Journal of Encapsulation and Adsorption Sciences
Vol.07 No.02(2017), Article ID:77243,12 pages
10.4236/jeas.2017.72008
Adsorptive Removal from Aqueous Solution of Cr(VI) by Green Moringa Tea Leaves Biomass
Candice C. Timbo1, Martha Kandawa-Schulz1, Marta Amuanyena2, Habauka M. Kwaambwa2*
1Department of Chemistry and Biochemistry, University of Namibia, Windhoek, Namibia
2Faculty of Health and Applied Sciences, Namibia University of Science and Technology, Windhoek, Namibia

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 18, 2017; Accepted: June 25, 2017; Published: June 28, 2017
ABSTRACT
Hexavalent chromium, Cr(VI), is a toxic metal present in industrial effluents. The study was carried to test the use of green Moringa leaves biomass as adsorbent for Cr(VI) from aqueous solutions. Batch adsorption method was used and the concentration of Cr(VI) measured using an ultraviolet-visible (UV-Vis) spectrophotometry. The effects of the adsorption contact time, adsorbent dosage, pH, initial adsorbate concentration and temperature were studied. Results show maximum removal of Cr(VI) of 99% ± 1%, with maximum adsorption capacity of 33.9 mg/g at a pH 2, 60 minutes contact time and 100 mg/l initial Cr(IV) concentration. The Langmuir and Freundlich adsorption isotherm models were used to fit the experimental data. The data showed that adsorption on green Moringa oleifera leaves tea biomass fitted well to Freundlich isotherm (r2 = 0.9432) compared to the Langmuir isotherm (r2 = 0.9122). M. oleifera leaves biomass can be used in water purification systems. The sludge of M. oleifera leaves is biodegradable, cost effective and environmentally friendly and therefore attractive in hexavalent chromium removal in water.
Keywords:
Adsorbent, Adsorption Isotherm, Heavy Metals, Hexavalent Chromium, Moringa oleifera

1. Introduction
Effluents from industrial activities are normally the culprits in pollution of water bodies by heavy metals such as cadmium (Cd), chromium (Cr), lead (Pb), mercury (Hg), nickel (Ni), etc. Heavy metals are non-biodegradable, carcinogenetic and bioaccumulate and hence they have been implicated in causing a wide range of diseases and disorders. Hexavalent chromium, Cr(VI), is one of the heavy metals that has been associated with health problems which include digestive tract and lungs cancer, diarrhea, nausea, vomiting, to mention but a few, Cr(VI), just like many other heavy metals, is discharged to the environment from industries such as electroplating, leather tanning and paints and pigments. Since Cr(VI) has carcinogenic, mutagenic and teratogenic properties, discharge from such industries has the potential to contaminate water bodies.
As result of the health problems associated with heavy metal pollution in water, there are several methods are being used to remove or recover them from industrial effluents. The techniques normally used include chemical precipitation, coagulation, flotation, ion-exchange, reverse osmosis, electrochemical treatments, cementation, membrane separation, hyperfiltration, evaporation, oxidation and solvent extraction [1] . Secondly, there are several disadvantages associated these techniques. Firstly, there is an issue of cost of the reagents and energy requirements which are serious problem for developing countries. Secondly, the operational costs involving disposal of toxic sludge generated because some of these techniques, such as precipitation, do not remove the heavy metals to acceptable limits and, in fact, produce waste that is difficult to treat. Techniques like ion exchange, although effective, require costly adsorbents materials for the removal of heavy metals from dilute aqueous streams. In view of the above-mentioned drawbacks, the use of low-cost materials of biological origin as adsorbents are being advocated by many researchers because they provide an economic solution through reduction of exorbitant costs for water and wastewater treatment. Many researchers are using these materials to study their adsorption properties and thus determine their potential in water and wastewater treatment. Adsorption is process by which impurities are selectively transferred from the fluid phase to the surface of particles suspended. The advantage is adsorption over other methods is that it requires a simple design with sludge free environment and hence can involve low investment in terms of both initial cost and land required.
