinted Organic-Inorganic Hybrid Sorbent for Selective Separation of Cadmium from Aqueous Solution,” Analytical Chemistry, Vol. 76, No. 2, 2004, pp. 453-457. http://dx.doi.org/10.1021/ac0347718
[10] M. E. Mahmoud and E. M. Soliman, “Study of the Selective Extraction of Iron (III) by Silica-Immobilized 5-Formyl-3-Arylazo-Salicylic Acid Derivatives,” Talanta, Vol. 44, No. 6, 1997, pp. 1063-1071. http://dx.doi.org/10.1016/S0039-9140(96)02194-7
[11] A. Tong, Y. Akama and S. Tanaka, “Selective Preconcentration of Au(III), Pt(IV) and Pd(II) on Silica Gel Modified with γ-Aminopropyltriethoxysilane,” Analytica Chimica Acta, Vol. 230, 1990, pp. 179-181. http://dx.doi.org/10.1016/S0003-2670(00)82778-6
[12] E. F. S. Vieira, J. A. Simoni and C. Airoldi, “Interaction of Cations with SH-Modified Silica Gel: Thermochemical Study through Calorimetric Titration and Direct Extent of Reaction Determination,” Journal of Materials Chemistry, Vol. 7, 1997, pp. 2249-2252. http://dx.doi.org/10.1039/a704286h
[13] E. F. S. Vieira, A. R. Cestari, J. A. Simoni and C. Airoldi, “Use of Calorimetric Titration to Determine Thermochemical Data for Interaction of Cations with MercaptoModified Silica Gel,” Thermochimica Acta, Vol. 328, No. 1-2, 1999, pp. 247-252. http://dx.doi.org/10.1016/S0040-6031(98)00649-2
[14] W. Wasiak, “Specific Interactions of n-Donor Adsorbates with N-Benzoylthiourea-Copper (II) Complexes Chemically Bonded to Silica Supports,” Chromatographia, Vol. 41, No. 1-2, 1995, pp. 107-111. http://dx.doi.org/10.1007/BF02274203
[15] S. Radi, Y. Toubi, M. Bacquet, S. Degoutin and F. Cazier, “1-(Pyridin-2-yl) Imine Functionalized Silica Gel: Synthesis, Characterization, and Preliminary Use in Metal Ion Extraction,” Separation Science and Technology, Vol. 48, No. 9, 2013, pp. 1349. http://dx.doi.org/10.1080/01496395.2012.726309
[16] S. Radi, Y. Toubi and M. Bacquet, “Synthesis of Pyridin- 3-Yl-Functionalized Silica as a Chelating Sorbent for Solid-Phase Adsorption of Hg(II), Pb(II), Zn(II), and Cd(II) from Water,” Research on Chemical Intermediates, Vol. 39, No. 8, 2013, pp. 3791-3802. http://dx.doi.org/10.1007/s11164-012-0881-6
[17] S. Radi, S. Tighadouini, Y. Toubi and M. Bacquet, “Polysiloxane Surface Modified with Bipyrazolic Tripodal Receptor for Quantitative Lead Adsorption,” Journal of Hazardous Materials, Vol. 185, No. 1, 2011, pp. 494- 501. http://dx.doi.org/10.1016/j.jhazmat.2010.09.016
[18] S. Radi, Y. Toubi, A. Attayibat and M. Bacquet, “New Polysiloxane-Chemically Immobilized C,C-Bipyrazolic Receptor for Heavy Metals Adsorption,” Journal of Applied Polymer Science, Vol. 121, No. 3, 2011, pp. 1393- 1399. http://dx.doi.org/10.1002/app.33541
[19] S. Radi and A. Attayibat, “Functionalized SiO2 With SDonor Thiophene: Synthesis, Characterization, and Its Heavy Metals Adsorption,” Phosphorus, Sulfur, and Silicon and the Related Elements, Vol. 185, No. 10, 2010, pp. 2003-2013. http://dx.doi.org/10.1080/10426500903440042
[20] S. Radi, A. Attayibat, M. Bacquet, “Surface Modification of Porous Silica with Bi-thiophene Tripodal Ligand and Aplication to Adsorption of Toxic Metal Cations,” Phosphorus, Sulfur, and Silicon and the Related Elements, Vol. 185, No. 3, 2010, pp. 232-241. http://dx.doi.org/10.1080/10426500902758469
[21] S. Radi, A. Attayibat, A. Ramdani and M. Bacquet, “Synthesis and Characterization of Novel Porous SiO2 Material Functionalized with C,C-Pyridylpyrazole Receptor,” Journal of Applied Polymer Science, Vol. 117, No. 6, 2010, pp. 3345-3349. http://dx.doi.org/10.1002/app.32021
