International Journal of Organic Chemistry
Vol.05 No.02(2015), Article ID:56878,5 pages
10.4236/ijoc.2015.52009
FeCl3 Catalyzed One Pot Synthesis of 1-Substituted 1H-1,2,3,4-Tetrazoles under Solvent-Free Conditions
Fatemeh Darvish*, Shima Khazraee
Department of Chemistry, K.N.Toosi University of Technology, P. O. Box 15875-4416, Tehran, Iran
Email: *darvish@kntu.ac.ir
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 15 April 2015; accepted 30 May 2015; published 3 June 2015
ABSTRACT
An efficient procedure for the preparation of 1-substituted-1H-1,2,3,4-tetrazoles via a three-com- ponent condensation of triethyl orthoformate, amine, and trimethylsilyl azide using inexpensive and environment-friendly FeCl3 as catalyst under solvent-free conditions has been reported. The reaction generates the corresponding 1-substituted tetrazole in excellent yields.
Keywords:
Iron(III) Chloride, 1-Substituted-1H-1,2,3,4-Tetrazoles, Amines, Triethyl Orthoformate, Solvent-Free, Trimethylsilyl Azide

1. Introduction
Tetrazoles have received considerable attention because of their wide application [1] . They have been used extensively in the synthesis of modified amino acids and peptidomimetic compounds as a metabolically stable equivalent of carboxylic acids [2] . Tetrazole moieties play a major role in material science like propellants and energetic compounds [3] . Furthermore, this nitrogen-rich ring system is an important synthon in organic synthetic and medicinal chemistry [4] . Due to interesting properties of tetrazole, the improvement of known methods for their preparation is still in demand.
The methods reported for the synthesis of 1-substituted tetrazoles involve acid-catalyzed cycloaddition between hydrazoic acid and isocyanides [5] or trimethylsilylazide [6] , cyclization between primary amines with an orthocarboxylic acid ester or ethyl orthoformate and sodium azide in the presence of acetic acid [7] , acidic ionic liquid [8] , ytterbium triflate [9] and natrolite zeolite [10] . Each of these reported methods has at least one or more of the following drawbacks, for instance, the use of expensive, toxic metal catalysts and excess amount of acetic acid or trifluoroacetic acid, utilization of organic solvents, harsh reaction conditions, tedious work-up, low yields, long reaction time and the presence of hydrazoic acid, which is highly toxic and volatile. The few methods that seek to avoid hydrazoic acid liberation during the reaction by avoiding acidic conditions require a very large excess of sodium azide. Thus, the quest for inexpensive, benign catalysts and mild reaction conditions is still a major challenge for the synthesis of 1-substituted tetrazoles.
FeCl3 is an efficient and green catalyst in modern organic synthesis [11] . It has been widely used in several environment-friendly and atom economical organic transformations. Recent reports on FeCl3 catalyzed arylation of benzyl alcohols and benzyl carboxylates [12] , hydroarylation of styrenes [13] , benzylation of 1,3-dicarbonyl compounds [14] and diasteroselective synthesis of cis-oxazolidines [15] have highlighted the applications of FeCl3 in organic synthesis. Herein, we report another remarkable catalytic activity of FeCl3 for the preparation of 1-substituted tetrazoles from a wide variety of primary amines with trimethylsilylazide and trimethylorthoformate under solvent-free conditions (Scheme 1).
2. Results and Discussion
Preliminary experiments were carried out in order to determine the best reaction conditions. We examined the reaction of trimethylsilyazide using several different catalysts and solvents as well as neat conditions (Table 1). Fortunately, most of the acid catalysts which were used, afforded the desired product while FeCl3 gave the best result under solvent-free conditions (Table 1, Entry 1).
Further studies showed that the optimum amount of FeCl3 was 0.2 mmol, an excess of FeCl3 did not lead to a substantial improvement in the yield while decreasing the catalyst reduced it (Table 2, Entry 2).
After optimizing the reaction conditions, this process was extended to other substituted anilines. A variety of amines possessing both electron-releasing and electron-withdrawing groups (such as chloro, nitro, bromo, methoxy, ethyl, methyl, and heterocyclic amine like 2-aminopyridine) were employed (Table 3). According to the table, the nature of substituent on the benzene ring did not affect the reaction time and the yields were excellent.
