International Journal of Organic Chemistry
Vol.4 No.3(2014), Article ID:49752,6 pages
DOI:10.4236/ijoc.2014.43021
An Efficient Synthesis of 2,3-Diaminoacid Derivatives Using Phosphine Catalyst
Yohei Oe1, Hiroaki Kishimoto2, Nahoko Sugioka2, Daisuke Harada2, Yukio Sato2, Tetsuo Ohta1, Isao Furukawa2
1Department of Biomedical Information, Faculty of Life and Medical Sciences, Doshisha University, Kyoto, Japan
2Department of Molecular Science and Technology, Faculty of Engineering, Doshisha University, Kyoto, Japan
Email: yoe@mail.doshisha.ac.jp, tota@mail.doshisha.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 29 June 2014; revised 17 August 2014; accepted 30 August 2014
ABSTRACT
Ethyl 2,3-diphthalimidoylpropanoate was effectively synthesized from ethyl propynoate with two equivalents of phthalimide catalyzed by triphenylphosphine in good yield. The choice of reaction media was important for selective synthesis of the desired 2,3-diaminocarboxylic acid derivatives. The reaction is considered to occur through a zwitterionic intermediate derived from the reaction of the α,β-unsaturated ester with triphenylphosphine.
Keywords:Addition, Alkynes, Amino Acids, Catalysis, Multi-Component Reaction

1. Introduction
2,3-Diaminoacids and their derivatives have attracted a great deal of attention due to their application as key structural fragments of biologically active compounds [1] -[7] , and/or as ligands for metal complexes [8] -[13] . Various methods to prepare these compounds have been reported [1] [2] [4] [14] -[19] .
While studying the PPh3-catalyzed three-component coupling of ethyl propynoate, a nitrogen nucleophile, and an aldehyde [20] , we found an efficient synthesis of 2,3-diaminoacid derivatives from ethyl propynoate and phthalimide catalyzed by triphenylphosphine. Further details on this synthesis are described in this report.
2. Results and Discussion
Reaction of ethyl propynoate (1) with phthalimide (2) in the presence of a stoichiometric amount of triphenylphosphine at room temperature gave ethyl 2,3-diphthalimidoylpropanoate (3) together with ethyl 2-phthalimidoylpropenoate (4) (Scheme 1). The ratio of 3 and 4 depended on the solvent used (Table 1). 3 was produced when highly polar solvents, such as DMSO, DMF, acetonitrile, and ketone were used. In these cases, 3 was obtained at more than 70% with no formation of 4. When 2-pyrrolidone was used, a mixture of 3 and 4 was obtained in 44 and 19% yields, respectively. However, the dehydroamino acid derivative 4 was mainly produced when a less polar solvent was used [21] . In esters, ethers, halogenated hydrocarbons, and aromatic solvents, 4 was obtained in moderate to good yields with no formation of 3.
DMSO and 2-butanone were selected to examine the effect of reaction temperature on the reaction (Table 2). The reaction in DMSO at room temperature for a short reaction time gave mostly 4. However, when the reaction time was increased, the yield of 4 began to decrease, while the yield of product 3 began to increase. Compound 3 was obtained in 97% yield at 100˚C and 87% yield at room temperature (Table 2, entry 8 and 11). In the 2-butanone solvent, it was also found that higher temperatures gave a better yield of 3.
The concentration of the substrates did not affect the yield of 3; the reaction of 1 mmol of substrate 1, 2 mmol of phthalimide (2), and 1 mmol of PPh3 in 1, 2, or 5 mL of DMSO gave 3 in 81%, 87%, and 88% yields, respectively, which were not significantly different.
This reaction also proceeded under other phosphines (Table 3). For example, the reaction progressed efficiently, when triphenylphosphine, tri(p-fluorophenyl)phosphine, and tri(p-methoxyphenyl)phosphine were used. These phosphines were found to act as catalysts [22] . For example, the reaction at room temperature for 24 h using 10 mol% triphenylphosphine resulted in 82% yield of 3. In contrast, the yield significantly decreased when tributylphosphine was used. Moreover, no reaction occurred when pyridine was used [23] .