The study was aimed at using M. oleifera tea leaf biomass waste as a possible sustainable technology to a global problem of water and wastewater treatment. M. oleifera is often referred to a multipurpose tree because it has been found to have nutritional, antimicrobial, medicinal, industrial and water treatment properties. For instance, Moringa leaves have been used as natural antihelmintic, antioxidant, antibiotic, detoxifer, and immune builders in some countries for the treatment of malnutrition and malaria. Because of the health and nutritional benefits of the leaves, there is an increase in the number of Moringa products on the market such as Moringa tea. As result, more and more people are buying Moringa products and therefore the cultivation of Moringa and consumption of Moringa tea will grow worldwide especially southern Africa [2] . The aim of this study is to find out the effectiveness of green M. oleifera tea leaf biomass waste as an adsorbent for the removal of Cr(VI) from water. Contact time, adsorbent dosage, pH, initial Cr(VI) concentration and temperature were the parameters used to study the adsorption behavior of Cr(VI) on M. oleifera tea leaf biomass.
2. Materials and Methods
2.1. Preparation of Adsorbent
Green M. oleifera leaves were obtained from a tree in Cimbebasia, Windhoek. The leaves were washed and air dried in an open space away from sunlight. After drying, the leaves were washed several times with hot to boiling distilled water until a colourless filtrate was obtained. The decolourized Moringa leaves were then dried in an oven at 105˚C. After drying, the leaves were ground to a fine powder using a mortar and pestle and then sieved to size range 250 - 500 µm. Crushed leaves powder was then stored in sealed bottles at room temperature for the adsorption studies.
2.2. Batch Adsorption Studies
A stock solution which contains 1000 mg/l of Cr(VI) was prepared using analytical grade potassium dichromate (K2Cr2O7) in distilled water. The adsorption experiments were carried out in 30 ml test tubes with the Moringa leaves adsorbent and the Cr(VI) solution. The concentration of chromium was then determined using an ultraviolet-visible spectrophotometer at 350 nm. The batch adsorption studies were then carried out by varying the experimental conditions, namely contact time, adsorbent dosage, initial concentration of chromium and pH. The percentage removal of Cr(VI) and adsorption capacity of the adsorbent for each concentration of Cr(VI) ions at equilibrium, qe, were calculated, respectively, using Equations (1) and (2):
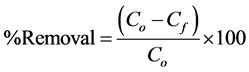 (1)
(1)
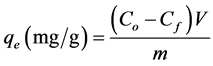 (2)
(2)
where Co is initial chromium concentration, Cf is final concentration, i.e. after treatment, V is the volume of solution (l) and m is the mass of adsorbent. To eliminate any instrumental error, the metal ion concentration before and after adding adsorbent was analyzed via a calibration curve with concentrations ranging from 10 to 100 mg/l prepared from the stock solution of K2Cr2O7. The results from the calibration curve (not shown here) indicated that absorbance increases linearly with increase in concentration. The correlation coefficient value, r2, calculated from the linear calibration curve data gave a value of 0.997 which shows that there is a significant linear relationship between the absorbance and the concentration.
To make the mixtures, a known amount of adsorbent was added to a solution of known metal ion concentration, and the resulting mixture was shaken using a mechanical shaker (Stuart Scientific). The mixture was then centrifuged and filtered using Whatman paper No. 1 filter paper. The concentration on metal ion in the filtrate, i.e. concentration of the un adsorbed metal ion in a solution, was the determined. The effects of contact time, adsorbent dosage, pH, initial Cr(VI) concentration and temperature were studied. To adjust pH of the mixtures as required, 0.1 M HCl and 0.1 M NaOH were used. The data reported here represent the mean of the two independent experiments (i.e. each experiment done in duplicate). The difference between two replicate experiments was less than 10% in all cases.
The Langmuir and Freundlich isotherm models were used to test the adsorption process of Cr(VI) on the Moringa tea leaf biomass. The linear forms of Langmuir and Freundlich equations are given in Equation (3) and (4), respectively [3] [4] [5] :
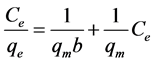 (3)
(3)
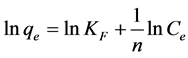 (4)
(4)
In Equations (3) and (4), Ce is the equilibrium concentration of adsorbate (mg/L) and qe is the amount in grams of Cr(VI) adsorbed per gram of adsorbent at equilibrium, qm and b are Langmuir constants related to adsorption capacity and rate of adsorption, respectively. The values of qm and b (with typical of units mg/g and l/mg, respectively) are calculated from the slope and intercept of the linear Langmuir plot of Ce/qe versus Ce. In the Freundlich equation, KF and n are the constants incorporating all factors affecting the adsorption process (adsorption capacity and intensity). The values of the constants KF and n are calculated from the intercept and slope of the Freundlich linear plot.