The suggested iron(III) chloride catalyzed transformation mechanism is shown in Scheme 2, in which the
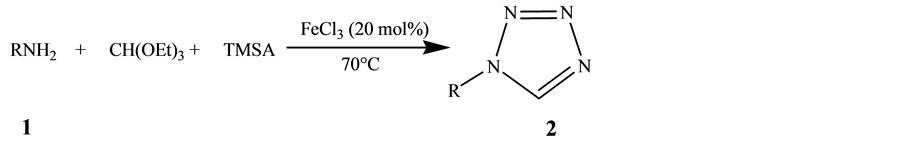
Scheme 1. Synthesis of 1-substituted 1H-1,2,3,4-tetrazoles.
Table 1. Effect of catalyst and solvent on the formation of tetrazole 2a.
aIsolated yields.
Table 2. Effect of the amount of catalyst on the formation of tetrazole 2a.
aIsolated yields.
Table 3. Synthesis of 1-substituted 1H-1,2,3,4-tetrazoles 2a-ia.
aReaction conditions: amine (1.0 mmol), triethylorthoformate (1.2 mmol), trimetylsilylazide (1 mmol), FeCl3 (20 mmol%), at 70˚C; bIsolated yields.

Scheme 2. Plausible mechanism for the formation of 1-substituted 1H-1,2,3,4-tetrazoles.
Lewis acidity of the catalyst probably has an important role in the promotion of the cyclization process. Apparently the role of FeCl3 is limited to activation of ethoxy groups and to breaking the CO bond, however the possible assistance of trimethylsilyl group in this cleavage should not be neglected. In the first step, carbocations are generated from the cleavage of methoxy group and stabilized by neighboring heteroatom O or N, the following nucleophilic displacements by amine and azide would explain the formation of intermediates A and B. By the assistance of FeCl3 and trimethylsilyl group the elimination of the last methoxy group becomes possible. Finally, 1-substituted tetrazoles will be produced upon the cyclization of intermediate C.
3. Conclusion
We have demonstrated that FeCl3 is an effective catalyst in promoting the reaction between amines, trimethylorthoformate and trimethylsilylazide that affords the corresponding 1-substituted-1H-1,2,3,4-tetrazole products. The process gave rise to excellent isolated yields of 1-substituted-1H-1,2,3,4-tetrazoles under solvent-free conditions and moderate temperature in shorter reaction times than many other reported methods.
4. Experimental
All the chemicals were purchased from the Merck Company and used without further purification. The melting points were taken in open capillary tubes with Electrothermal 9100 Apparatus. FT-IR (KBr) Spectra were recorded on an ABB FT-IR FTLA 2000 spectrometer. 1H NMR spectra were run on a Bruker DRX-300 (300 MHz) AVANCE instrument using TMS as an internal standard and CDCl3 as solvent. The chemical shifts (d) are reported in ppm relative to the TMS as an internal standard and J values are given in Hz. 13C spectra were recorded at 75 MHz. High-resolution mass spectra were recorded on a Mass-EI-POS (Apex Qe-FT-ICR instrument) spectrometer.
4.1. General Procedure
A mixture of amine (1 mmol), triethylorthoformate (1.2 mmol) and trimethylsilylazide (1 mmol) was stirred in the presence of FeCl3 (20 mol%) at 70˚C for an appropriate time under inert atmosphere (Table 3). The progress of the reaction was monitored by TLC (EtOAc/n-Hexane, 2:1). After completion, the reaction mixture was extracted with ethyl acetate (10 cm3 × 3) and washed with brine. The organic layer was dried over magnesium sulfate and the solvent was evaporated. The isolated product was pure (single spot on TLC) for all practical purposes. However, for characterization purposes it was further purified by plate chromatography (silica gel, eluent EtOAc/n-hexane2/1).
4.2. Characterization Data
4.2.1. 1-Phenyl1-H-1,2,3,4-tetrazole (2a)
Yield: 95%, m.p.: 65˚C - 66˚C (lit. [16] 65˚C - 66˚C); pale yellow needle. IR (KBr): v = 3121, 2926, 2844, 1596, 1439, 1463, 1396, 1206, 1093, cm−1. 1H-NMR (CDCl3): d = 7.47 - 7.58 (m, 3H, ArH), 7.70 (d, 2H, J = 7.3 Hz, ArH), 9.07 (s, 1H, CH) ppm. 13C-NMR (CDCl3): d = 121.2, 130.0, 130.2, 133.7, 140.6 ppm.
