International Journal of Organic Chemistry
Vol.3 No.2(2013), Article ID:33024,11 pages DOI:10.4236/ijoc.2013.32014
Modelling One-Pot Method for Synthesis of 2,3-Dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazine 5,5-Dioxides and Their Homologues
National University of Pharmacy, Kharkiv, Ukraine
Email: *aldry18@hotmail.com
Copyright © 2013 Oleksandr Yu Grevtsov et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 25, 2013; revised May 8, 2013; accepted May 22, 2013
Keywords: Sulfones; Lactims; Cyclization; Heterocycles; Nucleophilic Aromatic Substitution
ABSTRACT
A facile method for synthesis of 2,3-dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazines by interaction of methylenactive (2-fluorophenyl)sulfones with homologues of either 5-methoxy-3,4-dihydro-2H-pyrrole or 5-(methylthio)-3,4-dihydro- 2H-pyrrole has been developed.
1. Introduction
In recent years a substantial number of 1,1-dioxo-4H- 1,4-benzothiazines have been reported to possess pharmacological activity. They were mentioned as glycineNMDA receptor antagonists [1] and protein kinase inhibitors, which can be used to treat cancer and hyperproliferative disorders [2]. Similar compounds were patented as potent antivirals [3], potassium channel openers [4], antiischemics to cure heart diseases [5], 3-hydroxy- 3-methylglutaryl-CoA reductase inhibitors [6], some of them were reported to be diuretics [7]. Also they were known as highly effective antimicrobial agents against Streptococcus and Klebsiella [8].
1,1-Dioxo-4H-1,4-benzothiazines can be readily synthesized by the oxidation of 4H-1,4-benzothiazines [9- 13], intramolecular cyclization of N-[(2-alkylsulfonyl)- phenyl]amides of carboxylic acids [14-17], spontaneous cyclization of 1-[(2-nitrophenyl)sulfonyl]ketones during reduction [18,19].
The promising approach is also the cyclization of 2-[(2-halogenophenyl)sulfonyl]ethylenamines containing electronwithdrawing group A in the presence of bases [20-26] (Figure 1).
The last synthetic approach for 1,1-dioxo-4H-1,4- benzothiadiazines is the most convenient for the achievement of high range molecular diversity due to the variability of radicals in the aromatic ring (R1) and the positions 2, 3, 4 of 1,4-benzothiadiazine moiety (A, R3, R2). The most significant limitation of this method is mainly concerned with the leaving halogen (X) activity. For instance, the cyclization of orto-chloroderivatives (X = Cl) could be successfully carried out only in the presence of strong bases [20], silver nitrate [22,23], potassium carbonate-crown-ether [21] or requires the application of microwave technology [24,25]. In the case of fluoroderivatives (Х = F), the cyclization proceeds readily [26].
Though the cyclization of 2-[(2-halogenophenyl)sulfonyl]ethylenamines is a versatile methodology for 1,1- dioxo-4H-1,4-benzothiadiazines obtaining, the synthesis of the heterocyclic systems where R2 and R3 are the parts of the same cycle was not reported yet.
The purpose of this paper is to develop the convenient synthetic way for 2,3-dihydro-1H-pyrrolo[2,1-c][1,4]- benzothiazine 5,5-dioxides and their homologues, which could be interesting objects for further pharmacological
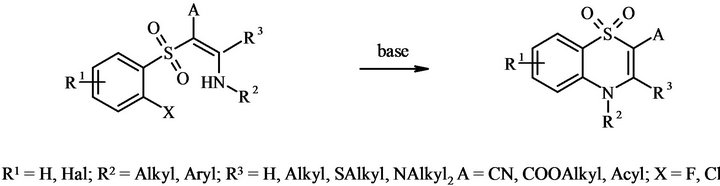
Figure 1. Cyclization of 2-[(2-halogenophenyl)sulfonyl]ethylenamines.
screening.
