Journal of Encapsulation and Adsorption Sciences
Vol.3 No.3(2013), Article ID:37168,5 pages DOI:10.4236/jeas.2013.33011
Preparation of Microcapsules with Liquid Droplet Coalescence Method Followed by Phase Separation
1Department of Management Information, Niigata University of Management, Niigata, Japan
2Department of Research & Development, Shyofu Inc., Kyoto, Japan
3Graduate School of Science and Technology, Niigata University, Niigata, Japan
Email: *tanaka@eng.niigata-u.ac.jp
Copyright © 2013 Yasushi Yokoyama et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 23, 2013; revised August 23, 2013; accepted August 30, 2013
Keywords: Core Shell Microcapsule; Liquid Droplet Coalescence Method; Phase Separation; Limonene Oil; Liquid-Liquid Dispersion
ABSTRACT
Novel preparation method of microencapsules was developed on the basis of the liquid coalescence method followed by phase separation. Oil droplets of limonene dissolving expanded polystyrene as a shell material were forced to collide and coalesce with the Isopar oil droplets of core material in the continuous wates phase. When two kinds of oil droplets are collided and coalesced with each other, expanded polystyrene dissolved in the limonene oil may be phase-separated in the oil droplets newly formed to form the microcapsule shell, because the Isopar oil was a poor solvent for expanded polystyrene but a good solvent for the limonene oil. In the experiment, the diameter (or number) of limonene oil droplets dissolving expanded polystyrene was mainly changed, because the coalescence frequency between the droplets is strongly dependent on the number of droplets. Favorable core shell types of microcapsules with the shell thickness from 1.0 to 5.0 μm were able to be prepared under all the experimental conditions adopted here.
1. Introduction
At present, microcapsules have been applied in the various fields such as cosmetics, pharmacy, foods, information recording materials, adhesives, coating materials, and so on.
These microcapsules have been prepared with the various preparation methods such as the chemical method, the physicochemical method and the mechanical method [1,2]. If the shell is able to be formed by chemical reaction, the chemical method is selected, and if the shell is able to be formed only by physicochemical process, the physicochemical method is selected. However, it is well known that each preparation method has some merits and demerits. Accordingly, these preparation methods are adopted in parallel or in series at occasional demands.
The preparation method has been mainly decided by the physical properties of both core, shell materials and the desired functions of microcapsules.
For example, if a core material is water soluble or hydrophilic, the oil soluble material or the hydrophobic material is generally selected as the shell material to improve the protection and isolation of core material microencapsulated [3-5]. In general, the in-situ polymerization method [6] and the interfacial polymerization method [7,8] belonging in the chemical method category are suitable to microencapsulation of the liquid core material. Also, the coacervation method and the phase separation method belonging in the physicochemical method category are often used for microencapsulating the liquid core. The microencapsulation methods stated just above are the complicated preparation methods and require much time to prepare the microcapsules with the desired functions.
Taking these into consideration, we tried to develop the novel preparation method for microencapsulating the oily core material.
This preparation method is on the basis of the liquid droplet coalescence method followed by phase separation [4] belonging in the physicochemical method category.
Namely, the oil droplets dissolving a shell material are forced to collide and coalesce with the core oil droplets in the continuous water phase. Here, the core oil droplet phase is a poor solvent for the shell material but a good solvent for the oil droplet phase dissolving the shell material.
When the oil droplets dissolving the shell material are forced to collide and coalesce with the core oil droplets, the shell material has to be separated in the newly formed oil droplets by phase separation, because the core oil phase is a poor solvent for the shell material.
If interfacial energy between the continuous phase and the shell material is lower than that between the continuous phase and the newly formed oil phase, the shell material separated in the newly formed oil droplets has to transfer to the outer region of the newly formed oil droplet and form the microcapsule shell.
The purpose of this study is to investigate whether the core shell microcapsules containing the core oil are able to be prepared with the liquid droplet coalescence method followed by phase separation or not and to discuss the formation mechanism of microcapsules.
2. Experimental
2.1. Materials
Polyvinyl alcohol (PVA: Polym. Degee: 500) was used as a water soluble stabilizer.
Limonene oil was used as the solvent dissolving expanded polystyrene (PS) which forms the microcapsule shell.
Isopar oil was used as the core material, in which Disperbyk 108 (BYK-Chemie Japan K.K.) of carboxylic acid ester was dissolved as a oil soluble stabilizer.
Distilled water was used as the continuous phase in which PVA is dissolved.
2.2. Preparation of Microcapsules
Figure 1 shows the microencapsulation mechanism with the liquid droplet coalescence method followed by phase separation presented in this work.
In this figure, the Isopar oil droplets of the core material play a role of mother droplets and the limonene oil droplets dissolving PS play a role of daughter droplets.
The limonene oil is a good solvent for PS and the Isopar oil is a poor solvent for PS and a good solvent for
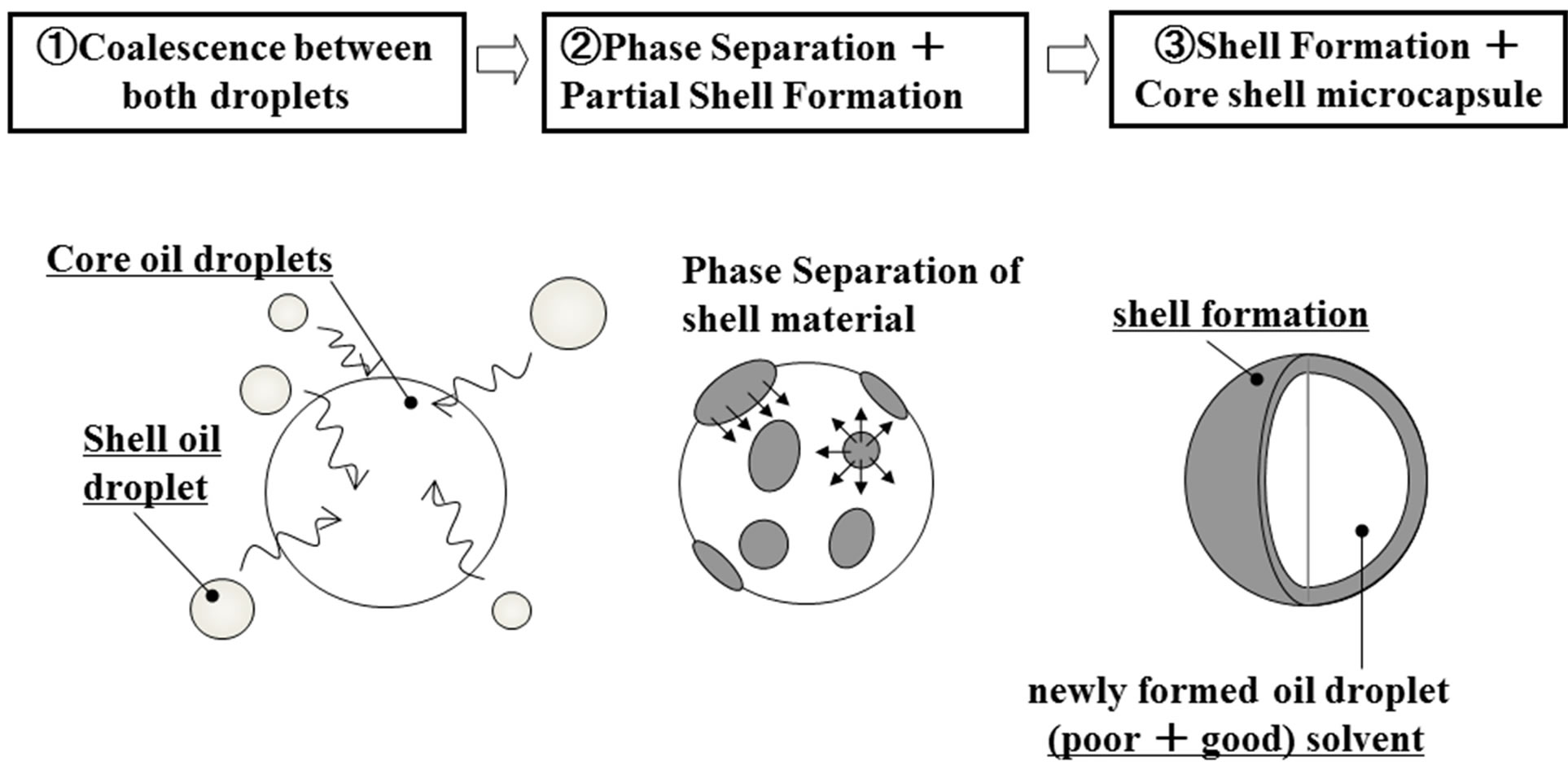
Figure 1. Microencapsulation mechanism with liquid droplet coalescence method followed by phase separation.