Other nucleophiles were also investigated. Trost et al. reported that 4 was formed in 95% yield from the reaction catalyzed by triphenylphosphine in a mixture of toluene and a buffer solution of acetic acid and sodium acetate at 105˚C [24] . They found that using p-toluenesulfoamide as a substrate gave a trace amount of 3. Di-
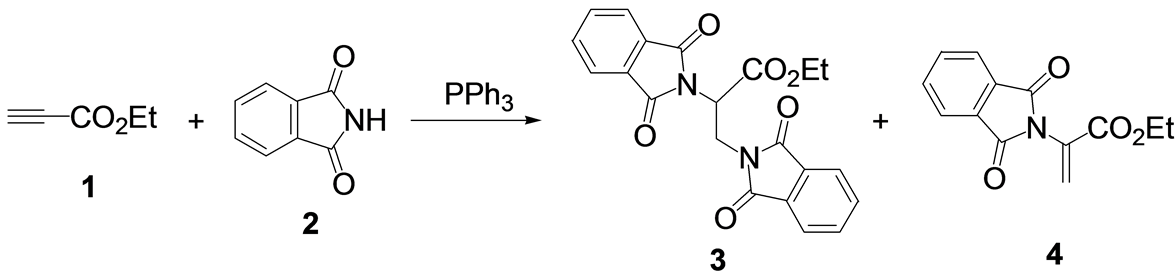
Scheme 1. Reaction of ethyl propynoate with phthalimide catalyzed by PPh3.
Table 1. Effect of solventa.
aA mixture of ethyl propynoate (1.0 mmol), phthalimide (2.0 mmol), PPh3 (1.0 mmol) was stirred in a solvent (2.0 mL) at room temperature for 24 h. bYields were determined by 1H NMR using internal standard (bibenzyl) method.
Table 2. Effect of reaction time and temperaturea.
aA mixture of ethyl propynoate (1.0 mmol), phthalimide (2.0 mmol), PPh3 (1.0 mmol) was stirred in a solvent (2.0 mL) at room temperature. bYields were determined by 1H NMR using internal standard (bibenzyl) method.
Table 3. Effect of phosphinea.
aA mixture of ethyl propynoate (1.0 mmol), phthalimide (2.0 mmol), phosphine (0.1 - 1.0 mmol) was stirred in dimethylsulfoxide (2.0 mL) at room temperature for 24 h. bYields were determined by 1H NMR using internal standard (bibenzyl) method.
acetamide, maleimide, benzylamine, allylamine, N-methyl-p-toluenesulfoamide, dimethyl malonate, and acetylacetone were also investigated, and they were found to give a 1:1 ratio of 3 and 4. Methyland phenylsubstituted propynoates were not converted to the desired 2,3-diaminoacid derivatives.
The reaction of 4 with phthalimide was also examined (Table 4). Without the presence of PPh3, 3 was not produced On the other hand, in the presence of PPh3, 3 was formed in good yield after 24 h. However, this reaction did not proceed in CH2Cl2.
The proposed reaction mechanism is shown in Scheme 2. First, triphenylphosphine attacks the propynoate to give zwitterionic intermediate 5 [25] -[39] . The intermediate subsequently remove a proton from phthalimide, giving a vinylphosphonium salt with a phthalimidate anion. The anion adds to the salt via Michael addition, followed by a proton transfer and elimination of phosphine to give 4. PPh3 attacks 4 again to give zwitterionic intermediate 7, which acts as a base to form another phthalimidate anion. This anion then attacks 4 by nucleophilic substitution to afford 3. Solvent effect is considered to stabilize zwitterionic intermediates 5 and 7.
3. Conclusion
α,β-Diamino acid derivatives are efficiently prepared by the reaction of propynoate with phthalimide in the
aA mixture of 4 (1.0 mmol), phthalimide (1.0 mmol), PPh3 (30 mol% or 0) was stirred in a solvent at room temperature for 24 h. bYields were determined by 1H NMR using internal standard (bibenzyl) method.