It was reported that the interaction of asymmetrical methylene active compounds with either 5-methoxy- 3,4-dihydro-2H-pyrrole or 5-(methylthio)-3,4-dihydro- 2H-pyrrole and their homologues led to formation of mixture E,Z-isomers of enamine-type products [27-29]. With regard to this fact, the interaction between (2- fluorophenyl)sulfones 1a-i and lactims 2а-f (Figure 2) should produce 2-{[(2-fluorophenyl)sulfonyl]methylene}- pyrrolidines and their homologues 3а-z (Figure 1).
The heating of sulfones 1 with excess of lactims 2 (30% for sulfonylacetonitriles 1а, d-i and 50% for other compounds 1) at 90˚C has been chosen as the standard reaction conditions. The reaction for acetonitriles 1а, d-i was held in DMF media, for other compounds 1 was carried out solvent-free. The process was monitored by TLC and disappearance of starting sulfone 1 spot has been controlled. The results of experiment demonstrated that the interaction of sulfones 1 with 5-methoxy-3,4-dihydro-2H-pyrrole and its homologues 2a-c in chosen conditions did not produces solely products 3. In some cases the products of further cyclization 4 were also present in the reaction mixture. The lactims 2 having larger cycle required more reaction time. Experimental data (Table 1)
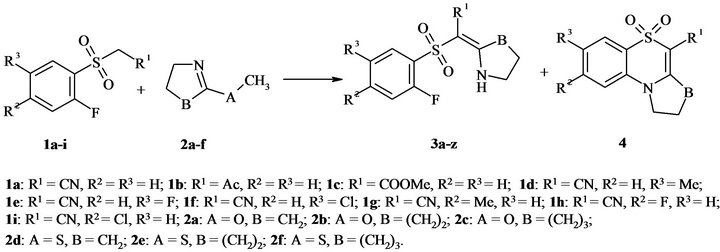
Figure 2. Interaction of sulfones 1 with lactims 2.
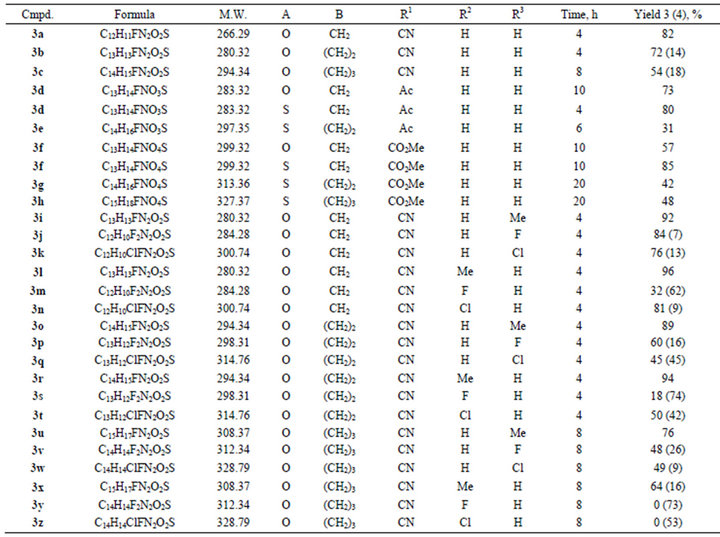
Table 1. 2-{[(2-Fluorophenyl)sulfonyl]methylene}pyrrolidines and their homologues 3а-z.
show that the compounds 3 with R1 = CN are the most susceptible for cyclization. Thus, they were chosen as the model objects to study the influence of the substituent in benzene ring on the cyclization of compounds 3. It was established that electrondonating groups interdict formation of 1,4-benzothiadiazine ring, at the same time electronwithdrawing groups (halogens) promote cyclization of enamines 3. For example, the combination of О-methyllactim 2с with sulfonylacetonitriles 1h, i, where R2 = Cl and F, only gave the product of cyclization 8,9, 10,11-tetrahydro-7H-azepino[2,1-c][1,4]benzothiazine-6-carbonitrile 5,5-dioxides 4y, z. The reaction of sulfonylacetone 1b (R1 = Ac) with О-methyllactim 2a resulted in selectively (1E)-1-[(2-fluorophenyl)sulfonyl]-1-(pyrrolidin-2-ylidene)acetone 3d, and its interaction with lactims 2b and 2c allowed us to isolate only 2-methyl- 1,4-benzoxathiine 4,4-dioxide 5 (Figure 3). Probably in the case of sterically hindered lactims 2b, c, the competing reaction of intramolecular cyclization to 2-methyl- 1,4-benzoxathiine-4,4-dioxide 5 became the preferable process.