the limonene oil. When the limonene oil droplets dissolving PS are forced to collide and coalesce with the Isopar oil droplets, PS dissolved in the limonene oil droplets may be separated in the newly formed oil droplets and transfered to the outer region of these oil droplets to form the microcapsule shell. In this case, it is necessary for interfacial energy between the shell material and the water phase to be smaller than that between the Isopar oil and the water phase. Thus, the core shell microcapsules have to be prepared on the basis of this formation mechanism.
Figure 2 shows the flow chart for preparing microcapsules. At first, two kinds of the (O/W) dispersions are prepared, namely, one is the (OA/W) dispersion, where the limonene oil droplets dissolving PS are dispersed in the continuous phase dissolving PVA and the other is the (OB/W) dispersion, where the Isopar oil droplets are dispersed in the continuous phase dissolving PVA.
When two kinds of the (O/W) dispersions are poured into the reactor under stirring, two kinds of oil droplets may begin to collide and coalesce with each other and then, the core shell microcapsules may be formed under the condition of interfacial energy stated above.
Figure 3 shows the schematic diagram of experimental apparatus used in this study. The reactor was a separable flask with the volume of 500 cm3, in which four baffles with 8.5 cm length made from aluminum plate were settled on the reactor wall. This reactor was set in the water bath to keep temperature of the reactor constant.
Two kinds of the (O/W) dispersions were prepared with the rotor stator type homogenizer. A six bladed disc type impeller as shown in Figure 3 was used in the microencapsulation process.
The experimental conditions are shown in Table 1.
Hereafter, the limonene oil droplets dissolving PS and the Isopar oil droplets may be called as the shell oil droplets and the core oil droplets, respectively.
3. Results and Discussion
In order to prepare the core shell microcapsules according
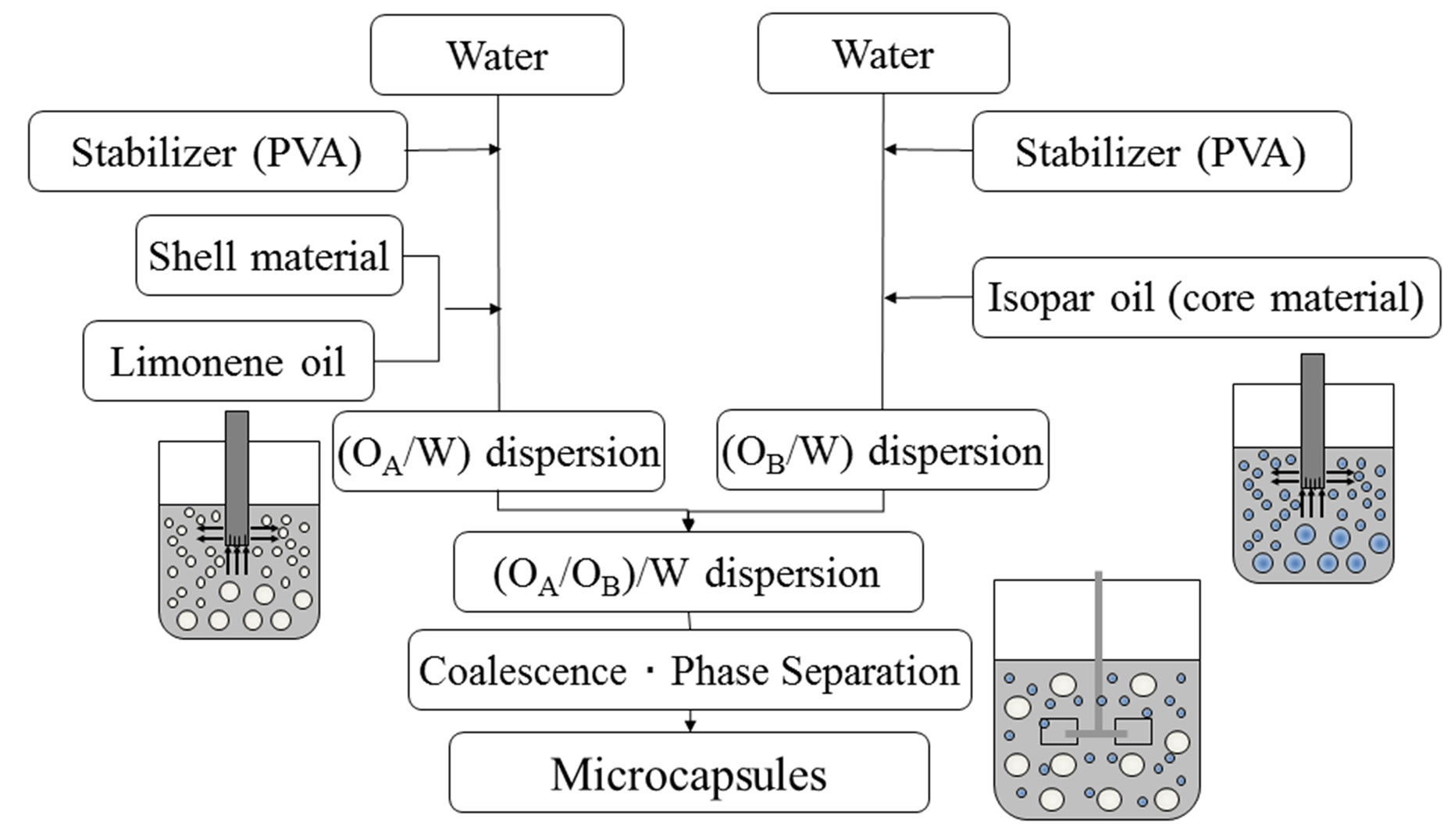
Figure 2. Flow chart for preparing microcapsules.
