New Journal of Glass and Ceramics
Vol.4 No.2(2014), Article ID:45420,6 pages DOI:10.4236/njgc.2014.42006
Influence of Reaction Conditions on Sol-Gel Process Producing SiO2 and SiO2-P2O5 Gel and Glass
Amany Mohamed Elnahrawy1, Ahmed Ibrahim Ali2,3
1National Research Center (NRC), Department of Solid State Physics, Giza, Egypt
2Energy Harvest-Storage Research Center and Department of Physics, University of Ulsan, Ulsan, South Korea
3Basic Science Department, Faculty of Industrial Education, Helwan University, Cairo, Egypt
Email: amany_physics_1980@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 9 November 2013; revised 17 February 2014; accepted 5 March 2014
ABSTRACT
The effect of H2O and the slow thermal annealing on the properties of pure silica (SiO2) and phosphosilicate (SiO2-P2O5) gel-glasses are presented. The monolithic samples have been prepared via sol-gel process using tetraethorthosilicate (TEOS), Si(C2H5O)4 and Triethylphosphate (TEP) (C2H5O)3P(O) as SiO2 and P2O5 precursors. Phosphate incorporates into the silicate network by substituting Si atoms and consequently, we observed changes in structural and spectroscopic properties for these systems. The structures of prepared samples were examined by observed weight loss, XRD and FTIR. It has been found that in the structure of pure silica and phosphosilicate glasses there are formed domains characterized by certain degree of ordering of the units present in their composition, while the structure of pure silica is still amorphous of these glasses. The changing character of domains structure may be the reason of different chemical activities of phosphosilicate glass.
Keywords:Silica Gel, Phosphosilicate Glass, Gelation, XRD, FTIR

1. Introduction
The sol-gel technique is considered to be excellent and the most practical chemical method in recent years for preparing chemically homogeneous monolithic, coatings and powders with a variety of useful applications such as optic, coating materials and biological [1] -[4] . The synthesis of organic and inorganic sol-gel materials consists of the following major stages: hydrolysis of the precursors (generally, metal alkoxides) to form a homogeneous sol, poly-condensation of the sol to form a non-crystalline gel network, several steps to remove excess solvent and unreacted precursors, then drying and calcination treatments to form end product. Metal alkoxides, the starting materials of the sol-gel process, are known for their relatively high reactivity, especially to water. Thus high rates of hydrolysis are required, which leads to the opining network of silicate system to overcome the agglomeration of particles. In these cases, it is necessary to modify the precursors so as to increase their stability toward hydrolysis [5] -[7] .
The behavior of silicate glasses is widely determined by the different other oxides they include which are classified as formers, modifiers or intermediates. Many different molecules are used as modifiers in silicate system, such as alcohols, acetylacetone, allyl acetoacetate and organic acids [8] . Phosphate doped silica gel and glasses, composed of two networks former oxides have acquired increasing relevance in glass technology because of their excellent structural and optical properties. Recently, there is a rapid development in silicate systems in various applications such as amplifier, lenses, biomedical research, catalysis, drug delivery and imaging due to their stability, low toxicity and ability to be functionalized with a range of molecules and polymers [9] - [11] . The first aim of this work is to relate the structural modifications with the positions of the vibrational modes. Structural changes induced by varying the H2O and annealing temperature of pure and doped silica glasses are investigated by Fourier transform infrared (FTIR) spectroscopies. The second aim of this work is to relate the peak frequencies shifts to structural modifications of the phosphosilicate (SiO2-P2O5) network.