To prove the formation of compound 5 in this reaction, we performed its alternative synthesis by heating of sulfonylacetone 1b in 1,4-dioxane at 90˚C in the presence of equimolar amount DBU for 4 hours.
The reaction of sulfonylacetone 1b (R1 = Ac) with S-methyllactims 2d and 2e lead to formations of enamine type products 3d and 3e, and S-methyllactim 2f (2Z)-2- {[(2-fluorophenyl)sulfonyl]methylidene}azepane 6 has been isolated with the yield 25% as mixture of Eand Zisomers.
The interaction of methyl [(2-fluorophenyl)sulfonyl]- acetate 1с with O-methyllactim 2a requires more time than interaction with sulfones 1a and 1b and results in the mixture of Eand Zisomers of enamine 3f. The reaction of sulfone 1с with larger O-methyllactims 2b, c is failed.
The only reaction of methyl [(2-fluorophenyl)sulfonyl]- acetate 1с with S-methyllactims 2d-f allowed us to obtain methyl (2E,Z)-[(2-fluorophenyl)sulfonyl](pyrrolidin-2- ylidene)acetate 3f and its homologues 3g and 3h with
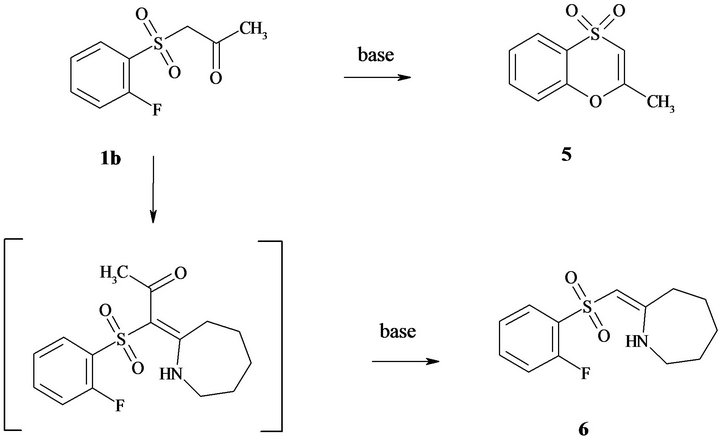
Figure 3. Transformations of sulfonylacetone 1b.
moderate yields.
The cyclization of enamines 3 to 2,3-dihydro-1Hpyrrolo[2,1-c][1,4]benzothiazine 5,5-dioxides and their homologues 4 has been performed by heating in 1,4- ioxane with equimolar amount of DBU at 50˚C - 60˚C according to Figure 4.
Since 2-fluorophenylsulfones 1 interaction with О- or S-methyllactims 2 was a satisfactory approach for enamines 3, and further cyclization of the condensation products 3 to the 2,3-dihydro-1H-pyrrolo[2,1-c][1,4]- benothiazine 5,5-dioxides and their homologues 4 occurred in numerous cases, it was interesting to develop “one-pot” method for synthesis of the products 4. For this purpose O-methyllactims 2a-c were chosen as the starting material for interaction with [(2-fluorophenyl)- sulfonyl]acetonitriles 1а, d-i, S-methyllactims 2d-f were used for condensation with 1-[(2-fluorophenyl)sulfonyl] acetone 1b and methyl [(2-fluorophenyl)sulfonyl] acetate 1c.
According to the proposed “one-pot”, procedure enamines 3 were not isolate. When the reaction of sulfones 1 with lactims 2 was complete, to the cool reaction mixture (20˚C) 1,4-dioxane and equimolar amount of DBU were added and the reaction mixture was heated at 60˚C for 2 - 4 hours. After dilution of reaction mixture with 2-propanol, the precipitate formed was filtered and crystallized from DMF-2-propanol mixture. The yields and some properties of obtained compounds are given in Table 2.