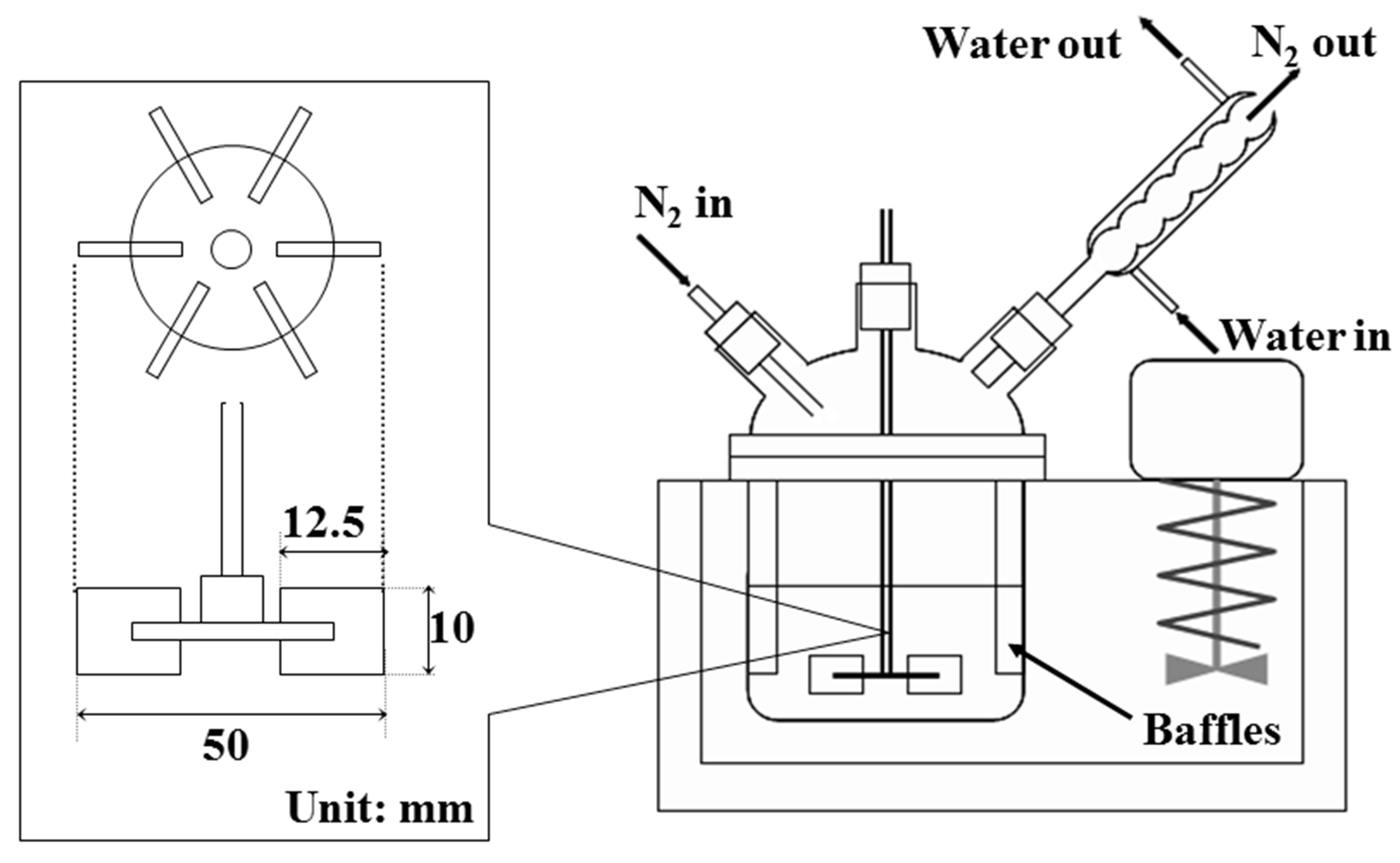
Figure 3. Schematic diagrams of experimental apparatus.
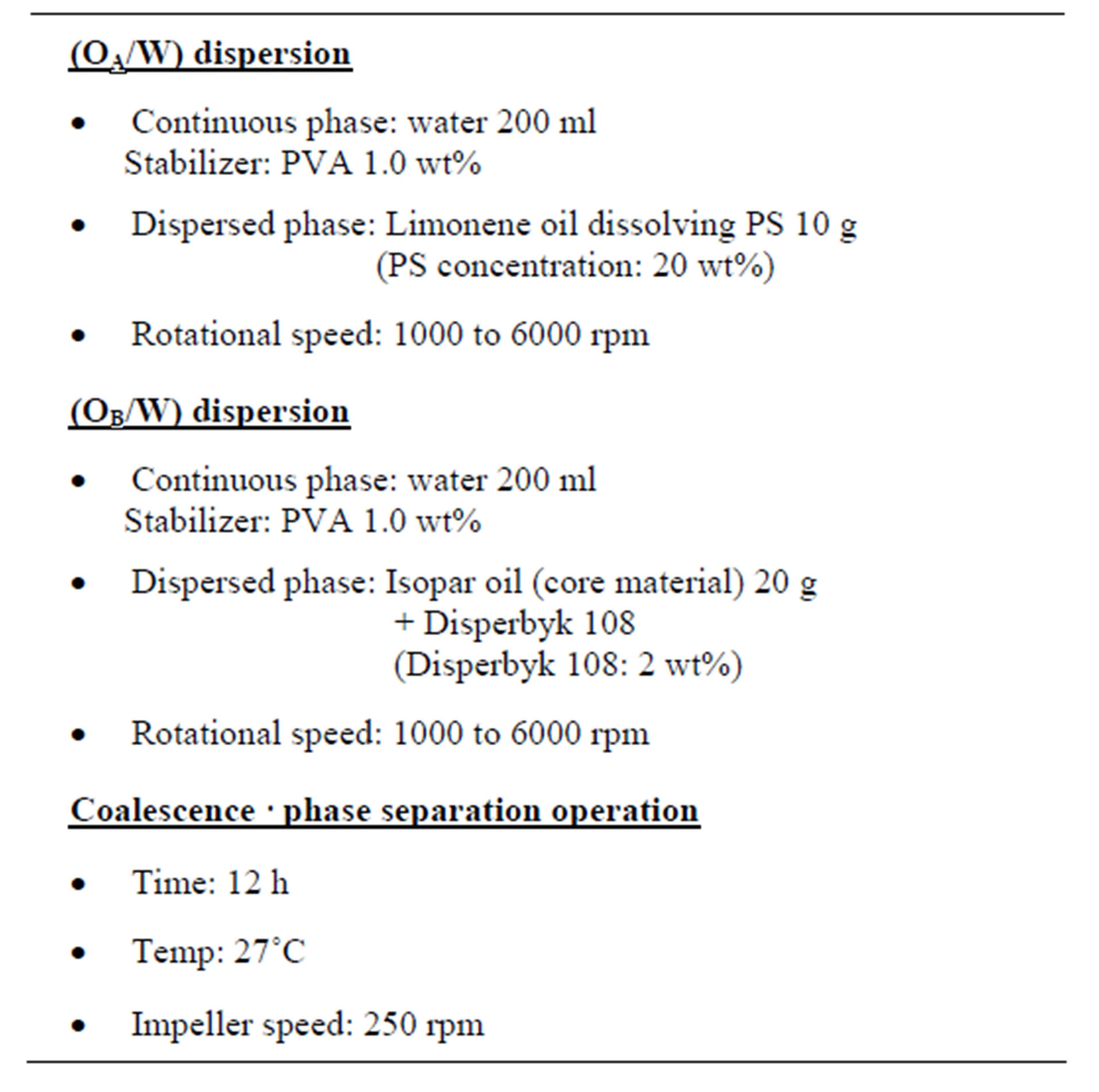
Table 1. Experimental conditions.
to the microencapsulation mechanism presented here, it is necessary for two kinds of oil droplets to collide and coalesce with each other. As the frequencies of collision and coalescence between the liquid droplets are strongly dependent on the rotational speed and the number and diameter of droplets, the diameters (namely, the number) of shell oil and core oil droplets are changed at first under the constant rotational speed [9,10].