2. Experimental
Pure silica (SiO2) and phosphosilicate (SiO2-7P2O5) systems were prepared using sol gel process at room temperature under different reaction condition. The synthesis of pure silica gel using Tetraethyorthosilicate (CH3CH2OH)4 (TEOS, purchased from Aldrich), with ethanol, distilled water and HCL, with different reaction condition of the chemical components (1:4:4:0.65) and (1:4:6:0.65), under vigorous stirring for 2 h. A (SiO2- 7P2O5) sol prepared using triethyphosphate with ethanol, distilled water and HCL, with different reaction condition of the chemical components (1:4:4:0.65) and (1:4:6:0.65), under vigorous stirring for 2 h. The final pH of the reaction mixtures was about 3. The resultant transparent and homogeneous solutions of silica gel and phosphosilicate gels were filled in a glass vials and aged two weeks at the room temperature then dried in oven type GFL 71.5, at different annealing temperatures, from 60˚C up to 500˚C for 3 h, as represented in the flow chart Figure 1. The final products were monolithic samples very clear, transparent and without cracks. X-ray diffraction (XRD) patterns were obtained on a Bruker D8 Advance diffractometer using Cu Kα radiation (k = 1.540 A˚), operating at 40 kV and 40 mA. Scans were performed with a detector step size of 0.02˚ over an angular range 2θ = 10 - 80. Fourier Transforms Infrared, were used to determine the individual frequencies and their intensities. FTIR (Range 400 - 4000 cm−1) spectrometer was used to confirm the formation of pure silica and phosphosilicate glass structures.
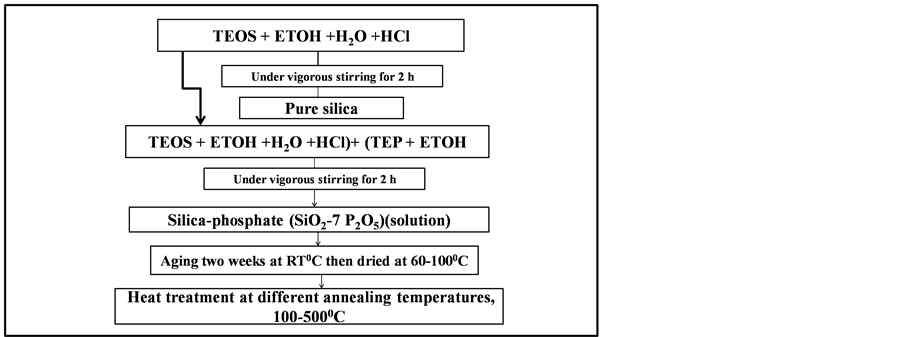
Figure 1. Flow chart for sol-gel processing for preparation of pure silica and phosphosilicate gel and glasses.
3. Results and Discussion
3.1. Observed Weight Loss
The synthesized solutions were homogeneous and transparent, the viscosity of pure silica and phosphosilicate which increased with the increasing aging time of the solutions. The gel prepared with higher water ratio taking long time in aging of the solution in RT and low thermal annealing as in Figure 2. It is apparent that the slow increases in viscosity for the samples containing higher ratio of distilled water with the aging time. In solution, pure silica (TEOS) reacted with H2O to form Si-OH bonds and also, in phosphosilicate to form Si-OH, Si-O-Si, Si-O-P and P-OH bonds. The Si-OH and P-OH bonds polymerize themselves and/or react with TEOS and TEP forming the macromolecules of the siloxane and phosphosilicate network structure. The formation of macromolecules leads to an increase of the viscosity, resulting in the increased the change in physicochemical properties of these systems.
The sol gel process consists of simultaneous hydrolysis and poly-condensation reactions that occur in silicate system and phosphosilicate system.
The hydrolysis reactions are shown below:
![]()
![]()
The gelation process starts by condensation polymerization of colloidal pure silica and phosphosilicate particles stabilized in aqueous solutions according to the condensation reaction as follows:
![]() .
.
 .
.