2. Experimental Section
The melting points (˚C) were measured with a Buchi В-520 melting point apparatus and were not corrected. IR spectra were recorded on FT-IR Bruker Tensor-27 spectrometer in KBr. Thin-layer chromatography (TLC) was performed on aluminum sheets precoated with silica gel (Merck, Kieselgel 60 F-254). LC/MS spectra were recorded with PE SCIEX API 150EX liquid chromatograph equipped with a UV detector (lmax 215 and 254 nm) and using a C18 column (100 × 4 mm). Elution started with water and ended with acetonitrile/water (95:5, v/v) and used a linear gradient at a flow rate of 0.15 mL/min and an analysis cycle time of 25 min. 1H NMR-spectra were
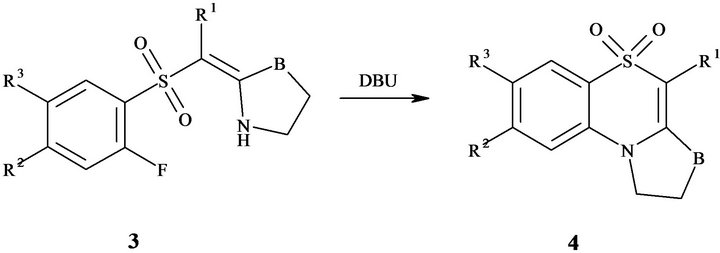
Figure 4. Cyclization of enamines 3 to 2,3-dihydro-1Hpyrrolo[2,1-c][1,4]benzothiazine 5,5-dioxides and their homologues 4.
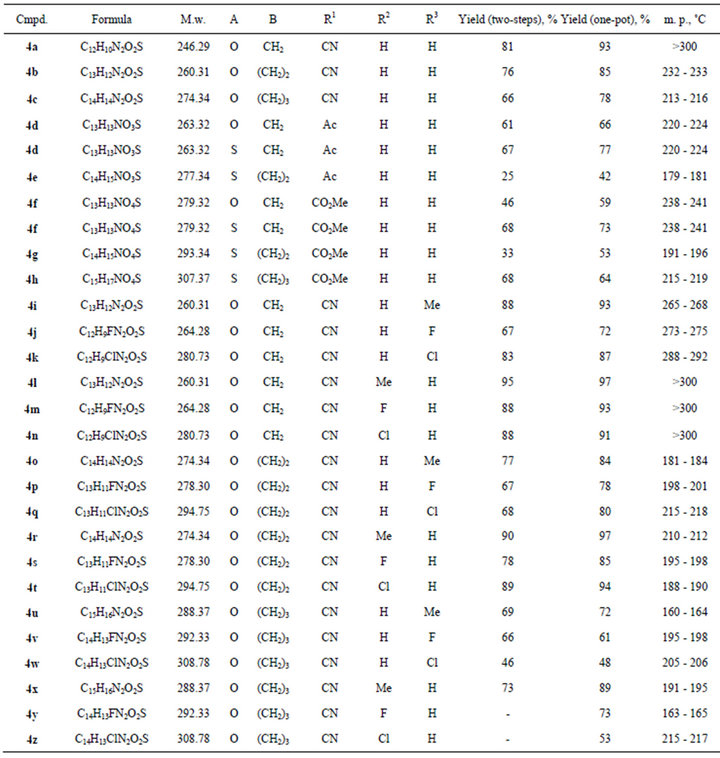
Table 2. 2,3-Dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazine 5,5-dioxides and their homologues 4.
recorded on Varian Mercury (200 MHz) spectrometer in DMSO-d6 using TMS as an internal standard (chemical shifts are reported in ppm). 13C NMR-spectra were recorded on Bruker DRX-300 (75 MHz) spectrometer in DMSO-d6 using TMS as an internal standard (chemical shifts are reported in ppm).
Starting (2-halogenophenyl)sulfones 1a-j [30-32], Oand S-methyl 2a-f [33,34] have been obtained as commercial substances similarly to the previously reported methods.
[(2-Fluorophenyl)sulfonyl](pyrrolidin-2-ylidene) acetonitriles and their homologues 3a-c, i-z; typical procedure.