Figure 4 shows the dependence of diameters of two kinds of oil droplets on the rotational speed of homogenizer. From this figure, it is found that the diameters of two kinds of oil droplets are proportional to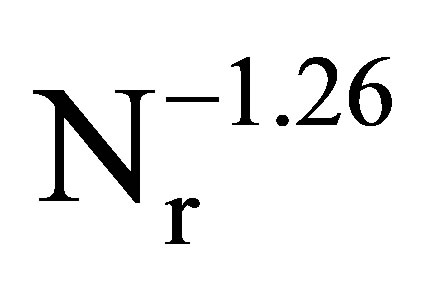 . This dependence is in agreement with that in the conventional dispersion, where the oil droplets have been formed under the condition of breakup dominant [10,11].
. This dependence is in agreement with that in the conventional dispersion, where the oil droplets have been formed under the condition of breakup dominant [10,11].
Figure 5 shows the optical microphotographs of shell oil droplets (a) and core oil droplets (b) prepared under the given conditions (Nr = 4500 rpm). It is found that the oil droplets with the broad diameter distribution are formed. In the experiment, microcapsules have been prepared by changing the diameters of both oil droplets.
Figure 6 shows the optical microphotographs and SEM photographs of a microcapsule prepared under the same conditions as those in Figure 5.
Microcapsule is found to be the core shell type and have the shell of the thickness of ca. 1 μm. In order to prepare the favorable microcapsules according to the microencapsulation mechanism presented in this study, the shell oil droplets should collide and coalesce preferentially
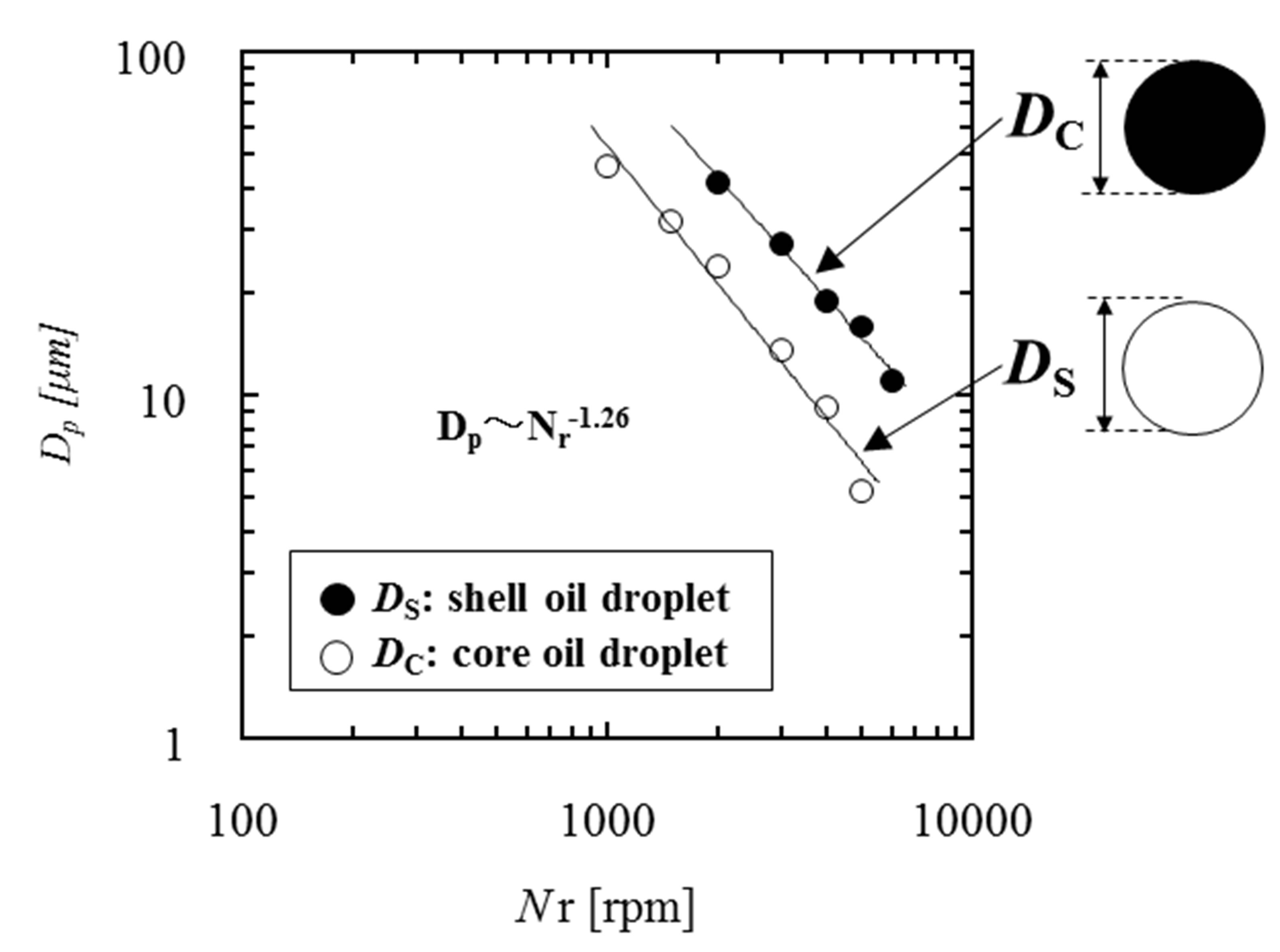
Figure 4. Dependence of mean diameters of two kinds of oil droplets on rotational speed.
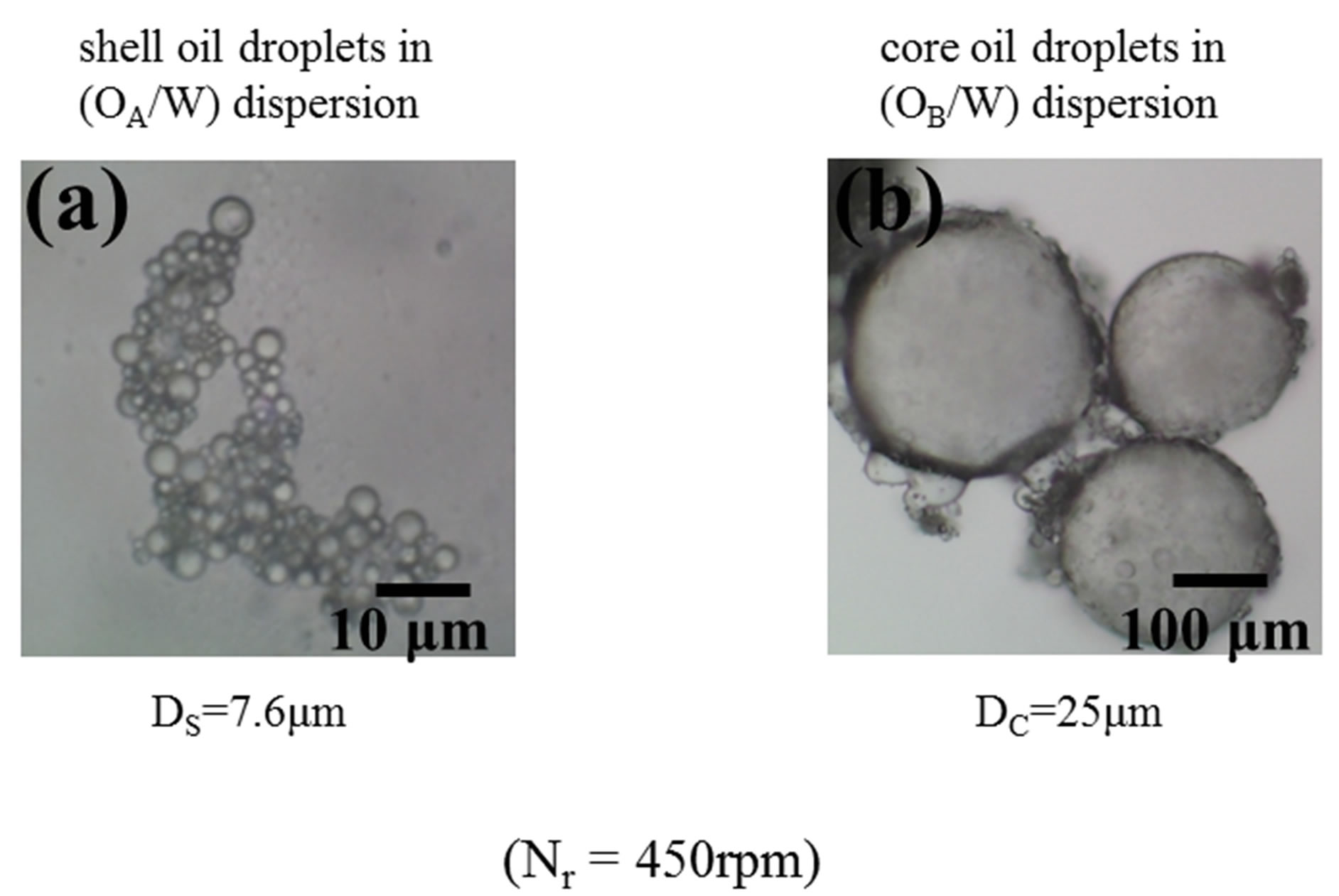
Figure 5. Observation of oil droplets.