3. Results and Discussion
3.1. Effect of Contact Time
The contact time required to reach adsorption equilibrium of Cr(VI) ions was studied between 10 to 100 minutes by carrying out the experiments at constant initial metal ion concentrations (100 mg/l), adsorbent dosage (0.4 g/50 ml), neutral pH, stirring speed (165 rpm) and room temperature. The results of the effect contact time on removal of Cr(VI) under these conditions are shown in Figure 1. As the contact time increased, the amount of Cr(VI) adsorbed also increased. After 60 minutes, the solution attained equilibrium. This result is in agreement with other adsorption studies on similar type of materials whereby the equilibrium time was attained within two hours [3] .
The progress of adsorption process can be described in two distinct stages. Typically, the first stage is involves a rapid adsorption, whereas the next stage is a slower adsorption process. Veena Devi et al. [4] explained that the rapid adsorption stage is as a result of a large numbers of vacant sites that are present, which ultimately leads to fast adsorption. There is then the slowing down of the process which can be attributed to the decrease in the heat of adsorption. The heat of adsorption is decreased because it is affected by the increase in work function (work done to overcome repulsion to the surface) with increase is surface
Figure 1. Effect of contact time of the Cr(VI) adsorption on green tea biomass (Dosage = 0.4 g).
coverage. The interaction between adsorbed species also results in less heat of adsorption being given out as more adsorbate molecules are adsorbed.
3.2. Effect of Solution pH
Solution pH is one of the important factors controlling the process of adsorption of metal ions as it affects the surface charge density of the adsorbent [5] [6] . It is known that the pH dependence of metal adsorption is largely related to the surface functional groups in the biosorbents and metal solution chemistry [7] .
A pH range of 1 - 11 was used to study the effect pH in adsorption experiments were carried out in the pH range of 1 - 11. As mentioned earlier, the adjustment was done using 0.1 M HCl and 0.1 M NaOH while keeping all other parameters constant, i.e. chromium concentration (100 mg/l), adsorbent dosage (0.4 g/50 ml), stirring speed (165 rpm), contact time (60 minutes), and room temperature.
Figure 2 shows that as the pH was increased, the percentage removal of Cr(VI) was decreased from about 97% at pH 2 to as low as 9.45% at pH 6. The maximum adsorption took place at lower pH and pH 2 was taken to be optimum value. Adsorption of Cr(VI) is clearly a function of solution pH and one of the species H2CrO4, 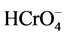 ,
, 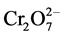 or
or 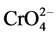 will be dominant depending on the pH [5] . The dominant ionic form of Cr(VI) at pH 2 is
will be dominant depending on the pH [5] . The dominant ionic form of Cr(VI) at pH 2 is  while
while  is dominant in the range of pH > 7 based on the pKa values [8] [9] .
is dominant in the range of pH > 7 based on the pKa values [8] [9] .
At acidic pH (~2), in which higher level Cr(VI) elimination was observed, adsorbent surface is highly protonated, facilitating 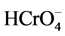 anion removal as a result of electrostatic interaction based adsorption. However, surface protonation decreases when pH is increased. There is strong competition between
anion removal as a result of electrostatic interaction based adsorption. However, surface protonation decreases when pH is increased. There is strong competition between 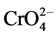 and OH− species present at high pH values. Thus, the number of positive sites on adsorbent surface becomes reduced at high pH, showing a decrease in Cr(VI) removal capacity.
and OH− species present at high pH values. Thus, the number of positive sites on adsorbent surface becomes reduced at high pH, showing a decrease in Cr(VI) removal capacity.
3.3. Effect of Adsorbent Dosage
The effect of adsorbent dosage on the adsorption of Cr(VI) is shown in Figure 3. Using 100 mg/l of Cr(VI), a fixed temperature (60˚C), optimum pH 2 and 60 minutes contact time, the solutions were agitated at 165 rpm with different Moringa dosages ranging from 0.05 g to 1.0 g in 50 ml.