To the solution of [(2-fluorophenyl)sulfonyl]acetonetrile 1а,d-i (10 mmol) in DMF (4 mL) the correspondent O-methyllactim 2a-c (13 mmol) had been added and the mixture was additionally heated for 4 to 8 hours (monitored by TLC, eluent—CHCl3) at 90˚C. The reaction mixture was diluted with 2-propanol after cooling. The precipitate formed was filtered and used for further transformation without any additional purification. The compounds were isolated as the mixture of E and Z isomers. The compounds 3b, c, j, k, m, n, p, q, s, t, v, w, x contained a great amount of the correspondent cyclized products 4b, c, j, k, m, n, p, q, s, t, v, w, x as inseparable mixtures, and their analytical samples were not isolated. The compounds 3y, z were not isolated but only the products of their further cyclization 4y, z.
1) (2E,Z)-[(2-fluorophenyl)sulfonyl](pyrrolidin-2- ylidene)acetonitrile (3a)
Yield: 8.2 mmol (82%); cream coloured solid.
LC/MS: m/z [M + H]+ calcd for C12H11FN2O2S: 266.29; found: 266.3.
2) (2E,Z)-[(2-fluoro-5-methylphenyl)sulfonyl](pyrrolidin-2-ylidene)acetonitrile (3i)
Yield: 9.2 mmol (92%); cream coloured solid.
LC/MS: m/z [M + H]+ calcd for C13H13FN2O2S: 280.32; found: 280.4.
3) (2E,Z)-[(2-fluoro-4-methylphenyl)sulfonyl](pyrrolidin-2-ylidene)acetonitrile (3l)
Yield: 9.6 mmol (96%); cream coloured solid.
LC/MS: m/z [M + H]+ calcd for C13H13FN2O2S: 280.32; found: 280.4.
4) (2E,Z)-[(2-fluoro-5-methylphenyl)sulfonyl](piperidin-2-ylidene)acetonitrile (3o)
Yield: 8.9 mmol (89%); cream coloured solid.
LC/MS: m/z [M + H]+ calcd for C14H15FN2O2S: 294.35; found: 294.2.
5) (2E,Z)-[(2-fluoro-4-methylphenyl)sulfonyl](piperidin-2-ylidene)acetonitrile (3r)
Yield: 9.4 mmol (94%); cream coloured solid.
LC/MS: m/z [M + H]+ calcd for C14H15FN2O2S: 294.35; found: 294.2.
6) (2E,Z)-azepan-2-ylidene[(2-fluoro-5-methylphenyl)- sulfonyl]acetonitrile (3u)
Yield: 7.6 mmol (76%); cream coloured solid.
LC/MS: m/z [M + H]+ calcd for C15H17FN2O2S: 308.37; found: 308.4.
(1E)-1-[(2-Fluorophenyl)sulfonyl]-1-(pyrrolidin-2- ylidene)acetone (3d) and (1e)-1-[(2-fluorophenyl) sulfonyl]-1-(piperidin-2-ylidene)acetone (3e); typical procedure.
The mixture of 1-[(2-fluorophenyl)sulfonyl]acetone 1b (10 mmol) with the corresponding S-methyllactim 2d,e (15 mmol) was heated at 90˚C for 6 to 8 hours (monitored by TLC, eluent—CHCl3). The reaction mixture was diluted with 2-propanol after cooling. The precipitate formed was filtered and used for further transformation without any additional purification. The interaction of the starting ketone 1b with 7-(methylthio)-3,4,5,6-tetrahydro-2H-azepine 2f resulted 2-methyl-1,4-benzoxathiine- 4,4-dioxide 5.
1) (1E)-1-[(2-fluorophenyl)sulfonyl]-1-(pyrrolidin-2- ylidene)acetone (3d)
Yield: 6.8 mmol (68%); cream coloured solid (This compound has been also obtained according to the similar procedure by the interaction between 1-[(2-fluorophenyl)sulfonyl]acetone 1b and 5-methoxy-3,4-dihydro- 2H-pyrrole 2a. Yield: 7.3 mmol (73%)).
