Journal of Surface Engineered Materials and Advanced Technology
Vol.2 No.3(2012), Article ID:21320,5 pages DOI:10.4236/jsemat.2012.23025
Diamond Particles Deposited among Nickel/Copper Particles in Energy Controlled CH4/H2 RF Discharge Plasmas
![]()
Department of Electrical Engineering, Graduate School of Engineering, Tohoku University, Sendai, Japan.
Email: iizuka@ecei.tohoku.ac.jp
Received April 20th, 2012; revised May 26th, accepted June 8th, 2012
Keywords: Diamond Microparticle; Diamond Growth; Self-Organization; Graphite; Cu Particle
ABSTRACT
Formation of diamond particles was investigated in an energy-controlled CH4/H2 radio-frequency (RF) discharge plasma. Here, in particular, it was examined how diamond particles grew on a nickel substrate under an influence of Cu vapor that was supplied from a heated Cu wire. Here, the plasma was generated by a hollow-magnetron-type (HMT) RF plasma source at the frequency of 13.56 MHz. Total pressure was kept at 100 mTorr. Diamond particles grew besides Ni and Cu particles. From Raman spectrum the substrate surface was covered with thin graphite film deposited as a background layer. It was shown that diamond could grow in a self-organized manner even when the other atomic gas species such as Ni and Cu were contained in the gas at the same time during the growth process.
1. Introduction
Self-organization process is quite interesting phenomenon because an ordered structure will evolve even in a chaotic background. Here, it is also quite interesting that the growth of diamond happens even under a presence of many other obstacle atomic species that have been generated in plasmas by the physical sputtering, thermal evaporation and chemical dissociation. Diamond has only sp3 carbon bonding. However, during its growth, the site of carbon bonding might be attacked by the other atomic species such as Mo, Cu, and Ni. This reaction may result in a formation of metallic carbon with different bonding except for diamond. Concerning to the growth of diamond on metallic substrates, an intermediate layer or an incubation layer, combined with carbon and metals, will be usually produced before the start of diamond growth. In any case, in order to construct pure crystal structure of diamond, reactions of carbons with the other atomic species should be restricted even when they are contained simultaneously in the plasma during the deposition. Carbon has to exclude those atoms during the growth of diamond.
Reactions of carbons with such metallic atoms were usually preceded on the metal surface. Diamond films grown on Cu substrate were studied since Cu was an excellent substrate candidate for hetero-epitaxial growth owing to its cubic structure and lattice mismatch of only 1.3%. However, the nucleation density of diamond growth on Cu surface was very low, so it was a problem for further application in the industry. Moreover, less adhesion of diamond on Cu substrate was attributed to an insufficient formation of interfacial carbide layer [1,2]. Nucleation and growth of diamond film were also investigated by using substrate of Mo [3]. Since diamond had superior characteristics for the heat conductivity, the growth of diamond on Cu had attracted considerable attention for the heat dissipation for the electronic and power devices [4,5]. The problem was that a large thermal stress caused poor adhesives between Cu and diamond boundaries [6]. On the other hand, the growth of diamond on Mo was relatively easy because of a formation of Mo2C layer, which could provide a favorable intermediate layer for diamond nucleation. The growth of diamond on Ni substrate was also studied [2,7]
Here, high quality diamond could be produced in a low electron temperature plasma, where a novel method for the control of electron energy distribution function has been developed in order to reduce higher order dissociation of CH4 [8]. This technique was applied to CH4/ H2 plasma for diamond deposition. High quality diamonds and nanocrystalline diamonds were produced in the low electron temperature plasma by employing a grid method [9,10].
In the present study, we investigated the growth of diamond on Ni substrate under an influence of Cu vapor evaporated from Cu wire connected to a heating system for Ni substrate in CH4/H2 plasma. The growth of diamond was carried out in the lower electron temperature plasma by employing the grid method. The properties of the depositions were expected to depend on the electron temperature in the CH4/H2 plasma.