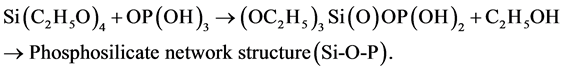
Finally, the produced clusters tend to aggregate to form a huge chains and networks of pure silica gel and phosphosilicate gel [12] -[14] . The gelation process in the first two weeks changed very slowly in this time for pure and doped silicate system and by increasing the drying temperature in daring oven from RT to 60, 80 and 100˚C the mass loss increased and obtained a dried gel. During the interval of transformation the drying process must be done at slowly low temperature to avoid fractures from capillary stresses due to the removal of the different solvent molecules from the liquid phase for silica and phosphosilicate systems. The chemical changes during the transform from sol to gel (agglomeration of dense colloidal particles) for the prepared systems due to the elimination of OHand alcohol groups to form Polymeric gels. After firing the samples at 100˚C the mass loss increased quickly due to the fast evaporation rate of the adsorbed water and alcohol molecules presented on the surface and in the porous of the prepared samples as in Figure 2.
3.2. X-Ray Diffraction
Pure and doped silicate glasses produced by sol gel method starts with a mixture of metal alkoxides with suitable solvent. Figure 3, Figure 4 show the XRD pattern of pure silica and phosphosilicate (SiO2-7P2O5) glasses calcined at different temperatures from 100˚C to 500˚C for 3 h. All the samples are amorphous up to 200˚C. However, the thermal analysis shows that heating at temperatures higher than 200˚C is necessary to remove most of the organic solvent and water. Therefore, the XRD analysis refers only to the samples calcined at 300 and 500˚C. XRD curve in Figure 3 shows the absence of any crystallinty in pure silica. While, phosphosilicate (SiO2-7P2O5) glass appears higher degree of crystallinty with increasing the calcinations temperature, ascribed to monoclinic SiP2O7 phase and rhombohedra Si5P6O25 as in Figure 4 and the average crystallite size is estimated at ~15 nm from the Scherer formula.. Where the introduction of P2O5 into pure silica system results in a distortion and rupture of the bonds and increases the content of non-bridge oxygen. With increasing the calcination temperatures up to 500˚C for 3 h as in Figure 3 the XRD patterns appeared the absence of any peaks in pure silica gel that was found to be still in amorphous phase.
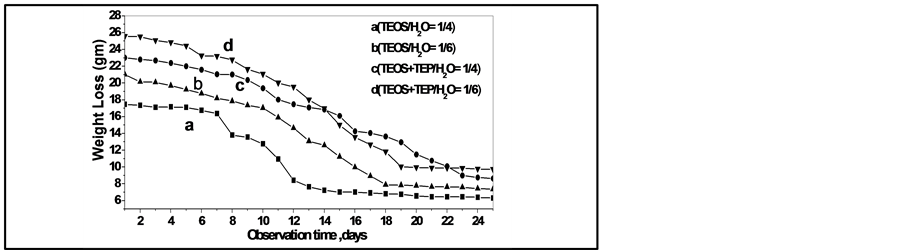
Figure 2. Observed weight loss in pure silica gel with ratio (a) TEOS/H2O = 1/4 and (b) TEOS/H2O = 1/6, for phosphosilicate gel (c) TEOS + TEP/H2O = 1/4 and (d) TEOS + TEP/H2O = 1/6, from room temperature to 100˚C.
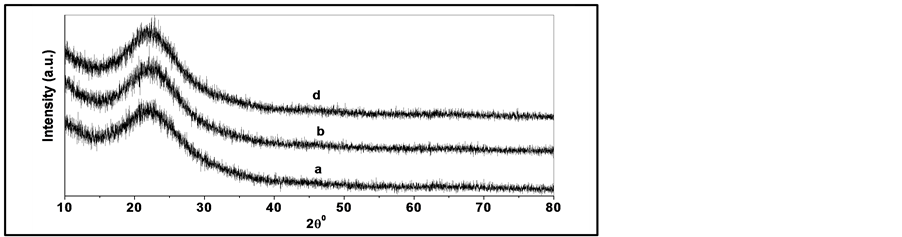
Figure 3. XRD patterns of pure silica gel and glass calcined at different temperatures (a) 100˚C (b) 300˚C and (c) 500˚C for 3 h.