The results show that Cr(VI) removal efficiency increases with increase in adsorbent dosage until it becomes constant (i.e. optimum percentage removal of 99% ± 1%) at around 0.5 g of the adsorbent. Jeyaseelan and Gupta [8] observed similar results with the removal efficiency reaching a constant value at 0.8 g/50 ml adsorbent dosage of green tea leaves of Camellia sinensis plant species. The results obtained obviously follow such a trend due to more adsorption sites being available with the as amount of adsorbent increases. Since adsorption sites adsorbent particles increase, it would be more probable for 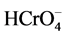 and
and 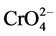 ions to be adsorbed and thus adsorption efficiency should also increase as stated
ions to be adsorbed and thus adsorption efficiency should also increase as stated
Figure 2. Effect of pH on Cr(VI) removal.
Figure 3. Effect of adsorbent dosage on adsorption of Cr(VI).
articulated by Bansal et al., [8] . In this particular case, the concentration of the Cr(VI) ions is constant and therefore increasing the adsorbent dosage increases the available adsorption surface area. The limiting constant maximum removal capacity could be explained from the fact that all adsorbents have a finite number of active sites which should become saturated at some adsorbate concentration.
3.4. Effect of Initial Metal Ion Concentration
A range of concentrations varying from 10 mg/l to 150 mg/l of Cr(VI) were taken, while keeping the dosage, pH, temperature and contact time fixed. It was observed that the removal of Cr(VI) is dependent on the initial concentration. Figure 4 shows that adsorption is higher at lower concentrations, i.e. increasing initial Cr(VI) concentration showed a decrease in metal removal. Changing the initial concentration of Cr(VI) in solution from 10 - 150 mg/l caused the percent removal to decrease from 75% to 8%. At low concentration, the metal ions interact with the binding sites, and result in maximum adsorption. This is because at low concentration, the ratio of available surface to the initial Cr(VI) concentration is larger, so the removal is higher. Therefore, at low initial metal ion concentrations, the removal capacity is higher. However, in the case of higher concentrations, this ratio is low and hence the percentage removal is also lesser. As the concentration increases, the metal ions start looking for free binding sites, and due to a lack of binding sites for complexation, the adsorption reduces [10] . In other words, when metal ion concentrations are increased, binding sites become saturated more quickly since the amount of biomass concentration remains constant.
3.5. Effect of Temperature
Adsorption of Cr(VI) on M. oleifera leaf biomass was studied using six different temperatures (25˚C, 30˚C, 40˚C, 50˚C, 60˚C and 70˚C) at fixed pH 2, chromium
Figure 4. Effect of initial concentration removal of Cr(VI).
concentration (100 mg/L), adsorbent dosage (0.4 g/50 ml), stirring speed (165 rpm) and contact time (60 minutes). Figure 5 shows the effect of temperature on the removal efficiency. Generally in the temperature range used, the percent removal of Cr(VI) increases from 84% at 25˚C reaching a maximum of 98% at 60˚C. Adsorption efficiency seems to decrease at temperatures higher than 60˚C. The increase in sorption capacity of the biosorbent is attributed to the enlargement of pore size and activation of the sorbent surface with rise in temperature [11] . It has been stated that a further rise in temperature increases the thermal motion of the metal ions and reduces the swelling effect in biosorbent, thus, enabling the metal ions to penetrate further [12] [13] . Similar results of optimal temperature have been observed for Cr(VI) adsorption with activated carbon [14] . The decrease in adsorption with rise in temperature may be due to desorption caused by the existing thermal energy.
3.6. Adsorption Isotherms
Langmuir and Freundlich adsorption isotherms were obtained and are given in Figure 6 and Figure 7, respectively, whereas the resulting corresponding maximum adsorption capacities and regression constants, r2, are tabulated in Table 1. The r2 value which is close to 1 indicates a good fit to given adsorption model. The adsorption of Cr(VI) on M. oleifera leaves tea biomass gives a better fit of the Freundlich isotherm in comparison to the Langmuir isotherm.
The essential feature of the Langmuir isotherm can be expressed in terms of separation factor (RL) which is a dimensionless constant also referred to equilibrium parameter. RL can be calculated by using equation:
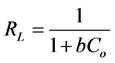 (5)
(5)
where Co is the initial adsorbate concentration (mg/L) and b can be obtained from Langmuir plot of Ce/qe versus Ce and the constant is related to the energy
Figure 5. Effect of temperature on Cr(VI) removal.
Figure 6. Linear Langmuir isotherm for adsorption of Cr(VI).
Figure 7. Linear Freundlich isotherm for adsorption Cr(VI).