IR (KBr): 3203, 2980, 2953, 2891, 1595, 1667, 1473, 1454, 1411, 1261, 1235, 1145, 1122, 1076, 994, 820, 761, 690, 595, 573, 529 cm−1.
1H NMR (200 MHz DMSO-d6): δ = 1.81 - 1.96 (m, 2 H, CH2), 2.15 (s, 3 H, COCH3), 3.09 (t, J = 8.0 Hz, 2 H, CH2), 3.58 (t, J = 7.2 Hz, 2 H, CH2), 7.35 - 7.44 (m, 2 H, CH), 7.63 - 7.74 (m, 1 H, CH), 7.85 - 7.94 (m, 1 H, CH), 11.50 (br s, 1 H, NH).
LC/MS: m/z [M + H]+ calcd for C13H14FNO3S: 283.32; found: 283.4.
2) (1E)-1-[(2-fluorophenyl)sulfonyl]-1-(piperidin-2- ylidene)acetone (3e)
Yield: 3.1 mmol (31%); cream coloured solid.
IR (KBr): 2975, 2940, 2873, 1606, 1580, 1466, 1444, 1304, 1216, 1152, 1111, 1018, 972, 867, 820, 765, 730, 688, 622, 598, 573, 531, 456 cm−1.
1H NMR (200 MHz DMSO-d6): δ = 1.49 - 1.71 (m, 4 H, CH2), 2.23 (s, 3 H, COCH3), 2.73 (t, J = 6.1 Hz, 2 H, CH2), 3.42 (t, J = 5.9 Hz, 2 H, CH2), 7.35 - 7.46 (m, 2 H, CH), 7.63 - 7.74 (m, 1 H, CH), 7.83 - 7.92 (m, 1 H, CH), 13.00 (br s, 1 H, NH).
LC/MS: m/z [M + H]+ calcd for C14H16FNO3S: 297.35; found: 297.4.
Methyl (2E,Z)-[(2-fluorophenyl)sulfonyl](pyrrolidin-2-ylidene)acetate and its homologues 3f-h; typical procedure.
The mixture of methyl 1-[(2-fluorophenyl)sulfonyl] acetate 1c (10 mmol) with the corresponding S-methyllactim 2d-f (15 mmol) was heated at 100˚C for 10 to 20 hours (monitored by TLC, eluent—CHCl3). The reaction mixture was diluted with 2-propanol after cooling. The precipitate formed was filtered and used for further transformation without any additional purification. The compounds were isolated as the mixture of E and Z isomers.
1) Methyl (2E,Z)-[(2-fluorophenyl)sulfonyl] (pyrrolidin-2-ylidene)acetate (3f)
Yield: 8.5 mmol (85%); cream coloured solid. (This compound has been also obtained according to the similar procedure by the interaction between methyl 1-[(2- fluorophenyl)sulfonyl]acetate 1c and 5-methoxy-3,4-dihydro-2H-pyrrole 2a. Yield: 5.7 mmol (57%)).
LC/MS: m/z [M + H]+ calcd for C13H14FNO4S: 299.32; found: 299.4.
2) Methyl (2E,Z)-[(2-fluorophenyl)sulfonyl](piperidin- 2-ylidene)acetate (3g)
Yield: 4.2 mmol (42%); cream coloured solid.
LC/MS: m/z [M + H]+ calcd for C14H16FNO4S: 313.36; found: 313.4.
3) Methyl (2E,Z)-azepan-2-ylidene[(2-fluorophenyl)- sulfonyl]acetate (3h)
Yield: 4.8 mmol (48%); cream coloured solid.
LC/MS: m/z [M + H]+ calcd for C15H18FNO4S: 327.37; found: 327.4.
General procedure for synthesis of compounds 4a-x (cyclization of compounds 3).
The mixture of the correspondent compound 3 (10 mmol), 1,4-dioxane (6 ml) and DBU (10 mmol) was heated at 60˚C for 2 to 4 hours (for R1 = CN 50˚C, 1 - 2 hours) (monitored by TLC, eluent—2-propanole-CHCl3, 1:30). In the case when the compound 3 contained the admixture of cyclization product 4, the ratio of 3 and 4 in the mixture was calculated on the basis of integral intensity of specific peaks in 1H NMR-spectra of mixture. The reaction mixture was diluted with 2-propanol after cooling. The precipitate formed was filtered and crystallized from 2-propanol-DMF mixture. The compounds 4y, z were formed at the first stage of reaction between 7-(methylthio)-3,4,5,6-tetrahydro-2H-azepine 2f and corresponding sulfones 1h, i.