[22] S. Radi, A. Attayibat, A. Ramdani, Y. Lekchiri and M. Bacquet, “Synthesis and Characterization of a New Material Based on Porous Silica—Chemically Immobilized C,N-Pyridylpyrazole for Heavy Metals Adsorption,” Materials Chemistry and Physics, Vol. 111, No. 2-3, 2008, pp. 296-300. http://dx.doi.org/10.1016/j.matchemphys.2008.04.011
[23] S. Radi, A. Attayibat, A. Ramdani and M. Bacquet, “Synthesis and Characterization of Novel Silica Gel Supported N-Pyrazole Ligand for Selective Elimination of Hg(II),” European Polymer Journal, Vol. 44, No. 10, 2008, pp. 3163-3168. http://dx.doi.org/10.1016/j.eurpolymj.2008.07.021
[24] S. Radi, A. Ramdani, Y. Lekchiri, M. Morcellet, G. Crini, L. Janus and M. Bacquet, “Immobilization of Pyrazole Compounds on Silica Gels and Their Preliminary Use in Metal Ion Extraction,” New Journal of Chemistry, Vol. 27, No. 8, 2003, pp. 1224-1227. http://dx.doi.org/10.1039/b301111a
[25] S. Radi, A. Ramdani, Y. Lekchiri, M. Morcellet, G. Crini, J. Morcellet and L. Janus, “Preparation of Pyrazole Compounds for Attachment to Chelating Resins,” European Polymer Journal, Vol. 36, No. 9, 2000, pp. 1885-1892. http://dx.doi.org/10.1016/S0014-3057(99)00274-8
[26] S. Radi, A. Ramdani, Y. Lekchiri, M. Morcellet, G. Crini, L. Janus and B. Martel, “Extraction of Metal Ions from Water with Tetrapyrazolic Macrocycles Bound to Merrifield Resin and Silica Gel,” Journal of Applied Polymer Science, Vol. 78, No. 14, 2000, pp. 2495-2499. http://dx.doi.org/10.1002/1097-4628(20001227)78:14<2495::AID-APP90>3.0.CO;2-S
[27] R. Qu, M. Wang, C. Sun, Y. Zhang, C. Ji, H. Chen, Y. Meng and P. Yin, “Chemical Modification of Silica-Gel with Hydroxylor Amino-Terminated Polyamine for Adsorption of Au(III),” Applied Surface Science, Vol. 255, No. 5, 2008, pp. 3361-3370. http://dx.doi.org/10.1016/j.apsusc.2008.09.055
[28] S. Brunauer, P. H. Emmett and E. Teller, “Adsorption of Gases in Multimolecular Layers,” Journal of the American Chemical Society, Vol. 60, No. 2, 1938, pp. 309-319. http://dx.doi.org/10.1021/ja01269a023
[29] I.A. Banerjee, L. Yu and H. Matsui, “Cu Nanocrystal Growth on Peptide Nanotubes by Biomineralization: Size Control of Cu Nanocrystals by Tuning Peptide Conformation,” Proceedings of the National Academy of Sciences of the United States, Vol. 100, No. 25, 2003, pp. 14678-14682. http://dx.doi.org/10.1073/pnas.2433456100
[30] P. Roumeliotis, A. A. Kurganov and V. A. Davankov, “Effect of the Hydrophobic Spacer in Bonded [Cu(lHydroxyprolyl)alkyl]+ Silicas on Retention and Enantioselectivity of α-Amino Acids in High-Performance Liquid Chromatography,” Journal of Chromatography A, Vol. 266, 1983, pp. 439-450. http://dx.doi.org/10.1016/S0021-9673(01)90915-X
[31] G. V. Kudryavtsev, D. V. Milchenko, S. Z. Bernadyuk, T. E. Vertinskaya and G. V. Lisichkin, “Synthesis and Properties of Phosphate Cation-Exchangers Based on Silica,” Theoretical and Experimental Chemistry, Vol. 23, No. 6, 1988, pp. 658-663. http://dx.doi.org/10.1007/BF00534608
[32] X. Li and F. Xue, “Removal of Cu(II) from Aqueous Solution by Adsorption onto Functionalized SBA-16 Mesoporous Silica,” Microporous and Mesoporous Materials, Vol. 116, No. 1-3, 2008, pp. 116-122. http://dx.doi.org/10.1016/j.micromeso.2008.03.023
NOTES
*Corresponding author.


