New Journal of Glass and Ceramics
Vol.05 No.03(2015), Article ID:58224,5 pages
10.4236/njgc.2015.53007
Thermal and FT-IR Properties of Semiconducting SnO2-PbO-V2O5 Glass System
Ponnada Tejeswara Rao*, Balireddy Vasundhara
Department of Physics, GITAM Institute of Technology, GITAM University, Visakhapatnam, India
Email: *teja_msc_phy@yahoo.co.in
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 19 June 2015; accepted 20 July 2015; published 23 July 2015
ABSTRACT
Melt quenched “SnO2(50−x)PbO:50V2O5” glass system containing x = 5, 10, 15 in molar ratio has been investigated. Effects of heating rate, glass transition, crystallization, melting temperature and infrared spectra of SnO2 substituted PbO-V2O5 glass system are reported. XRD results show that perfect vitrification has been achieved for all the glass samples after annealing at 150˚C. DSC results have indicated that eutectic composition of the lead metavanadate has been maintained for all the glass systems up to 15 mole% of substitution. IR spectra for a SnO2 substitution of 5 mole% V=O stretching frequency occur at 966 cm−1 without appearance of any additional peak. But for 10 mole% and 15 mole% SnO2 substituted samples, additional peaks appear at 1023 and 1005 cm−1 indicating the effect of SnO2 in the vanadate crystalline matrix such that there is an elongation of V=O bond. Since the crystalline matrix is affected, we can expect similar effect in the glass matrix also.
Keywords:
DSC, XRD, FT-IR, Lead Vanadate Glasses

1. Introduction
Transport properties of semiconducting glasses are very interesting and provide useful information on the conduction mechanisms. Modern glasses are of crucial importance for electronics and have been widely used in industry, space explorations, computer memory units, etc. IR spectroscopy is a particularly suitable method for the structural studies of vanadate system in glass and crystalline forms because of the characteristic vanadate group’s vibrational frequencies which can be easily identified. Structural models for PbO-V2O5 glass systems have been discussed on the basis of IR spectroscopy [1] by comparing with those of known crystal structures. From IR and NMR spectroscopy, Hayakwa et al. [2] have proposed the existence of VO4 tetrahedral and VO5 trigonal bipyramids. The local structure around Pb in lead vanadate glasses mainly consists of PbO3 trigonal and PbO4 square pyramids. This study does not contradict the results obtained in earlier IR studies [3] that 50PbO:50V2O5 glass system contains affected VO5 groups but gives additional information regarding the local environment of vanadium as well as lead ions. The lead ions are located between the vanadate chains and layers. These ions affect the isolated V=O bonds by elongating them, thus reducing their frequency. In the present studies IR spectra have been recorded in order to verify if the substituent metal oxide is replacing PbO in the glass network of the equimolar lead vanadate i.e., 50PbO:50V2O5 glass system.
2. Experimental
The glasses were obtained by melting a chemically pure PbO, V2O5 and SnO2 in amounts varying from 5 to 15 mole% PbO and SnO2 in glazed silica crucibles in the 800˚C - 1200˚C temperature range. Vitrification was achieved by rapid cooling of the melt using a roller technique. The glasses were subjected to crystallization at 380˚C. The prepared samples were grounded into fine powder for X-ray, DSC and FT-IR studies. X-ray studies were carried out on a PAN Alytic X’Pert-PRO diffractrometer using CuKα radiation at 1.5418Å and diffractrometer settings in the 2θ range from 10˚C - 70˚C by changing the 2θ with a step size of 0.020. Differential Scanning calorimetry investigation of glass specimens was performed using DuPont, USA make model 2000 DSC instrument. DSC scans were conducted using 5 - 10 mg ground as-cast glass specimens which heats up at the rate of 10˚C/min between 0˚C and 600˚C in a platinum crucible and alumina powder was used as the reference material. The density of the glass samples was determined by the Archimedes principle, using toluene as immersion liquid. In the present studies IR spectra were recorded for both the vitreous and non-vitreous samples in order to study the effect of a different metal oxide substitution in the place of PbO in the lead metavanadate glass systems.
3. Results and Discussion
The X-ray diffractograms annealed at 150˚C containing (x = 5, 10, 15 mole%) of SnO2 showed no trace of crystallinity and are shown in Figure 1.
DSC investigations were conducted on the SnO2-PbO-V2O5 glasses. The DSC patterns for these glass systems shown in Figure 2 are slightly different when compared to the un-substituted system [4] . Values of glass transition temperature Tg, crystallization temperature Tc, melting temperature Tm, glass forming tendency Kg and densities of the xSnO2(50−x)PbO:50V2O5 are given in Table 1.