Scheme 2. Proposed mechanism.
presence of catalytic amount of triphenylphosphine. This method is very simple and the yield of the product is almost quantitative. Furthermore, if optically active amino acids could be prepared, this method might become more usable. Our first trial of the reaction using chiral phosphines gave racemic product. But now preliminary results using chiral additives indicate the possibility to yield the optically active product. Now establishment of asymmetric reaction is underway.
4. Experimental
4.1. General
Proton nuclear magnetic resonance (1H NMR) spectra were measured using a JEOL JNM A-400 (400 MHz) spectrometer using tetramethylsilane as the internal standard. IR spectra were measured on a Shimadzu IR-408 spectrometer. Mass spectral (GC-MS) data were recorded on a Shimadzu QP2000A instrument. Melting points were measured on a Yanako Model MP and were not corrected. All substrates were purchased and used without further purification except triphenylphosphine and phthalimide (recrystalization from methanol). Solvents were purified according to the literature method and stored under Ar atmosphere [40] .
4.2. Typical Experimental Procedure
Into a dry 80 mL Schlenk tube were added phthalimide (2.0 mmol), triphenylphosphine (0.3 mmol), and DMSO (2 mL). To this mixture, ethyl propynoate (1.0 mmol) was added dropwise, and the mixture was stirred for 24 h. The yield was determined by internal standard (bibenzyl) method. That is, bibenzyl (0.25 mmol) was added to the reaction mixture, and then concentrated mixture was analyzed by 1H NMR. The integration area of 5.17 ppm (product 3), 5.97 ppm (byproduct 4), and 2.91 ppm (bibenzyl) was used to determining the yields of 3 and/or 4. Reaction mixture was purified by column chromatography (silica gel 60, 200 - 400 mesh, hexane-ethyl acetate) to give the product.
4.3. Identification of the Products
Ethyl 2,3-diphthalimidoylpropanoate (3): Yellow solid, 1H NMR (CDCl3) δ 7.82 - 7.81 (m, 4H), 7.77 - 7.67 (m, 4H), 5.17 (dd, J = 9.2 , 5.6 Hz, 1H), 4.53 - 4.45 (m, 2H), 4.28 (q, J = 7.2 Hz, 2H), 1.27 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3) δ 167.8, 167.2, 166.8, 134.2, 134.1, 131.5, 62.3, 50.4, 36.8, 14.0; IR (KBr) 2960, 1760, 1724, 1600, 1380, 1220, 1085, 1030, 720, cm−1; GC-MS(m/z) 392; mp. 132˚C - 133˚C.
Ethyl 2-phthalimidoylpropenoate (4): White solid, 1H NMR (CDCl3) δ 7.91 - 7.87 (m, 2H), 7.79 - 7.75 (m, 2H), 6.66 (s, 1H), 5.97 (s, 1H), 4.26 (q, J = 7.14 Hz, 2H), 1.28 (t, J =7.12 Hz, 3H); 13C NMR (CDCl3) δ 166.5, 162.3, 134.5, 131.8, 129.4, 127.8, 123.9, 61.9, 13.9; IR (KBr) 3150, 2960, 1790, 1724, 1639, 1400, 1380, 1250, 1070, 1030, 720, cm−1; GC-MS(m/z) 245; mp. 71˚C - 72˚C.
References
- Viso, A., de la Pradilla, R.F., García, A. and Flores, A. (2005) α,β-Diamino Acids: Biological Significance and Synthetic Approaches. Chemical Reviews, 105, 3167-3196. http://dx.doi.org/10.1021/cr0406561
- Viso, A., de la Pradilla, R.F., Tortosa, M., García, A. and Flores, A. (2011) Update 1 of: α,β-Diamino Acids: Biological Significance and Synthetic Approaches. Chemical Reviews, 111, PR1-PR42. http://dx.doi.org/10.1021/cr100127y
- Qian, H., Fu, Z., Huang, W., Zhang, H., Zhou, J., Ge, L., Lin, R., Lin, H. and Hu, X. (2010) Synthesis and Preliminary Biological Evaluation of Capsaicin Derivatives as Potential Analgesic Drugs. Journal of Medicinal Chemistry, 6, 205-210.