2. Experimental
Figure 1 shows a schematic of the experimental apparatus. There were two regions in a vacuum chamber, i.e., regions I and II. Separation mesh grid was made of stainless wires, to which dc voltage VG was biased. The plasma was generated in region I by a 13.56 MHz radio-frequency (RF) power source. Here, a hollow-type magnetron (HTM) RF plasma source was adopted, because it was expected that the plasma could be created by a synergy between the hollow-cathode effect and the magnetron effect. The magnetic field for providing the magnetron effect was supplied by two magnet rings wound around the plasma source. The plasma produced diffused in the axial z direction across the diverging magnetic field. Here, a cylindrical RF electrode of 16 cm or 9 cm in diameter was employed, to which RF power of 300 W was supplied through an impedance matching circuit with a blocking condenser for an efficient plasma production. By using the HTM plasma source, a highdensity plasma was efficiently produced.
A nickel substrate, which was kept at a floating potential, was set up in region II and was heated up to about 700˚C - 800˚C. In the heating section a tungsten W wire coil was placed in the ceramic holder and connected to Cu wires to feed the electric heating power. The temperature of W coil was increased up to about 1300˚C, therefore the Cu wires were heated to at least 500˚C - 600˚C, which was enough to sublimate Cu though lower than its melting point of 1083˚C. Nickel, of course, could deform its structure though its temperature was lower
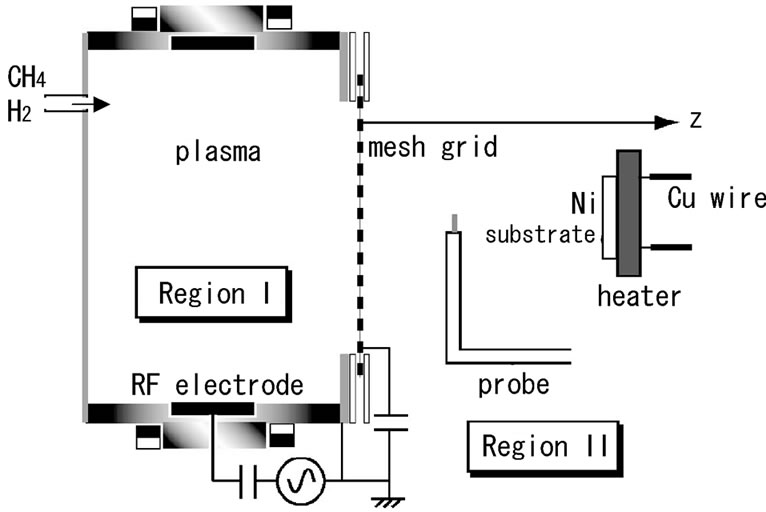
Figure 1. Schematic of the experimental apparatus.
than its melting point of 1453˚C. Before the deposition experiments, the surface of Ni substrate was not scratched artificially. Total flow rate of CH4 and H2 was fixed at 200 sccm. Total pressure was kept at 100 mTorr throughout the whole deposition experiment. The properties of the deposited films were characterized by a scanning electron microscopy (SEM) and Raman spectroscopy.
3. Experimental Results
The low electron temperature plasma was produced when the grid was kept at its floating potential. It was because that in our case the floating potential was decreased to about –20 V, which was sufficiently low enough for the electrons to be expelled towards the plasma source. Hence, those high-energetic electrons passing over such potential barriers could ionize neutral gas in downstream region. So, the low electron temperature plasma was produced by such ionization. In this case, the electron temperature Te in region II was kept constant, being almost independent of the contamination deposited on the mesh grid. Therefore, the plasma parameters were kept almost constant in time during the depositions. The electron temperature was an important key factor to produce diamond particles. When the electron temperature was high, disordered graphite and/or diamond-like carbon with broad D and G bands in Raman spectrum were simply obtained. On the other hand, when the electron temperature was decreased to be 1 eV or less, by the floating grid, it was possible to produce diamond particles.
Figure 2 shows a typical scanning electron microscopy image (SEI: secondary electron imaging) of the surface of Ni substrate, where many microparticles were observed. Though the shape of almost all particles was irregular, some of them were small spherical particles. Henceforth, the labels “a” and “c” were given to the bigger particles with irregular shape, and the label “b” was given to the small spherical particles, as shown in Figure 2.

Figure 2. SEI image of microparticles deposited on Ni substrate. Particles “a”, “b” and “c” are consisted of nickel, copper, and diamond, respectively. Pressure is 100 mTorr.