Figure 1. X-ray diffractograms of xSnO2(50−x)PbO: 50V2O5 glass system annealed at 150˚C. (a) x = 5 mole%; (b) x = 10 mole%; (c) x = 15 mole%.
Figure 2. Differential scanning calorimetry curves of xSnO2(50−x)PbO:50V2O5 glass system. (a) x = 5 mole%; (b) x = 10 mole%; (c) x = 15 mole%.
Table 1. Values of glass transition temperature (Tg), crystallization temperature (Tc), melting temperature (Tm) and glass forming tendency (Kg) for the xSnO2(50−x)PbO:50V2O5.
There is a slight change in Tg along with an increase in the number of crystallization peaks, the Tg values decrease with increasing SnO2 contents, these results suggest that SnO2 acts as a network modifier where as PbO acts as a network former [5] . Crystallization temperature Tc is the maximum temperature of the exotherm and onset of crystallization temperature, Tx is the beginning of the first exothermic reaction where the crystallization starts. As seen in Figure 2 up to x = 15 mole% there is only one endothermic peak corresponding to melting point. This indicates that the substituted samples behave like the eutectic composition upto x = 15 mole%. In order to understand the devitrification tendency and thermal stability of the glass samples, glass forming tendency values Kg are calculated using the below equation.
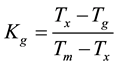 (1)
(1)
Lower Kg suggests higher tendency of crystallization and lower thermal stability. Kg represents the temperature interval during nucleation [6] . These values indicate that the 5SnO245PbO:50V2O5 glass samples have lowest thermal stability among four compositions, with a Kg value of 0.099. The densities seem to be increasing with an increase in SnO2 substitution. The IR spectra for the glass as well as crystalline forms are for xSnO2(50−x)PbO:50V2O5 glass systems given in Figure 3. The observed IR band positions are given in Table 2 and Table 3.
A consequence of disorder in the amorphous or glass systems when compared to crystalline forms is the
Figure 3. IR spectra of xSnO2(50−x)PbO:50V2O5 glass system; ____ glass; ……crystalline. (a) x = 5 mol%; (b) x = 10 mol%; (c) x = 15 mol%.
Table 2. FT-IR data of xSnO2(50−x)PbO:50V2O5 glass system in both amorphous and crystalline forms.
Table 3. FT-IR data of xSnO2(50−x)PbO:50V2O5 glass system in both amorphous and crystalline forms.
υ (V=O) symmetric stretching, υ (VO2) υ (VO3) asymmetric, stretching, υ (VOV) bending frequency (symmetric and antisymmetric), C.V. combination vibration of (VO3)n single chain, G indicates glass.
breakdown of the wave vector selection (k-selection) rules, which allows electromagnetic radiation to couple with vibrations other than k = 0. As a result, unlike the crystalline case in which narrow well defined lines are observed, broad and diffuse bands representing a continuum of IR absorption result. Even though bands due to individual, localized structural units are observable, the identification of IR spectra of glasses alone is rather difficult unless crystalline spectra are also present. Hence the present discussion mainly corresponds to the IR bands observed in the devitrified samples. SnO2 substituted samples for x = 10 and 15 mole% , there is an indication of additional bands at 1023 cm−1 beside 966 cm−1 and 1005 cm−1 beside 962 cm−1 the appearance of additional bands can be understood as the splitting of the original V=O stretching band into affected and unaffected components. The affected components arises due to the effect of dopant i.e. SnO2 in the present case on V=O bond the glass matrix. In this case from the IR spectral evidence we can summarize the following.
As SnO2 is substituted for PbO, SnO2 is replacing PbO in the glass network in such a way that it affects V=O bond frequency, as evident by the shift in V=O band (at 1023 cm−1) whose intensity increases with the increase of SnO2 concentration. The affected V=O band (at 966 cm−1) indicates the presence of meta vanadate phase containing PbO in the usual evidence we can summarize the following. As SnO2 is substituted for PbO, SnO2 is replacing PbO in the glass network in such a way that it affects V=O bond frequency, as evident by the shift in V=O band (at 1023 cm−1) whose intensity increases with the increase of SnO2 concentration. The affected V=O band (at 966 cm−1) indicates the presence of metavanadate phase containing PbO in the usual glass network. The occurrence of the band at 1005 cm−1 is due to the indirect effect of SnO2 on the V=O band in the new stable phase that is being formed. In x(TiO2):(100−x)(V2O5) glass containing x = 20 mole%, Dimitriev et al. [3] reported that the isolated V=O band is unaffected at 1020 cm−1. It was observed by Dimitrov et al. [1] that in the IR spectra of xPbO:(1−x)V2O5 glass systems as x is varied from 0 to 75 mole% , there appears a new band in the range 970 - 955 cm−1 beside 1020 cm−1 band. It has been suggested that [3] Pb2+ ions occupy a position between the V-O-V layers. They exercise a direct influence of the isolated V=O bonds of VO5 groups according to the scheme Pb2+…O=V5+. This causes an elongation of the affected V=O bond and a decrease in the vibrational frequency to 970 - 950 cm−1. There may be unaffected V=O bonds whose vibrational frequency is still around 1020 cm−1. With the increase of PbO, their number decreases and for eutectic composition 50PbO:50V2O5 (i.e. PbV2O6 metavanadate) only one type of VO5 polyhedron results as is evidenced by the presence of a single high frequency band in the region 955 - 970 cm−1 [7] . This band is observed at 966 cm−1 in 5SnO2:45PbO:50V2O5 system. As SnO2 concentration is increased to 10 mol% and 15 mole%, the appearance of 1023 cm−1 band indicates that SnO2 is replacing PbO in such a way that the V=O bond in the glass network of the eutectic composition is elongated [8] .