4.2.2. 1-(4-Metoxyphenyl)1-H-1,2,3,4-tetrazole (2b)
Yield: 88%, m.p.: 119˚C - 120˚C (lit. [16] 116˚C - 117˚C); Colorless solid. IR (KBr): v = 3132, 3019, 1606, 1597, 1514, 1463, 1389, 1257, 1156, 1093, 1201, 831 cm−1. 1HNMR (CDCl3,): d = 3.86 (s, 3H, OCH3), 7.06 - 7.03 (d, 2H, J = 6.8 Hz, ArH), 7.59 (d, 2H, J = 6.8 Hz, ArH), 8.94 (s, 1H, CH) ppm. 13C NMR (CDCl3): d = 115.2, 122.9, 126.8, 140.6, 160.7 ppm.
4.2.3. 1-(4-Ethyllphenyl)1-H-1,2,3,4-tetrazole (2c)
Yield: 90%, m.p. 97˚C - 98˚C; pale yellow solid. IR (KBr): v = 3137, 2962, 2921, 2880, 1519, 1475, 1367, 1247, 1089, 1024, 838 cm−1; 1H NMR (CDCl3): d = 1.25 - 1.30 (3H, t, J = 7.6 Hz, CH3), 2.78 - 2.70 (2H, q, J = 7.6 Hz, CH2), 7.41 - 7.38 (2H, d, J = 8.4 Hz, ArH), 7.62 - 7.59 (2H, d, J = 8.4 Hz, ArH), 8.97(1H, s, CH) ppm. 13C NMR (CDCl3): d = 15.3, 28.5, 121.2, 129.5, 131.6, 140.5, 146.7 ppm. HRMS (EI+) Calcd for [C9H10N4]+: 174.0907, found: 174.0901.
4.2.4. 1-(4-Dimethyllphenyl)1-H-1,2,3,4-tetrazole (2d)
Yield: 93%. m.p. 57˚C - 58˚C; colorless solid. IR (KBr): v = 3120, 2957, 2911, 2852, 1619, 1505, 1100 cm−1. 1H NMR (CDCl3): d = 2.32 (3H, s, CH3), 2.34 (3H, s, CH3), 7.30 - 7.26 (1H, d, J = 8.1 Hz, ArH), 7.41 - 7.38 (1H, d, J = 8.1 Hz, ArH), 7.46 (1H, s, ArH), 8.97 (1H, s, CH) ppm. 13C NMR (CDCl3, d, ppm): 19.5, 19.9, 118.4, 122.2, 130.9, 131.6, 138.9, 139.0, 140.5. HRMS (EI+) Calcd for [C9H10N4]+: 174.0907, found: 174.0896.
4.2.5. 1-(4-Bromophenyl)1-H-1,2,3,4-tetrazole (2e)
Yield: 87%. m.p. 184˚C - 185˚C (lit. [17] 133˚C - 134˚C); pale yellow solid. IR (KBr): v = 3130, 2917, 2849, 1501, 1462, 1385 cm−1. 1H NMR (DMSOd6): d = 7.87 (s, 4H, ArH), 10.11 (s, 1H, CH) ppm.13C NMR (DMSOd6): d = 122.5, 123.1, 132.9, 142.3, 142.4.
4.2.6. 1-(4-Chlorophenyl)1-H-1,2,3,4-tetrazole (2f)
Yield: 88%. m.p. 158˚C - 159˚C (lit. [16] 155˚C - 156˚C); colorless crystal. IR (KBr): v = 3125, 3105, 2917, 2849, 1505, 1462, 1385, 1201, 1088, 995, 831 cm−1. 1H NMR (DMSOd6): d = 7.75 - 7.72 (d.t, 2H, J = 8.8 Hz, ArH), 7.97 - 7.94 (d.t, 2H, J = 8.8 Hz, ArH), 10.11 (s, 1H, CH) ppm; 13C NMR (DMSOd6): d = 122.9, 130.1, 132.6, 134.1, 142.3, 142.5.