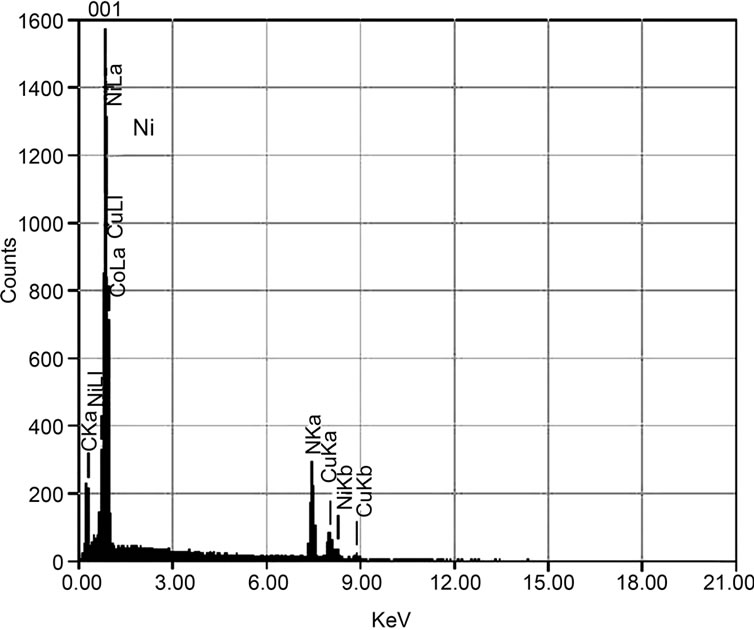
In order to examine the atomic components of these particles, energy disperse X-ray (EDX) spectroscopy was first carried out. Figures 3(a), (b), and (c) showed the typical EDX spectra of the particles “a”, “b”, and “c”, respectively. As can be seen in Figure 3(a), the microparticle “a” had a dominant peak of Ni. This meant that these particles were made of Ni, which might come from Ni substrate. By the heat, the Ni atoms could evaporate from the Ni substrate and/or migrate on the Ni surface. In fact, the surface of Ni substrate was changed a little from flat to coarse by the effect of heat even in the case of no plasma production. That is, Ni substrate temperature was sufficiently raised up to make its surface changed and eventually microparticle structure of Ni came out. In Figure 3(a) a small signal of carbon was also found, indicating that a small amount of carbon might be included in the Ni microparticles as a contamination. Otherwise, for another possibility, it was considered that the surface of Ni particles had been covered with a thin carbon film.
The EDX spectrum in Figure 3(b) showed that spherical microparticles “b” were mainly made of Cu. In this case, at the same time, a small signal of carbon was also detected. As in the case of Figure 3(a), there was a possibility that these spherical microparticles might either include carbon or be covered by a thin carbon film. However, it was quite difficult to separate these possibilities by only using the results of EDX. We will discuss this fact in the next section. Figure 3(c) shows that the particle “c” was made from carbon. A peak of Ni was also detected, but its intensity was considerably small, compared with those of the cases in Figures 3(a) and (b). So, it was confirmed that the particle “c” had been made of carbons.
 (a)
(a) (b)
(b) (c)
(c)
Figure 3. EDX spectra of microparticles “a”, “b”, and “c” in Figure 2 are shown in (a), (b) and (c), respectively. Dominant peaks in (a), (b) and (c) are Ni, Cu, and C, respectively.
In order to specify the carbon material, Raman spectrum of the particle “c” was taken as shown in Figure 4. The spot diameter of the laser beam for the Raman spectroscopy was about 1 μm, therefore, the spatial resolution was comparable to the particle size. Trace 1 was taken at the middle of the particle “c”. Here, a strong peak was detected at 1332 cm–1, which corresponded to diamond. Therefore, the carbon-dominated particle “c” was identified as a diamond. Half-width of the peak signal was less than 1 cm–1, showing that the purity of diamond was quite good. Trace 2 corresponded to the Raman signal at the edge of the diamond particle “c”. The peak intensity at 1332 cm–1 was reduced, but conversely a small peak came out at 1580 cm–1. The small peak at 1580 cm–1 corresponded to the G band of graphite. Therefore, the edge region of diamond particles was covered with graphite film. Trace 3 showed the Raman spectrum at the position about 5 μm away from the diamond particle. The signal showed that the surface outside the diamond particle was also covered with simple graphite. Note that the microparticles “a” and “b” were located near diamond “c” as shown in Figure 2. Therefore, the signals of carbon, detected in Figures 3(a) and (b), were considered as a thin graphite film deposited on these particles.