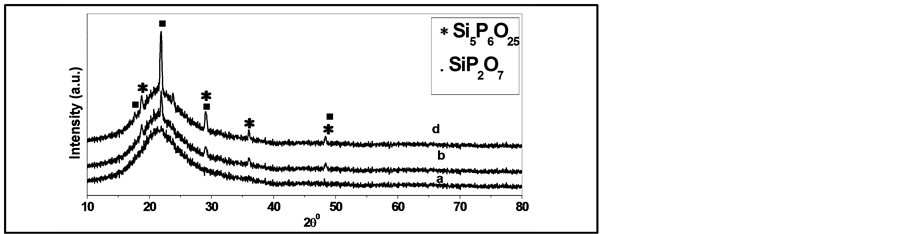
Figure 4. XRD patterns of phosphosilicate glasses calcined at different temperatures (a) 100˚C (b) 300˚C and (c) 500˚C for 3 h, • SiP2O5 phase and * Si5P6O25 phase.
3.3. The Fourier Transform Infrared (FTIR)
FTIR spectroscopy shows bands assigned to silica and phosphosilicate glasses as in (Figure 5, Figure 6) within the range 400 - 4000 cm−1. The broad bands in the range 3400 to 3700 cm−1 were attributed to the presence of water molecule due to silanol and phosphanol groups (free and structured), to hydroxyl groups and to the stretching modes of water [13] [15] [16] . The presence and intensity of the deformation mode of HOH around 1600 - 1640 cm−1, in both samples and can be confirmed the presence of OH in the pours structures [16] -[19] . The vibration bands at 1050 - 1090 cm−1 from asymmetric stretching Si-O-Si and Si-O-P in pure silica and SiO2-7P2O5 network structure [20] .
In silica gel doped with phosphate the symmetric mode of PO and PO2 are observed at 950 - 1200 cm−1 [21] . O-P-O stretching modes are observed at 792 cm−1 and 807 cm−1. Additional the weak banda at around 550 to 590 cm−1 is due to the bending vibrations of O-P-O bond as in Figure 5, Figure 6(B), and found to decrease gradually and change to weak shoulder with increasing the temperature due to the rearrangement of phosphosilicate systems [22] . Finally, the characteristic bands for silicate system are amperes at 440 - 466 cm−1 is assigned
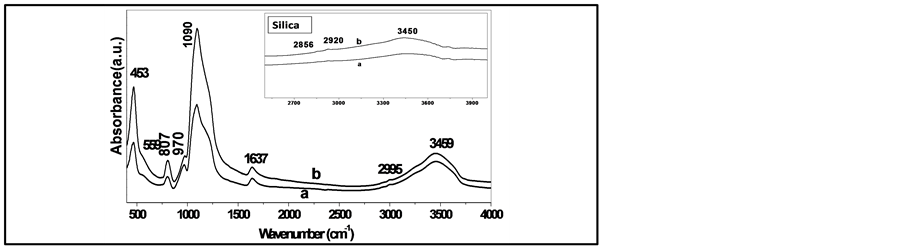
Figure 5. The FTIR absorbance spectra in wide spectral region 400 - 4000 cm−1 of (a) phosphosilicate with two different molar ratio (a) (TEOS + TEP/H2O = 1/4), (b) (TEOS + TEP/H2O = 1/6) and the inset for pure silica gel in the range 2700 - 4000 cm−1, calcined at temperature 100˚C, 200˚C, for 3 h.