Table 1. Langmuir and Freundlich isotherm constants for the adsorption Cr(VI) on Moringa tea leaf biomass.
of adsorption. The separation factor, RL, the isotherm shape as unfavorable adsorption is when RL > 1, linear for RL = 1, favorable when 0 < RL < 1 and irreversible when RL = 0.
In this study, the value of RL was found to be 0.00862. From the result, it is evident that the value of RL on removal of chromium using Moringa tea leaves biomass as adsorbent lies between 0 and 1 and hence, adsorption of the adsorbate seems to be favorable.
In the case of the Freundlich isotherm, KF and n are constants at a particular temperature and for a particular adsorbent and adsorbate. The magnitude of the exponent, 1/n, is a heterogeneity parameter or gives an indication of the favorability of adsorption; the smaller 1/n, the greater the expected heterogeneity. If n lies in the range 1 - 10, it indicates a favorable sorption process. From the data in Table 1, the value of 1/n = 0.373 and hence n = 2.68, indicating that the sorption of Cr(VI) on to Moringa tea leaf biomass is favorable.
4. Conclusions
The study showed the enormous potential of green Moringa tea leaves biomass for Cr(VI) species removal found in water and wastewater. The following conclusions are drawn from the above results:
・ Adsorbent prepared from Moringa leaves biomass could be used for the removal of Cr(VI) from aqueous solutions. Adsorption of Cr(VI) on Moringa tea leaf biomass yielded maximum adsorption capacity of 33.9 mg/g at solution pH 2. In comparison to other adsorbents such as the ones shown in Table 2, Moringa leaves biomass has comparable or higher adsorption capacity.
・ The equilibrium time for the adsorption of Cr(VI) on the adsorbent prepared from Moringa tea leaf biomass in the present study from aqueous solutions is found to be 60 minutes.
・ The percentage removal of Cr(VI) using optimum conditions, i.e. pH 2, contact time of 60 minutes, a temperature of 60˚C and a dosage of 0.5 g, was found to be 99% ± 1% using initial concentration of 100 mg/l.
・ Increase of adsorbent dosage causes an increase in the removal of Cr(VI).
・ The adsorption process of Cr(VI) can be described by Langmuir and Freundlich isotherm models. However, Freundlich isotherm model shows a better agreement with the equilibrium data.
Table 2. Comparison of adsorbent capacity of various adsorbents.
The characterization of the surface of the green Moringa tea leaves biomass by some analytical techniques such as infrared spectroscopy, scanning electron microscopy (SEM), BET surface area, and surface charge by measuring zeta potential may have provided more insight the adsorption mechanism of Cr(VI).
Acknowledgements
The authors wish to acknowledge the research funding from NCRST and NRF under the Namibia - South Africa Research Partnership Bilateral Agreement programme.
Cite this paper
Timbo, C.C., Kandawa-Schulz, M., Amuanyena, M. and Kwaambwa, H.M. (2017) Adsorptive Removal from Aqueous Solution of Cr(VI) by Green Moringa Tea Leaves Biomass. Journal of Encapsulation and Adsorption Sci- ences, 7, 108-119. https://doi.org/10.4236/jeas.2017.72008
References
- 1. Fu, F. and Wang, Q.I. (2011) Removal of Heavy Metal Ions from Wastewaters: A Review. Journal of Environmental Management, 92, 407-418.
https://doi.org/10.1016/j.jenvman.2010.11.011 - 2. Kwaambwa, H.M., Chimuka, L., Kandawa-Schulz, M., Munkombwe, N.M. and Thwala, J.M. (2012) Situational Analysis and Promotion of the Cultivation and Utilisation of the Moringa oleifera Tree in Selected Sub-Saharan Africa Countries. Progress Multi-Disciplinary Research Journal, 4, 9-40.
- 3. Vargas-Nieto, C., Carriazo, J.G. and Castillo, E. (2011) A Study of Low-Cost Adsorbent Materials for Removing Cr(VI) from Aqueous Waste Effluent. Ingeniería E Investigación, 31, 154-162.
- 4. Veena Devi, B., Jahagirdar, A.A. and Zulfiqar Ahmend, M.N. (2012) Adsorption of Chromium on Activated Carbon Prepared from Coconut Shell. International Journal of Engineering Research and Applications, 2, 364-370.