In order to distinguish these particles more clearly we took an image of composition mode (COMPO) in the backscattered electron imaging (BEI). This image could generate a compositional map of the sample. The COMPO image is shown in Figure 5" target="_self"> Figure 5. The microparticles “a”, “b”, and “c” were corresponding to those in Figure 2, respectively. In Figure 5" target="_self"> Figure 5 the dark image of microparticles “c” corresponded to diamond and could be separated clearly from the other microparticles “a” and “b”. This result showed that diamond particles were created on Ni substrate in spite of the fact that other particles made of Ni and Cu had been generated simultaneously. That is, the growth of diamond particles “c” seemed to be independent of those of the other kinds of particles “a” and “b”. Actually, the diamond grew on the surface of Ni substrate. Further, Ni and Cu particles also grew independently during the growth of diamond particles.
4. Discussion
Spherical microparticle “b” was found to be made of Cu. Here, the copper atoms were supplied from heated Cu wires connected to W wire installed in the Ni substrate holder made of ceramics. Therefore, in the gas phase, Cu atoms were mixed with CHn (n = 1 - 3) radicals produced in CH4/H2 plasma and had a chance to react with CHn radicals. However, EDX result showed that the microparticle “b” was mainly consisted of Cu. As previously reported [1,2], a less reactivity among Cu, Ni and CHn radicals was related with a low nucleation density of diamond on the Cu substrate. The shape of the sphere
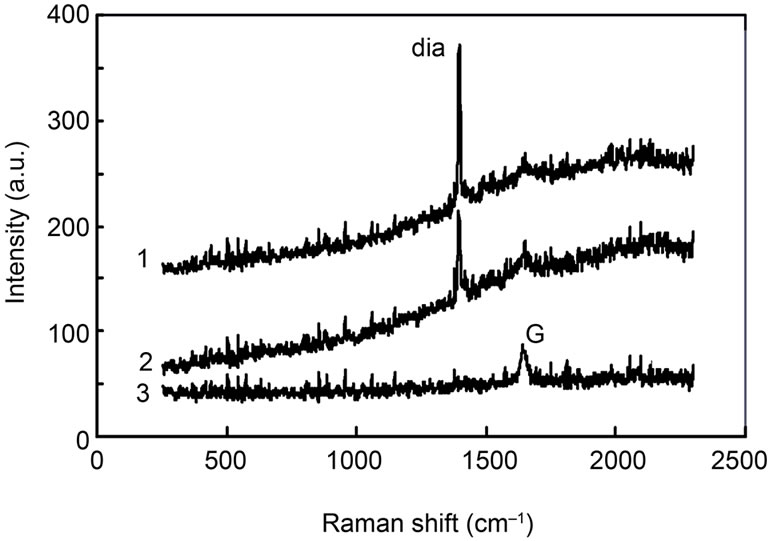
Figure 4. Raman spectra (1) at the center of particle “c”; (2) at the edge of “c”; and (3) at the position 5 μm away from particle “c”. The peaks of diamond at 1332 cm–1 and G band at 1580 cm–1 are denoted by “dia” and “G”, respectively.

Figure 5. COMPO image of microparticles, deposited on Ni substrate. Particles “a”, “b” and black colored “c” are consisted of nickel, copper, and diamond, respectively. Pressure is 100 mTorr.
indicated that these Cu particles had grown in a space of the plasma without touching any Ni substrate surface. If these particles were grown on the Ni surface from the beginning, their shape should not be sphere but something like hemisphere.
The formation process of Cu spheres was considered as follows. If Cu vapor was contained in the plasma, Cu atoms could react each other to make Cu clusters and finally they could coagulate each other to make a bigger Cu particle. This was possible because the reaction ability of C and Cu was less [1,2]. It was also well known that these particles were negatively charged in the plasma by the effect of plasma electrons and able to be trapped and levitated in the plasma by the electrostatic force evolved at the plasma sheath [8]. Moreover, these particles, levitated in the plasma, could be heated up by an ion bombardment by the ions accelerated toward the Cu particle by the sheath electric field produced around the Cu particle. Then, the Cu particles would be in a quasi-liquid state. Therefore, a point-symmetric structure, i.e., a spherecal structure, could be built up. These particles were eventually cooled down by the collision with neutral gas and fell down by chance on the Ni substrate, where the diamond particles were growing. Note that Cu atoms could also arrive at the diamond surface, but they could not easily react with carbon [1,2]. Therefore, the growth of diamond was not disturbed by the Cu atoms.