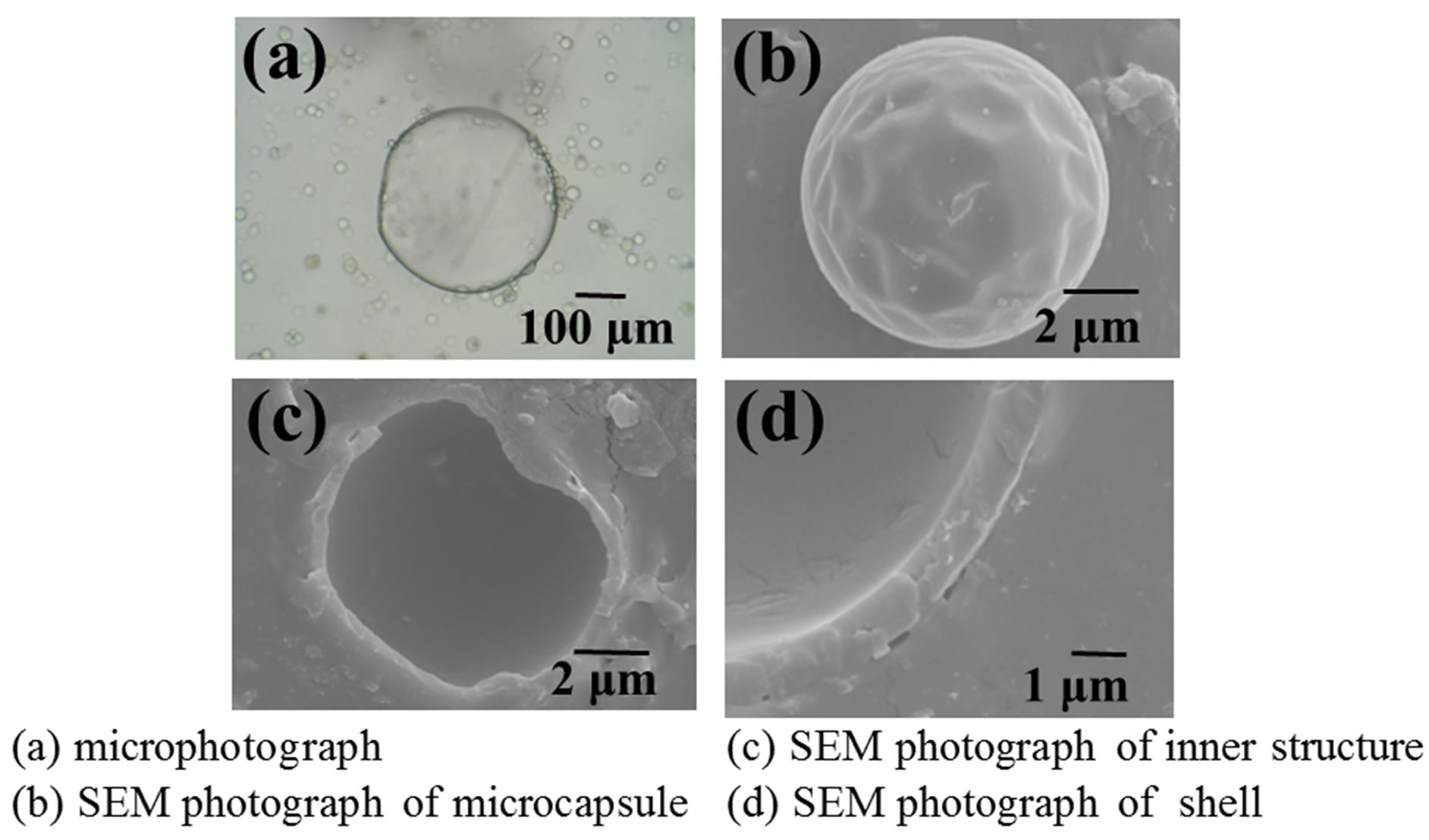
Figure 6. Optical microscopic and SEM photographs of microcapsule.
with the core oil droplets. For this purpose, it is necessary that the number (ns) of shell oil droplets is very larger than that (ns) of core oil droplets.
Taking this into consideration, it is tried to prepare microcapsules by changing the diameter of shell oil droplets.
Figure 7 shows the SEM photographs of microcapsules prepared by changing the diameter of shell oil droplets, namely the number of shell oil droplets. The number of core oil droplets with DC = 20.7 μm is 2.6 × 102 number/cm3 and those of shell oil droplets with DS = 9.6, 14.3, 26.1, 42.1 μm are 3.1 × 102, 2.1 × 102, 1.1 × 102, 7 × 10 number/cm3, respectively. It is found that the favorable microcapsules are able to be prepared under all the experimental conditions.
Figure 8 shows the effect of the diameter of shell oil droplets on the diameters (DM) and shell thickness (H) of microcapsules in the case of DC = 20.7 μm. It is found that the diameters (DM) of microcapsules linearly increase with those (DS) of shell oil droplets.
As the shell oil droplets coalesce with the core oil droplets and the new oil droplets are formed, the diameters of microcapsules have to become larger than those of core oil droplets.
Here, the diameter of microcapsule can be expressed with the diameters of shell oil droplet and core oil droplet as follows.