- 5. Venkateswarlu, P., Venkata Ratnam, M., Rao, D. and Rao, M. (2007) Removal of Chromium from an Aqueous Solution Using Azadirachta indica (neem) Leaf Powder as an Adsorbent. International Journal of Physical Sciences, 2, 188-195.
http://www.academicjournals.org/IJPS - 6. Bansal, M., Singh, D., Garg, V.K. and Rose, P. (2008) Mechanisms of Cr(Vi) Removal from Synthetic Wastewater by Low Cost Adsorbents. Journal of Environmental Research and Development, 3, 228-243.
- 7. Chen, P., Xiong, Z., Luo, J., Lin, J. and Tan, K.L. (2002) Interaction of Hydrogen with Metal Nitrides and Imides. Nature, 420, 302-304.
https://doi.org/10.1038/nature01210 - 8. Jeyaseelan, C. and Gupta, A. (2016) Green Tea Leaves as a Natural Adsorbent for the Removal of Cr(VI) from Aqueous Solutions. Air, Soil and Water Research, 9, 13-19. https://doi.org/10.4137/ASWR.S35227
- 9. Dehghani, M.H., Sanaei, D., Ali, I. and Bhatnagar, A. (2016) Removal of Chromium(VI) from Aqueous Solution Using Treated Waste Newspaper as a Low-Cost Adsorbent: Kinetic Modeling and Isotherm Studies. Journal of Molecular Liquids, 215, 671-679.
https://doi.org/10.1016/j.molliq.2015.12.057 - 10. Gebrehawaria, G., Hussen, A. and Rao, V.M. (2015) Removal of Hexavalent Chromium from Aqueous Solutions Using Barks of Acacia albida and Leaves of Euclea schimperi. International Journal of Environmental Science and Technology, 12, 1569-1580.
https://doi.org/10.1007/s13762-014-0530-2 - 11. Taheryan, P., Shahidi, A. and Najafi Mood, M.H. (2015) Removal Performance Assessment of Chromium (VI) in Solution Using Grape Leaves Powder and Carbon as Adsorbent. International Journal of Research Studies in Agricultural Sciences, 1, 21-28.
- 12. Mckay, G., Blair, H.S. and Gardener, J.K. (1982) Adsorption of Dyes on Chitin. Equilibrium Studies. Journal of Applied Polymer Science, 27, 3043-3057.
https://doi.org/10.1002/app.1982.070270827 - 13. Guo, Y.P, Yang, S.F, Yu, K.F, Wang, Z.C. and Xu, H.D. (2002) Adsorption of Cr(VI) on Micro- and Mesoporous Rice Husk-Based Active Carbon. Materials Chemistry and Physics Journal, 78, 132-137.
- 14. Mohanty, S., Bal, B. and Das, A.P. (2014) Adsorption of Hexavalent Chromium onto Activated Carbon. Austin Journal of Biotechnology & Bioengineering, 1, 5.
- 15. Gupta, S. and Babu, B.V. (2006) Adsorption of Cr(VI) by a Low-Cost Adsorbent Prepared from Neem Leaves. Proceedings of National Conference on Environmental Conservation, 1-3, 175-180.
- 16. Vinodhini, V. and Das, N. (2010) Relevant Approach to Assess the Performance of Sawdust as Adsorbent of Chromium (VI) Ions from Aqueous Solutions. International Journal of Environmental Science and Technology, 7, 85-92.
https://doi.org/10.1007/bf03326120 - 17. Munir, K., Yusuf, M., Noreen, Z., Hameed, A., Hafeez, F.Y. and Faryal, R. (2010) Isotherm Studies for Determination of Removal Capacity of Bi-Metal (Ni and Cr) Ions by Aspergillus niger. Pakistan Journal of Botany, 42, 593-604.
- 18. Dakiky, M., Khamis, M., Manassra, A. and Mereb, M. (2002) Selective Adsorption of Chromium (VI) in Industrial Wastewater Using Low-Cost Abundantly Available Adsorbents. Advances in Environmental Research Journal, 6, 533-540.
- 19. Dhanakumar, S., Solaraj, G., Mohanraj, R. and Pattabh, S. (2007) Removal of Cr (VI) from Aqueous Solution by Adsorption Using Cooked Tea Dust. Indian Journal of Science and Technology, 1, 1-6.
http://www.indjst.org