- Moura, S. and Pinto, E. (2010) Synthesis of Cyclic Guanidine Intermediates of Anatoxin-a(s) in Both Racemic and Enantiomerically Pure Forms. SYNLETT, 967-969. http://dx.doi.org/10.1055/s-0029-1219559
- Ellsworth, B.A., Wang, Y., Zhu, Y., Pendri, A., Gerritz, S.W., Sun, C., Carlson, K.E., Kang, L., Baska, R.A., Yang, Y., Huang, Q., Burford, N.I., Cullen, M.J., Johnghar, S., Behnia, K., Pelleymounter, M.A., Washburn, W.N. and Ewing, W.R. (2007) Discovery of Pyrazine Carboxamide CB1 Antagonists: The Introduction of a Hydroxyl Group Improves the Pharmaceutical Properties and in Vivo Efficacy of the Series. Bioorganic Medicinal Chemistry Letters, 17, 3978-3982. http://dx.doi.org/10.1016/j.bmcl.2007.04.087
- Bostrom, J., Berggren, K., Elebring, T., Greasley, P.J. and Wilstermann, M. (2007) Scaffold Hopping, Synthesis and Structure-Activity Relationships of 5,6-Diaryl-Pyrazine-2-Amide Derivatives: A Novel Series of CB1 Receptor Antagonists. Bioorganic Medicinal Chemistry Letters, 15, 4077-4084. http://dx.doi.org/10.1016/j.bmc.2007.03.075
- Adediran, S.A., Cabaret, D., Flavell, R.R., Sammons, J.A., Wakselman, M. and Pratt, R.F. (2006) Synthesis and β-Lactamase Reactivity of α-Substituted Phenaceturates. Bioorganic Medicinal Chemistry Letters, 14, 7023-7033. http://dx.doi.org/10.1016/j.bmc.2006.06.023
- Huang, Z., Hwang, P. Watson, D.S., Cao, L. and Szoka Jr., F.C. (2009) Tris-Nitrilotriacetic Acids of Subnanomolar Affinity toward Hexahistidine Tagged Molecules. Bioconjugate Chemistry, 20, 1667-1672. http://dx.doi.org/10.1021/bc900309n
- Zangl, A., Kluefers, P., Schaniel, D. and Woike, T. (2009) Photoinduced Linkage Isomerism of {RuNO}6 Complexes with Bioligands and Related Chelators. Dalton Transactions, 1034-1045. http://dx.doi.org/10.1039/b812246f
- Luts, T., Suprun, W., Hofmann, D., Klepel, O. and Papp, H. (2007) Epoxidation of Olefins Catalyzed by Novel Mn(III) and Mo(IV) Salen Complexes Immobilized on Mesoporous Silica Gel. Journal of Molecular Catalysis A: Chemical, 261, 16-23. http://dx.doi.org/10.1016/j.molcata.2006.07.035
- Liu, Y., Pak, J.K., Schmutz, P., Bauwens, M., Mertens, J., Knight, H. and Alberto, R. (2006) Amino Acids Labeled with [99mTc(CO)3]+ and Recognized by the L-Type Amino Acid Transporter LAT1. Journal of the American Chemical Society, 128, 15996-15997. http://dx.doi.org/10.1021/ja066002m
- Takashima, H., Hirai, C. and Tsukahara, K. (2005) Selective and Monofunctional Guanosine 5’-Monophosphate Binding by Chloro[3-(2,3-diaminopropionylamino)propionic Acid](Dimethyl Sulfoxide)platinum(II) Complex. Bulletin of the Chemical Society of Japan, 78, 1629-1634. http://dx.doi.org/10.1246/bcsj.78.1629
- Rattat, D., Eraets, K., Cleynhens, B., Knight, H., Fonge, H. and Verbruggen, A. (2004) Comparison of Tridentate Ligands in Competition Experiments for Their Ability to Form a [99mTc(CO)3] Complex. Tetrahedron Letters, 45, 2531-2534. http://dx.doi.org/10.1016/j.tetlet.2004.02.006