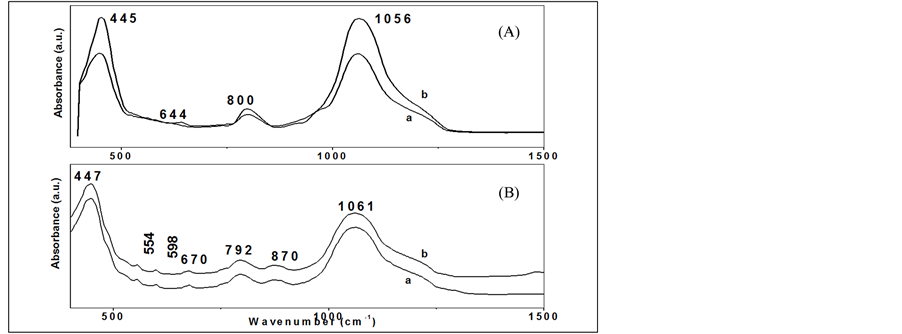
Figure 6. The FTIR absorbance spectra in wide spectral region 400 - 4000 cm−1 of (A) pure silica with two different molar ratio (TEOS/H2O = 1/4), (b) (TEOS/H2O = 1/6) and (B) phosphosilicate with two different molar ratio (a) (TEOS+TEP/H2O = 1/4), (b) (TEOS+TEP/H2O = 1/6), calcined at temperature 300˚C, 400˚C, for 3 h.
to Si-O-Si bending vibration and symmetric stretching modes of Si-O-Si [16] [22] . In summary, the introduction of a phosphate and the change in HOH ratio in the silica gel is likely to inhibit the condensation of silanols and phosphanols groups which will lead to a more cross-linked network.
4. Conclusion
We have prepared by sol-gel pure silica and SiO2-7P2O5 monolithic with good adherence, using tetraethyorthosilicate and triethyphosphate as a silica and phosphorus precursor. Observed weight loss, XRD, and FTIR analyses confirmed the expected chemical composition of the prepared samples and demonstrated the influence of reaction condition on the chemical bonds in the SiO2 and SiO2-7P2O5 glass. The densification of SiO2 and SiO2-7P2O5 with increasing annealing temperature was also noticed through XRD and the higher intensity of the FTIR spectra. The FTIR spectra reveal the formation of SiO2 and SiO2-7P2O5 network.
Acknowledgements
Dr. A. M. Elnahrawy thanks Prof. G. Turky, from Microwave Physics and Dielectrics Department, National Research Centre, Cairo, Egypt for their support in this work.
References
- Kannan, G.A., Choudhury, R.N. and Dutta, K.N. (2010) Electrochemical Performance of Sol-Gel Derived PhosphoSilicate-Methacrylate Hybrid Coatings. Journal of Electroanalytical Chemistry, 641, 28-34.
- Brinker, C.J., Hurd, A.J., Schunk, P.R., Frye, G.C. and Ashley, C.S. (1992) Review of Sol Gel Thin Film Formation. Journal of Non-Crystalline Solids, 147-148, 424-436.
- Randy, M., Raf, M., Pieterjan, K., Pieter, A., Jan, V., Guy, V.D., Johan, M.A. and Patrick, A. (2008) Ordered Mesoporous Silica Induces pH-Independent Supersaturation of the Basic Low Solubility Compound Itraconazole Resulting in Enhanced Transepithelial Transport. International Journal of Pharma-Ceutics, 357, 169-179. http://dx.doi.org/10.1016/j.ijpharm.2008.01.049
- Nandiyanto, A.B.D., Kim, S. G, Iskandar, F. and Okuyama, K. (2009) Synthesis of Silica Nanoparticles with Nanometer-Size Controllable Mesopores and Outer Diameters. Journal of Microporous and Mesoporous Materials, 120, 447-453. http://dx.doi.org/10.1016/j.micromeso.2008.12.019
- Chiola, V., Ritsko, E.J. and Vanderpool, D.C. (1971) Process for Producing Low-Bulk Density Silica. Application No. US 3556725D, Publication No. US 3556725.
- Chandler, C.D., Roger, C. and Hampden-Smith, M.J. (1993) Chemical Aspects of Solution Routes to Preovskite Phase Mixed Metal Oxides from Metal Organic Precursors. Journal of Chemical Reviews, 93, 1205.