4. Conclusion
Perfect vitrification has been achieved for all the glass samples as can be seen from their X-ray diffractograms of the prepared samples after annealing at 150˚C for two hours. DSC recordings show that eutectic composition of the lead metavanadate has been maintained for all the glass systems up to 15 mole% of substitution. The DSC data also indicate that all the glass systems are characterized by more than one crystallization peak. This can be thought as an evidence for the existence of more than one meta-stable phase in the glass systems. The dopant SnO2 is not divalent oxide like PbO. Besides SnO2 is known to be glass former unlike PbO which is replacing PbO in the glass network in such a way that the eutectic composition is maintained. In the present studies the IR spectra of SnO2 substituted samples of 5 mole% V=O stretching frequency occur at 966 cm−1 without the appearance of any additional peak. But for 10 mole% and 15 mole% SnO2 substituted samples, additional peaks appear at 1023 and 1005 cm−1 indicating the effect of SnO2 in the vanadate crystalline matrix such that there is an elongation of V=O bond. Since the crystalline matrix is affected, we can expect similar effect in the glass matrix also.
Cite this paper
Ponnada TejeswaraRao,BalireddyVasundhara, (2015) Thermal and FT-IR Properties of Semiconducting SnO2-PbO-V2O5 Glass System. New Journal of Glass and Ceramics,05,53-58. doi: 10.4236/njgc.2015.53007
References
- 1. Dimitrov, V. and Dimitriev, Y. (1990) Structure of Glasses in PbO-V2O5 System. Journal of Non-Crystalline Solids, 122, 133-138.
http://dx.doi.org/10.1016/0022-3093(90)91058-Y - 2. Hayakawa, S., Yoko, T. and Sakka, S. (1995) IR and NMR Structural Studies on Lead Vanadate Glasses. Journal of Non-Crystalline Solids, 183, 73-84.
http://dx.doi.org/10.1016/0022-3093(94)00652-0 - 3. Dimitrov, V. (1995) Structural Changes in Vitreous Vanadate Systems. Journal of Non-Crystalline Solids, 192, 183- 186.
http://dx.doi.org/10.1016/0022-3093(95)00349-5 - 4. Ramesh, K.V. (2000) Thermal, Electrical and Spectroscopic Studies of CuO, ZnO and TiO2 Substituted for PbO in Eutectic Lead Vanadate Glass System. Ph.D. Thesis, Andhra University, Visakhapatnam.
- 5. Oz, B., Kabaci, I., Ovecoglu, M.L. and Ozen, G. (2007) Thermal Properties and Crystallization Behavior of Some TeO2-K2O Glasses. Journal of the European Society, 27, 1823-1827.
- 6. Murugan, G.S. and Ohishi, Y.J. (2004) TeO2-BaO-SrO-Nb 2O5 Glasses: A New Glass for Waveguide Device Applications. Journal of Non-Crystalline Solids, 341, 86-92.
http://dx.doi.org/10.1016/j.jnoncrysol.2004.04.006 - 7. Tejeswara Rao, P. (2013) Preparation and Characterization of Some Lead Meta Vanadate Semi-Conducting Glass Systems with GeO2, Bi2O3 and SnO2 Substituted for Lead Oxide. Ph.D. Thesis, Andhra University, Visakhapatnam.
- 8. Tejeswara Rao, P., Ramesh, K.V. and Sastry, D.L. (2012) Electrical and Spectroscopic Studies of the CdO Substituted Lead Vanadate Glass System vs Crystalline Form. New Journal of Glass and Ceramics, 2, 34-40.
http://dx.doi.org/10.4236/njgc.2012.21006
NOTES
*Corresponding author.