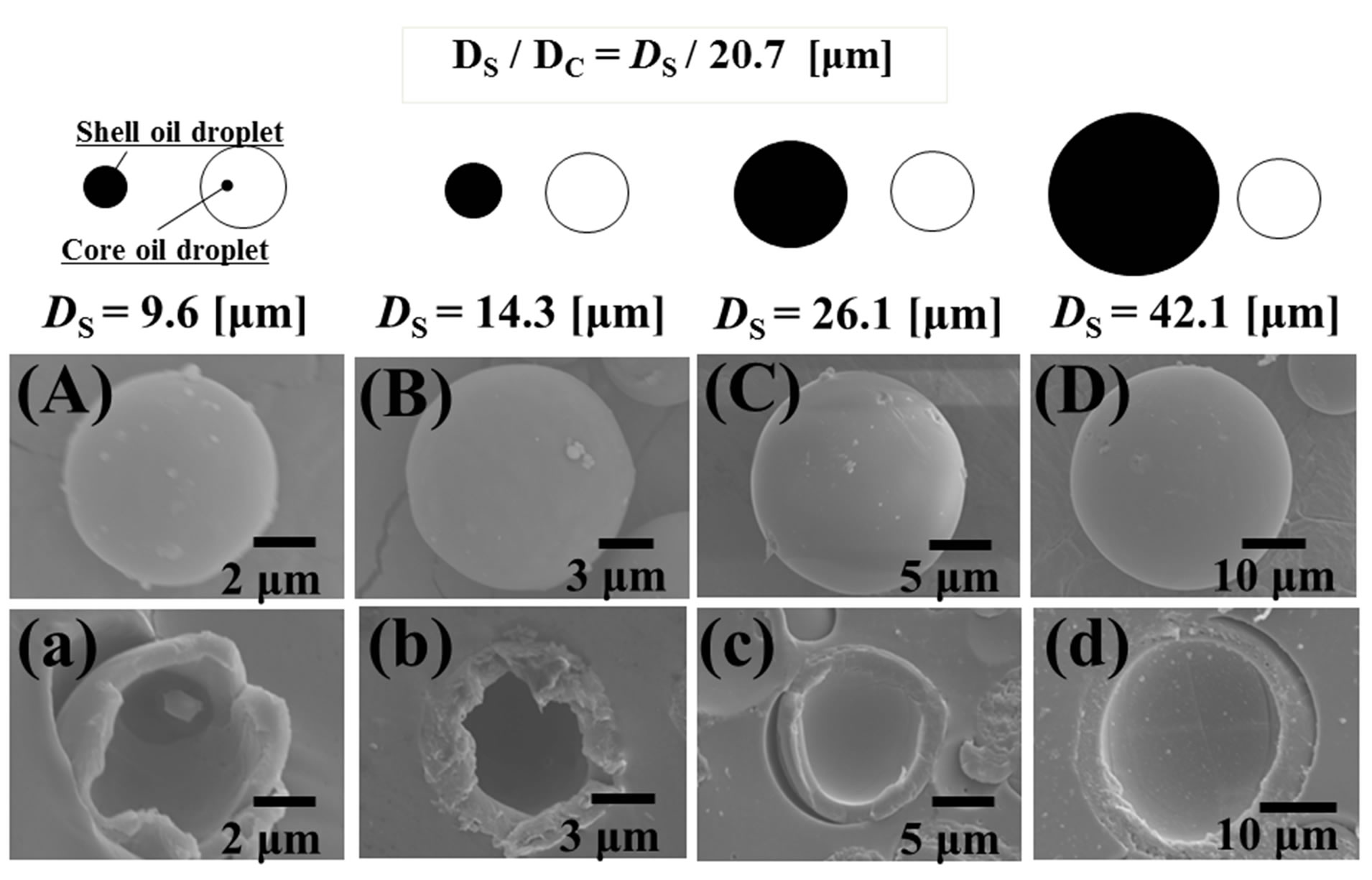
Figure 7. Effect of shell oil droplet diameter on structure of microcapsule.
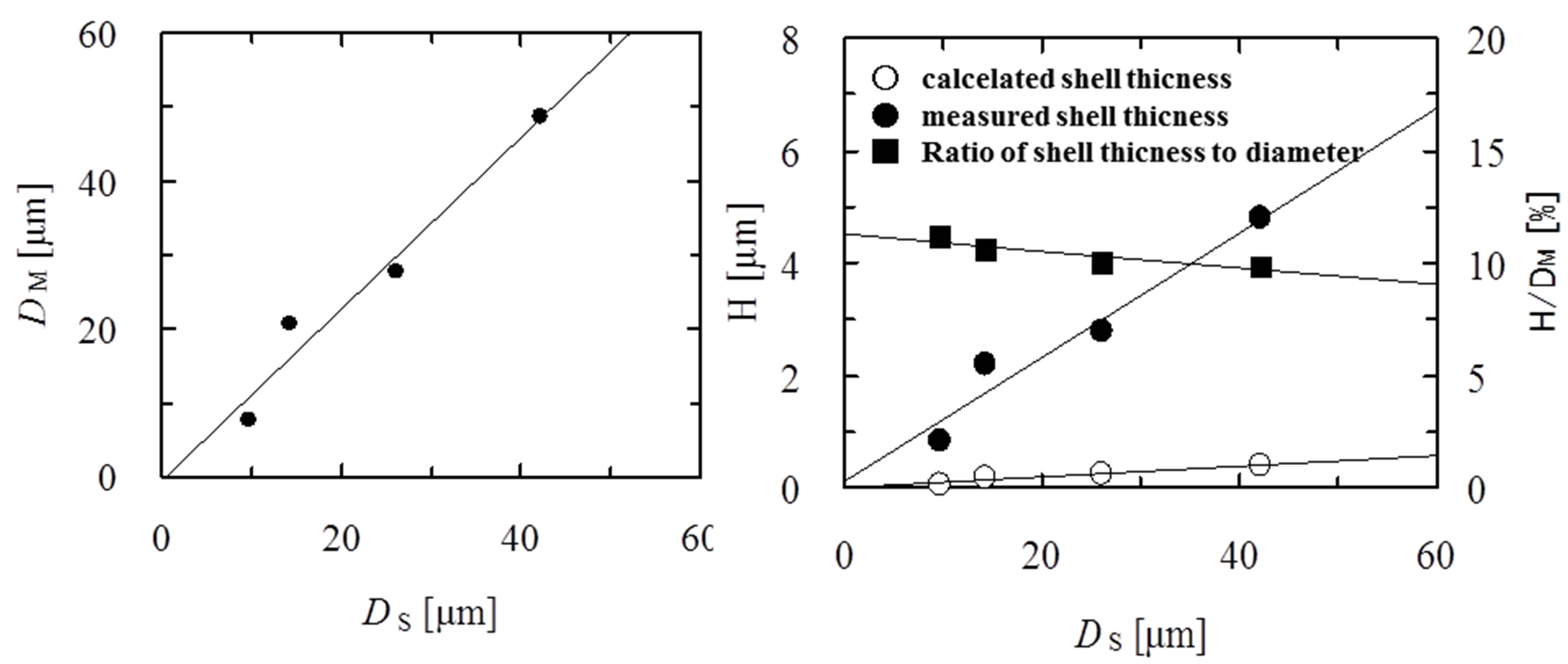
Figure 8. Effect of shell oil droplet diameter on diameter and shell thickness of microcapsule.
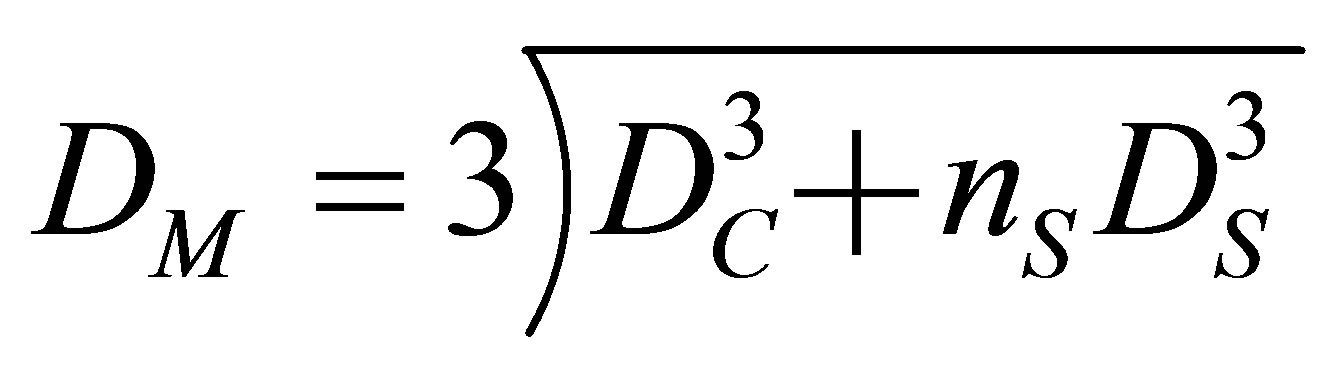 (1)
(1)
Then, Equation (2) can be derived.