4.2.7. 1-(3-Nitrophenyl)1-H-1,2,3,4-tetrazole (2g)
Yield: 92%. m.p. 110˚C - 111˚C (lit. [18] 108˚C - 109˚C); colorless crystal crystal. IR (KBr): v = 3132, 3091, 2911, 2854, 1596, 1519, 1463, 1344, 1211, 1088, 990, 857 cm−1. 1H NMR (DMSOd6): 8.26 - 8.22 (m, 3H, ArH), 8.53 - 8.49 (d, 1H, J = 6.9, ArH), 10.20 (s, 1H, CH) ppm; 13C NMR (DMSOd6): 122.0, 126.2, 137.5, 141.7, 144.2, 148.3 ppm.
4.2.8. 1-(4-Nitrophenyl)1-H-1,2,3,4-tetrazole (2h)
Yield: 90%. m.p. 207˚C - 208˚C (lit. [16] 202˚C - 204˚C); pale yellow crystal. IR (KBr): v = 3132, 3091, 2911, 2854, 1611, 1596, 1519, 1463, 1344, 1211, 1088, 990, 857 cm−1. 1H NMR (DMSOd6): 8.26 - 8.22 (d, 2H, J = 7.0 Hz, ArH), 8.53 - 8.49 (d, 2H, J = 6.9, ArH), 10.30 (s, 1H, CH) ppm; 13C NMR (DMSOd6): 121.9, 125.7, 138.2, 142.7, 142.8, 147.4 ppm.
4.2.9. 1-(4-Nitrophenyl)1-H-1,2,3,4-tetrazole (2i)
Yield: 92%. m.p. 128˚C - 129˚C (lit. [19] 125˚C - 126˚C); Colorless needle; IR (KBr): ν = 1597, 1576, 1472, 1391, 1212, 1182, 1151, 1090, 1006 cm−1.1H NMR (DMSOd6): d = 7.61 - 7.65 (m, 1H), 8.07 - 8.05 (d, 1H, J = 8.1 Hz), 8.21 - 8.15 (td, 1H, J = 1.7 Hz), 8.66 - 8.64 (dd, 1H, J = 0.7 Hz), 10.18 (s, 1H) ppm. 13C NMR (DMSOd6): d = 115.6, 125.3, 140.6, 141.6, 146.5, 149.3 ppm.
References
- Butler, R.N. (1996) Comprehensive Heterocyclic Chemistry: Five-Membered Rings with More Than Two Heteroatoms and Fused Carbocyclic Derivatives. Katrisky, A.R. and Scriven E.F.V., Eds., Academic Press, Elsevier, Waltham, Vol. 4, 621-678.
- Herr, R. (2002) 5-Substituted-1H-tetrazoles as Carboxylic Acid Isosteres: Medicinal Chemistry and Synthetic Methods. Bioorganic & Medicinal Chemistry, 10, 3379-3393. http://dx.doi.org/10.1016/S0968-0896(02)00239-0
- Klapötke, T.M., Sabaté, C.M. and Stierstorfer, J. (2009) Neutral 5-Nitrotetrazoles: Easy Initiation with Low Pollution. New Journal of Chemistry, 33, 136-147. http://dx.doi.org/10.1039/B812529E
- Ichikawa, T., Yamada, M., Yamaguchi, M., Kitazaki, T., Matsushita, Y., Higashikawa, K. and Itoh, K. (2001) Optically Active Antifungal Azoles. XIII. Synthesis of Stereoisomers Metabolites of 1-[(1R,2R)-2-(2,4-Difluorophenyl)-2-hy- droxy-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]-3-(4-(1"-1tetrazolyl)phenyll-2-imidazolidinone (TAK-456). Chemical & Pharmaceutical Bulletin, 49, 1110-1119. http://dx.doi.org/10.1248/cpb.49.1110
- Zimmerman, D.M. and Olofson, R.A. (1969) The Rapid Synthesis of 1-Substituted Tetrazole. Tertrahedron Letters, 58, 5081-5084. http://dx.doi.org/10.1016/S0040-4039(01)88889-4
- Jin, T., Kamijo, S. and Yamamoto, Y. (2004) Synthesis of 1-Substituted Tetrazoles via the Acid-Catalyzed [3 + 2] Cycloaddition between Isocyanides and Trimethylsilyl Azide. Tetrahedron Letters, 45, 9435-943. http://dx.doi.org/10.1016/j.tetlet.2004.10.103
- Satoh, Y. and Marcopulos, N. (1995) Application of 5-Lithiotetrazoles in Organic Synthesis. Tetrahedron Letters, 36, 1759-1762. http://dx.doi.org/10.1016/0040-4039(95)00117-U