It should be also noted that the entire part of the substrate surface had been covered with graphite as shown by Raman spectrum in Figure 4. No significant signal of D band, corresponding to the disordered structure of graphite, appeared in Raman spectrum. This meant that relatively pure graphite had deposited on the surface. In the region near the substrate, the electron temperature was reduced to be about 1 eV [10]. So, CH4 will not be so much dissociated to provide enough C, CH, and CH2 radicals. Main species was supposed to be CH3 in this region. It was also known that the species CH3 would play a key role on the formation of diamond and the less amount CH2 would play a key role on the graphite formation.
5. Conclusion
A formation of diamond particles was investigated in an energy-controlled CH4/H2 RF discharge plasma. Here, it was examined in particular how the diamond particles grew on a nickel substrate under influence of Cu vapor that was supplied from heated Cu wire. Diamond particles have grown together with the growth of Ni and Cu particles. Less reaction ability among C, Cu, and Ni was important for their independent growth. It was also thought that the spherical shape of Cu particles had been produced via Cu droplets heated by ion bombardment. From Raman spectrum the substrate surface was covered with thin graphite film deposited as a background layer. It was shown that the diamond could grow in a self-organized manner even when the other atomic species such as Ni and Cu were contained in the processing gas at the same time during the growth process.
6. Acknowledgements
The work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- W. E. Picket, M. R. Pederson, K. A. Jackson and S. C. Erwin, “Theoretical Electronic Structure Studies of Diamond: Surfaces, Adsorbates, Defects and Heterointerfaces,” Materials Science and Engineering: B, Vol. 14, No. 1, 1992, pp. 87-92. doi:10.1016/0921-5107(92)90334-6
- P. C. Yang, W. Zhu and J. T. Glass, “Nucleation of Oriented Diamond Films on Nickel Substrates,” Journal of Materials Research, Vol. 8, No. 8, 1993, pp. 1773-1776. doi:10.1557/JMR.1993.1773
- M. Ece, B. Oral and J. Patscheider, “Nucleation and Growth of Diamond Films on Mo and Cu Substrates,” Diamond and Related Materials, Vol. 5, No. 3-5, 1996, pp. 211-216.
- Y. J. Chen and T. F. Young, “Thermal Stress and Heat Transfer Characteristics of a Cu/Diamond/Cu Heat Spreading Device,” Diamond and Related Materials, Vol. 18, No. 2-3, 2009, pp. 283-286.
- P. Hui and H. S. Tan, “Temperature Distributions in a Heat Dissipation System Using a Cylindrical Diamond Heat Spreader on a Copper Heat Sink,” Journal of Applied Physics, Vol. 75, No. 2, 1994, pp. 748-757. doi:10.1063/1.356480
- A. Fernandes, A. Neves, R. F. Silva and M. H. Nazare, “Evaluation of MPCVD Diamond Film Adhesion on Hard Metal Substrates by Micro Raman Spectroscopy,” Diamond and Related Materials, Vol. 6, No. 5-7, 1997, pp. 769-773.
- T. Shimizu, S. Iizuka, K. Kato and N. Sato, “High Quality Diamond Formation by Electron Temperature Control in Methane-Hydrogen Plasma,” Plasma Sources Science and Technology, Vol. 12, No. 4, 2003, pp. 821-825. doi:10.1088/0963-0252/12/4/316
- G. Nishimura, S. Iizuka, G. Uchida and N. Sato, “Diamond-Particles Levitated in a Reactive Plasma,” Diamond and Related Materials, Vol. 12, No. 3-7, 2003, pp. 374-377.
- R. Ikada, G. Nishimura, K. Kato and S. Iizuka, “Production of High Density and Low Electron-Temperature Plasma by a Modified Grid-Biasing Method Using Inductively Coupled RF Discharge,” Thin Solid Films, Vol. 457, No. 1, 2004, pp. 55-58. doi:10.1016/j.tsf.2003.12.013
- K. Kato, S. Iizuka, N. Sato, “Electron-Temperature Control for Plasmas Passing through a Negatively Biased Grid,” Applied Physics Letters, Vol. 65, No. 7, 1994, pp. 816-818. doi.org/10.1063/1.112240