- Moura, S. and Pinto, E. (2010) Synthesis of Cyclic Guanidine Intermediates of Anatoxin-a(s) in both Racemic and Enantiomerically Pure Forms. Synlett, 2010, 967-969. http://dx.doi.org/10.1055/s-0029-1219559
- Becerril, A., León-Romo, J.L., Avina, J., Castellanos, E. and Juaristi, E. (2002) Diastereoselective Alkylation of a Chiral 1,4-benzodiazepine-2,5-dione Containing the α-Phenethyl Group. Attempted Asymmetric Synthesis of α,β-diamino-propionic Acid. ARKIVOC, 2002, 4-14. http://dx.doi.org/10.3998/ark.5550190.0003.c02
- Brown, E.G. and Turan, Y. (1995) Pyrimidine Metabolism and Secondary Product Formation; Biogenesis of Albizziine, 4-Hydroxyhomoarginine and 2,3-Diaminopropanoic Acid. Phytochemistry, 40, 763-771. http://dx.doi.org/10.1016/0031-9422(95)00317-Z
- Fouques, D. and Landry, J. (1991) Study of the Conversion of Asparagine and Glutamine of Proteins into Diaminopropionic and Diaminobutyric Acids Using [Bis(trifluoroacetoxy)iodo]benzene Prior to Amino Acid Determination. Analyst, 116, 529-531. http://dx.doi.org/10.1039/an9911600529
- Hellmann, H. and Haas, G. (1957) Acylaminomethylation of CH-Acidic Compounds; Syntheses of β-Aminocarboxylic Acids. Chemische Berichte, 90, 1357-1363. http://dx.doi.org/10.1002/cber.19570900733
- Hellmann, H. (1957) Neuere Methoden der praparativen organischen Chemie II. 8. Amidomethylierungen. Angewandte Chemie, 69, 463-471. http://dx.doi.org/10.1002/ange.19570691305
- Oe, Y., Inoue, T., Kishimoto, H., Sasaki, M., Ohta, T. and Furukawa, I. (2012) Three-Component Coupling Catalyzed by Phosphine: Preparation of α-amino γ-Oxo Acid Derivatives. International Journal of Organic Chemistry, 2, 111-116.
- Riddick, J.A. and Bunger, W.B. (1970) Organic Solvent. 3rd ed., Wiley-Interscience, New York.
- Ross, J., Chen, W., Xu, L. and Xiao, J. (2001) Ligand Effects in Palladium-Catalyzed Allylic Alkylation in Ionic Liquids. Organometallics, 20, 138-142. http://dx.doi.org/10.1021/om000712y
- Nair, V., Sreekanth, A.R. and Vinod, A.U. (2001) Novel Pyridine-Catalyzed Reaction of Dimethyl Acetylenedicarboxylate with Aldehydes: Formal [2+2] Cycloaddition Leading to 2-Oxo-3-benzylidinesuccinates. Organic Letters, 3, 3495-3497. http://dx.doi.org/10.1021/ol016550z
- Trost, B.M. and Dake, G.R. (1997) Nitrogen Pronucleophiles in the Phosphine-Catalyzed γ-Addition Reaction. The Journal of Organic Chemistry, 62, 5670-5671. http://dx.doi.org/10.1021/jo970848e
- Rychnovsky, S.D. and Kim, J. (1994) Triphenylphosphine-Catalyzed Isomerizations of Enynes to (E,E,E)-Trienes: Phenol as a Cocatalyst. The Journal of Organic Chemistry, 59, 2659-2660. http://dx.doi.org/10.1021/jo00088a067
- Xu, Z. and Lu, X. (1999) Phosphine-Catalyzed [3+2] Cycloaddition Reactions of Substituted 2-Alkynoates or 2,3-alkenoates with Electron-Deficient Olefins and Imines. Tetrahedron Letters, 40, 549-552.http://dx.doi.org/10.1016/S0040-4039(98)02405-8