- Potewar, T.M., Siddiqui, S.A., Lahoti, R.J. and Srinivasan, K.V. (2007) Efficient and Rapid Synthesis of 1-Substituted- 1H-1,2,3,4-tetrazoles in the Acidic Ionic Liquid 1-n-Butylimidazolium Tetrafluoroborate. Tetrahedron Letters, 48, 1721-1724. http://dx.doi.org/10.1016/j.tetlet.2007.01.050
- Su, W., Hong, Z., Shan, W. and Zhang, X. (2006) A Facile Synthesis of 1-Substituted-1H-1,2,3,4-tetrazoles Catalyzed by Ytterbium Triflate Hydrate. European Journal of Organic Chemistry, 12, 2723-2726. http://dx.doi.org/10.1002/ejoc.200600007
- Habibi, D., Nasrollahzadeh, M. and Kamali, T.A. (2011) Green Synthesis of the 1-Substituted 1H-1,2,3,4-Tetrazoles by Application of the Natrolite Zeolite as a New and Reusable Heterogeneous Catalyst. Green Chemistry, 13, 3499- 3504. http://dx.doi.org/10.1039/c1gc15245a
- Diaz, D.D., Miranda, P.O., Padron, J.I. and Martin, V.S. (2006) Recent Uses of Iron(III) Chloride in Organic Synthesis. Current Organic Chemistry, 10, 457-476. http://dx.doi.org/10.2174/138527206776055330
- Zhan, Z.-P. and Liu, H.-J. (2006) FeCl3-Catalyzed Coupling of Propargylic Acetates with Alcohols. Synlett, No. 14, 2278-2280. http://dx.doi.org/10.1055/s-2006-949645
- Kischel, J., Iovel, I., Mertins, K., Zapf, A. and Beller, M.A. (2006) Convenient FeCl3-Catalyzed Hydroarylation of Styrenes. Organic Letters, 8, 19-22.
- Komeyama, K., Morimoto, T., Nakayama, Y. and Takaki, K. (2007) Cationic Iron-Catalyzed Intramolecular Hydroalkoxylation of Unactivated Olefins. Tetrahedron Letters, 48, 3259-3261. http://dx.doi.org/10.1016/j.tetlet.2007.03.004
- Cornil, J., Guérinot, A., Reymond, S. and Cossy, J. (2013) FeCl3・6H2O, a Catalyst for the Diastereoselective Synthesis of cis-Isoxazolidines from N-Protected δ-Hydroxylamino Allylic Acetates. Journal of Organic Chemistry, 78, 10273- 10287. http://dx.doi.org/10.1021/jo401627p
- Fallon, F.G. and Herbst, R.M. (1957) Synthesis of 1-Substituted Tetrazoles. Journal of Organic Chemistry, 22, 933- 936. http://dx.doi.org/10.1021/jo01359a020
- Horwitz, J.P. and Grakauskas, V.A. (1957) The Reactions of Diazonium Salts with Some Substituted Hydrazines. II. 1,6-Bisaryl-3,4-diacetyl-1,5-hexazadienes. Journal of the American Chemical Society, 79, 1249-1253. http://dx.doi.org/10.1021/ja01562a055
- Nishiyama, K., Oba, M. and Watanabe, A. (1987) Reactions of Trimethylsilyl Azidewith Aldehydes: Facile and Convenient Syntheses of Diazides Tetrazoles, and Nitriles. Tertrahedron, 43, 693-700. http://dx.doi.org/10.1016/S0040-4020(01)90003-1
- Grunert, C.M., Weinberger, P., Schweifer, J., Hampel, C., Stassen, A.F., Mereiter, K. and Linert, W. (2005) Synthesis and Characterisation of Tetrazole Compounds: 3 Series of New Ligands Representing Versatile Building Blocks for Iron(II) Spin-Crossover Compounds. Journal of Molecular Structure, 733, 41-52. http://dx.doi.org/10.1016/j.molstruc.2004.07.036
NOTES
*Corresponding author.