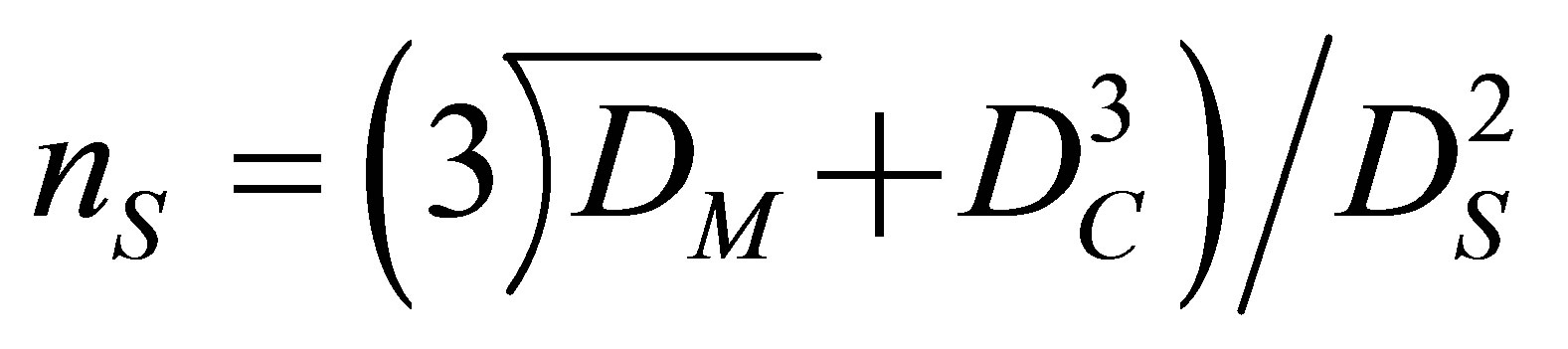 (2)
(2)
Accordingly, the number of shell oil droplets coalesced with a core oil droplet can be calculated by interpolating the values of DM, DC and DS into Equation (2).
Table 2 shows the values of nS calculated by Equation (2).
For a example, nS = 13.6 at DM = 20, DC = 20.7 and DS = 18 means that the shell oil droplets of number of 13.6 with the diameter of 18 μm coalesce with a core shell oil droplet with the diameter of 20.7 μm and form a microcapsule with the diameter of 20 μm.
As there is the broad distribution in the diameters of core oil droplets and shell oil droplets, the values of nS calculated here may be a measure for estimating the number of coalescence between the core oil droplets and the shell oil droplets.
The dependence of the shell thickness of microcapsule on the diameters of shell oil droplets is also shown in Figure 8.
The values of the shell thickness calculated are found to increase with the diameter of shell oil droplet and to be largely different from those measured.
This result is thought to be attributable to the fact that the calculated values are derived by assuming coalescence of a core oil droplet with many shell oil droplets of uniform droplet diameter. Furthermore, two kinds of oil droplets have the broad diameter distribution and coalescence between the core oil droplets, between the shell oil droplets and between microcapsules formed are ignored completely. The ratio of the shell thickness to the diameter of microcapsule is found to decrease with the diameter of shell oil droplet. This result is thought to be attibutable to the fact that the increasing rate of diameters of microcapsules is larger than that of the shell thickness.
Figure 9 shows the formation mechanism of microcapsules on the basis of the results obtained above.
Namely, if the diameters of shell oil droplets (dark circle) are very smaller than those of core oil droplet (white circle), the shell may be formed preferentially on the
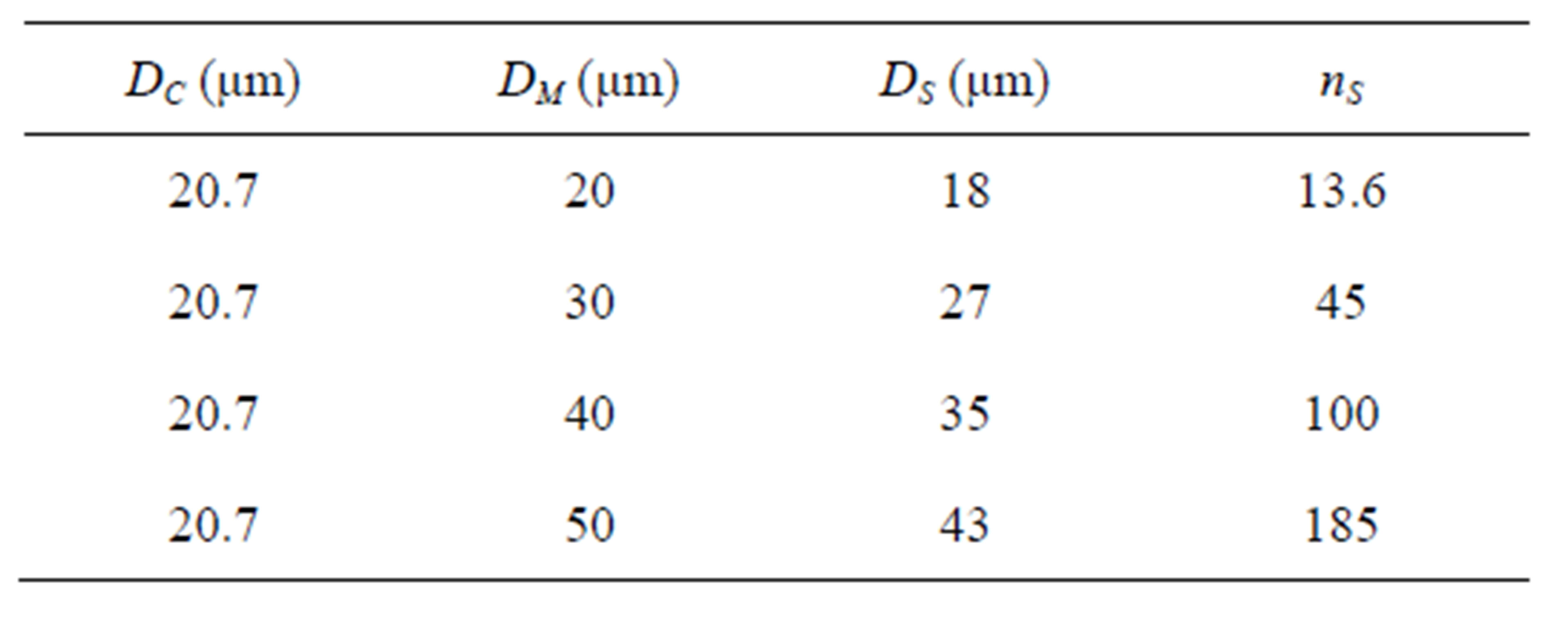
Table 2. Calculated values of numbers of shell oil droplets together with DS, DC and DM.