- Zhang, C. and Lu, X. (1995) Phosphine-Catalyzed Cycloaddition of 2,3-Butadienoates or 2-Butynoates with Electron-Deficient Olefins. A Novel [3+2] Annulation Approach to Cyclopentenes. The Journal of Organic Chemistry, 60, 2906-2908. http://dx.doi.org/10.1021/jo00114a048
- Xu, Z. and Lu, X. (1997) Phosphine-Catalyzed [3+2] Cycloaddition Reaction of Methyl 2,3-Butadienoate and N-Tosylimines. A Novel Approach to Nitrogen Heterocycles. Tetrahedron Letters, 38, 3461-3464.http://dx.doi.org/10.1016/S0040-4039(97)00656-4
- Guo, C. and Lu, X. (1993) Rein-vestigation on the Catalytic Isomerization of Carbon-Carbon Triple Bonds. Journal of the Chemical Society, Perkin Transactions, 1993, 1921-1923. http://dx.doi.org/10.1039/p19930001921
- Trost, B.M. and Li, C.J. (1994) Phosphine-Catalyzed Isomerization-Addition of Oxygen Nucleophiles to 2-Alkynoates. Journal of the American Chemical Society, 116, 10819-10820. http://dx.doi.org/10.1021/ja00102a071
- Trost, B.M. and Kazmaier, U. (1992) Internal Redox Catalyzed by Triphenylphosphine. Journal of the American Chemical Society, 114, 7933-7935. http://dx.doi.org/10.1021/ja00046a062
- Trost, B.M. and Dake, G.R. (1997) Nucleophilic α-Addition to Al-kynoates. A Synthesis of Dehydroamino Acids. Journal of the American Chemical Society, 119, 7595-7596. http://dx.doi.org/10.1021/ja971238z
- Trost, B.M. and Li, C.J. (1994) Novel “Umpolung” in C-C Bond Formation Catalyzed by Triphenylphosphine. Journal of the American Chemical Society, 116, 3167-3168. http://dx.doi.org/10.1021/ja00086a074
- Kuroda, H., Hanaki, E. and Kawakami, M. (1999) A Convenient Method for the Preparation of Furans by the Phosphine-Initiated Reactions of Enynes Bearing a Carbonyl Group. Tet-rahedron Letters, 40, 3753-3756. http://dx.doi.org/10.1016/S0040-4039(99)00601-2
- Nozaki, K., Sato, N., Ikeda, K. annd Takaya, H. (1996) Synthesis of Highly Functionalized γ-Butyrolactones from Activated Carbonyl Compounds and Dimethyl Acetylenedicarboxylate. The Journal of Organic Chemistry, 61, 4516-4519. http://dx.doi.org/10.1021/jo951828k
- Yavari, I. and Mosslemin, M.H. (1998) An Efficient One-Pot Synthesis of Dialkyl 2,5-dihydrofuran-2,3-dicarboxylates Mediated by Vinyltriphenylphosphonium Salt. Tetrahedron, 54, 9169-9174. http://dx.doi.org/10.1016/S0040-4020(98)00554-7
- Yavari, I., Hekmat-Shoar, R. and Zonouzi, A. (1998) A New Efficient Rout to 4-Carboxymethylcoumarins Mediated by Vinyltriphenylphosphonium Salt. Tetrahedron Letters, 39, 2391-2392.
- Yavari, I. and Baharfar, R. (1997) Vinylphosphonium Salt Mediated One-Pot Synthesis of Functionalized-3-(triphenylphosphoranylidene)butyrolactones. Tetrahedron Letters, 38, 4259-4262.
- Caddick, S., Aboutayab, K., Jenkins, K. and West, R.I. (1996) Intramolecular Radical Substitution Reactions: A Novel Approach to Fused [1,2-a] Indoles. Journal of the Chemical Society, Perkin Transactions, 1996, 675-682. http://dx.doi.org/10.1039/p19960000675
- Perrin, D.D. and Armarego, W.L.F. (1988) Purification of Laboratory Chemicals. 3rd Edition, Pergamon, Oxford.