- Ohya, Y. , Kume, T. and Ban, T. (2005) Fabrication of Zinc Oxide Transparent Thin-Film Transistor with ZrO2 Insulating Layer by Sol-Gel Method, Journal of Applied Physics, 44, 1919-1922. http://dx.doi.org/10.1143/JJAP.44.1919
- Sayilkan, H., Arpac, E. and Sener, E. (1997) The Modification of Aluminium Tri-Sec-Butoxide with Different Alcohols and Chelating Ligands: Hydrolysis and Condensation of the Products. Journal of Synthesis and Reactivity in Inorganic, Metal-Organic, 27, 1437-1452.
- Portales, H., Mattarelli, M., Montagna, M., Chiasera, A., Ferrari, M., Martucci, A., Mazzoldi, P., Pelli, S. and Righini, G.C. (2005) Optoelectronics—Rare-Earth Ion Activated Glasses (Investigation of the Role of Silver on Spectroscopic Features of Er3+-Activated Ag-Exchanged Silicate and Phosphate Glasses). Journal of Non-Crystalline Solids, 351, 1738-1742. http://dx.doi.org/10.1016/j.jnoncrysol.2005.04.006
- Anderson, C. and Bard, A.J. (1995) An Improved Photocatalyst of TiO2/SiO2 Prepared by a Sol-Gel Synthesis. Journal of Physical Chemistry, 99, 9882-9885. http://dx.doi.org/10.1021/j100024a033
- Trewyn, B.G., Nieweg, A.J., Zhao, Y.N. and Victor, Y.S. (2007) Biocompatible Mesoporous Silica Nanoparticles with Different Morphologies for Animal Cell Membrane Penetration. Chemical Engineering Journal, 137, 23-29. http://dx.doi.org/10.1016/j.cej.2007.09.045
- Scherer, G.W., Hæreid, S., Nilsen, E. and Einarsrud, M.A. (1996) Shrinkage of Silica Gels Aged in TEOS. Journal of Non-Crystalline Solids, 202, 42-52.
- Iler, K.R. (1979) The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry. John Wiley & Sons, New York.
- Patwardhan, V.S., Mukherjee, N. and Clarson, J.S. (2001) Formation of Silica Structures Utilizing a Cationically Charged Synthetic Polymer. Academic Poster Session, ACS Rubber Division Meeting.
- Stolen, H.R. and. Walrafen, E.G. (1976) Water and Its Relation to Broken Bond Defects in Fused Silica. Journal of Chemistry and Physics, 64, 2623-2631.
- Efimov, A.M. and Pogareva, V.G. (2006) IR Absorption Spectra of Vitreous Silica and Silicate Glasses: The Nature of Bands in the 1300 to 5000 cm−1 Region. Chemical Geology, 229, 198-217. http://dx.doi.org/10.1016/j.chemgeo.2006.01.022
- Wang, S.L., Johnston, C.T., Bish, D.L., White, J.L. and Hem, S.L. (2003) Water-Vapor Adsorption and Surface Area Measurement of Poorly Crystalline Boehmite. Journal of Colloid and Interface Science, 260, 26-35, http://dx.doi.org/10.1016/S0021-9797(02)00150-9
- Iller, R.K. (1979) The Chemistry of Silica. John Wiley & Sons, New York, 866.
- Johnston, C.T., Sposito, G. and Erickson, C. (1992) Vibrational Probe Studies of Water Interactions with Montmorillonite. Clays Clay Minerals, 40, 722-730. http://dx.doi.org/10.1346/CCMN.1992.0400611
- Sitarz, M. and Szumera, M. (2008) Crystallization of Silica-Phosphate Glasses. Journal of Thermal Analysis and Calorimetry, 91, 255-260.
- Carta, D., Pickup, D.M., Knowles, J.C., Smith, M.E. and Newport, R.J. (2005) Sol-Gel Syntheses of P2O5-CaO-Na2OSiO2 System as a Novel Bioresorbable Glass. Journal of Materials Chemistry, 15, 2134-2140. http://dx.doi.org/10.1039/b414885a
- Roman, J., Padilla, S. and Vallet-Regi, M. (2003) Sol-Gel Glasses as Precursors of Bioactive Glass Ceramics. Journal of Chemical Materials, 15, 798-806.