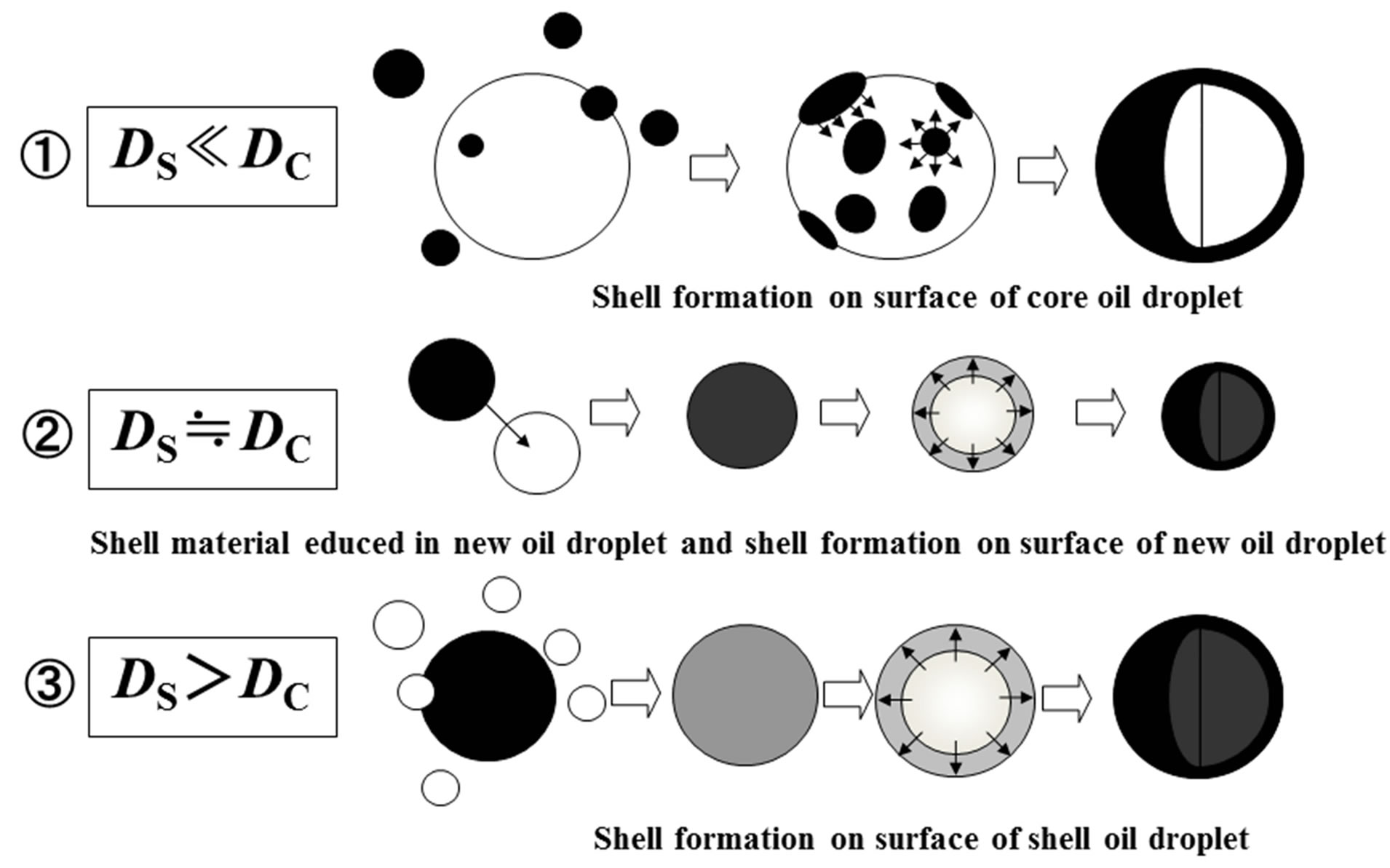
Figure 9. Microencapsulation mechanism depending on diameters of shell oil and core oil droplets.
surface area of a core oil droplet, because the smaller shell oil droplets have to collide and coalesce mainly with the surface of larger core droplet (case ①).
If the diameter of shell oil droplet is nearly equal to that of the core oil droplet, a new oil droplet may be formed when the shell oil droplets and the core oil droplets of the same number may collide and coalesce and the shell material may be separated in a new oil droplet. And then, the shell material has to be transfered toward the surface area of a oil droplet due to lower interfacial free energy to form the microcapsule shell (case ②).
If the diameter of shell oil droplet is larger than that of core oil droplet, the microcapsule shell has to be formed on the surface of shell oil droplet, because the smaller core oil droplets have to collide and coalesce preferentially on the surface of a larger shell oil droplet (case ③).
As a result, it is found that the core shell microcapsules are able to be prepared irrespective of the diameters of shell oil droplets and core oil droplets by the liquid droplet coalescence followed by phase separation developed in this work.
4. Conclusions
It was tried to prepare microcapsules by the novel microencapsulation method. The liquid droplets dissolving the shell material were forced to collide and coalesce with the core oil droplets. Microcapsules were able to be prepared with collision and coalescence between both oil droplets with any droplet diameter.
The microencapsulation mechanism presented here is suitable to the preparation of core shell microcapsules.
REFERENCES
- T. Kondo, “Saishin Makurokapseruka Gijutsu (Microencapsulation Technique) (in Japanese),” ETS, Tokyo, 1967.
- T. Tanaka, “Key Point of Preparation of Nano/Microcapsules,” Techno System Publishing Co. Ltd., Tokyo, 2008.
- M. Takahashi, Y. Taguchi and M. Tanaka, “Microencapsulation of Hydrophilic Solid Powder as a Fire Retardant by the Method of in Situ Gelation in Droplets Using a Non-Aqueous Solvent as the Continuous Phase,” Polymers & Polymer Composites, Vol. 17, No. 2, 2009, pp. 83-90.
- M. Takahashi, Y. Taguchi and M. Tanaka, “Microencapsulation of Hydrophilic Solid Powder as Fire Retardant Agent with Epoxy Resin by Droplet Coalescence Method,” Journal of Applied Polymer Science, Vol. 110, No. 3, 2008, pp. 1671-1676. doi:10.1002/app.28211
- M. Takahashi, Y. Taguchi and M. Tanaka, “Microencapsuration of Hydrophilic Solid Powder as a Flame Retardant with Epoxy Resin by Using Interfacial Reaction Method,” Polymers for Advanced Technologies, Vol. 21, No. 3, 2010, pp. 224-228.
- A. Branko, S. Urska and K. Matjaz, “Differential Scanning Calorimetric Examination of Melamine-Formaldehyde Microcapsules Containing Decane,” Journal of Applied Polymer Science, Vol. 119, 2011, pp. 3687-3695.
- X.-X. Zhang and X.-M. Tao, “Structure and Thermal Stability of Microencapsulated Phase-Change Materials,” Colloid & Polymer Science, Vol. 282, 2004, pp. 330-336.
- T. Takahashi, Y. Taguchi and M. Tanaka, “Preparation of Polyurea Microcapsules Containing Pyrethroid Insecticide with Hexamethylene Diisocyanate Isocyanurate,” Journal of Applied Polymer Science, Vol. 107, No. 3, 2008, pp. 2000-2006. doi:10.1002/app.27238
- E. O’shima and M. Tanaka, “Coalescence and Breakup of Droplets in Suspension Polymerization,” Kagaku Kogaku Ronbunshu, Vol. 8, No. 1, 1982, pp. 86-90. doi:10.1252/kakoronbunshu.8.86
- M. Tanaka, “Local Droplet Diameter Variation in a Stirred Tank,” The Canadian Journal of Chemical Engineering, Vol. 63, No. 5, 1985, pp. 723-727. doi:10.1002/cjce.5450630504
- K. Hosogai and M. Tanaka, “Study of Suspension Polymerization of Styrene with a Circular Loop Reactor,” Polymer Engineering and Science, Vol. 32, No. 6, 1992, pp. 431-437. doi:10.1002/pen.760320608