General procedure for synthesis of compounds 4a-z (“one-pot” method).
To the solution of 1 (10 mmol) in DMF (4 mL) the correspondent lactim 2 (13 mmol) had been added and the mixture was additionally heated for 4 to 8 hours (monitored by TLC, eluent—CHCl3) at 90˚C. After the reaction mixture was cooled to room temperature, 1,4- dioxane and equimolar amount of DBU were added and the reaction mixture was heated additionally at 60˚C for 2 - 4 hours. The precipitate formed after dilution of reaction mixture with 2-propanol, was filtered and crystallized from DMF-2-propanol mixture.
1) 2,3-Dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazine- 4-carbonitrile 5,5-dioxide (4a)
Yield: 8.1 mmol (81%), 9.3 mmol (93%) (“one-pot” method); cream coloured solid.
IR (KBr): 2206, 1609, 1591, 1554, 1481, 1419, 1279, 1152, 1132, 1074, 769, 607, 575 cm−1.
1H NMR (200 MHz DMSO-d6): δ = 2.16 - 2.31 (m, 2 H, CH2), 3.23 (t, J = 7.8 Hz, 2 H, CH2), 4.26 (t, J = 7.2 Hz, 2 H, CH2), 7.51 - 7.59 (m, 2 H, CH), 7.82 (m, 1 H, CH), 8.02 (d, J = 8.1 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 19.59, 33.74, 53.40, 81.92, 112.63, 117.91, 122.61, 125.13, 126.00, 133.73, 134.25, 161.32.
LC/MS: m/z [M + H]+ calcd for C12H10N2O2S: 246.29; found: 246.1.
2) 7,8,9,10-Tetrahydropyrido[2,1-c][1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4b)
Yield: 7.6 mmol (76%), 8.5 mmol (85%) (“one-pot” method); cream coloured solid.
IR (KBr): 2957, 2881, 2203, 1581, 1533, 1469, 1350, 1278, 1139, 1073, 767, 609, 572 cm−1.
1H NMR (200 MHz DMSO-d6): δ = 1.73 - 2.01 (m, 4 H, CH2), 3.02 (t, J = 6.7 Hz, 2 H, CH2), 4.09 (t, J = 5.9 Hz, 2 H, CH2), 7.55 - 7.66 (m, 1 H, CH), 7.78 - 7.85 (m, 2 H, CH), 8.01 (d, J = 8.0 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 17.40, 21.56, 29.84, 48.07, 84.63, 112.65, 117.98, 121.78, 126.05, 126.36, 133.50, 138.13, 159.89.
LC/MS: m/z [M + H]+ calcd for C13H12N2O2S: 260.31; found: 260.1.
3) 8,9,10,11-Tetrahydro-7H-azepino[2,1-c][1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4c)
Yield: 6.6 mmol (66%), 7.8 mmol (78%) (“one-pot” method); cream coloured solid.
IR (KBr): 2940, 2862, 2204, 1580, 1535, 1457, 1415, 1299, 1215, 1167, 1073, 777, 717, 636, 588 cm−1.
1H NMR (200 MHz DMSO-d6): δ = 1.72 (br s, 4 H, CH2), 1.83 (br s, 2 H, CH2), 3.18 (br s, 2 H, CH2), 4.31 - 4.35 (m, 2 H, CH2), 7.54-7.62 (m, 1 H, CH), 7.75 - 7.88 (m, 2 H, CH), 7.98 (d, J = 8.1 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 24.97, 26.14, 27.44, 34.37, 50.84, 86.61, 112.73, 118.29, 121.69, 125.76, 126.02, 133.72, 138.62, 164.44.
LC/MS: m/z [M + H]+ calcd for C14H14N2O2S: 274.34; found: 274.2.
4) 1-(5,5-Dioxido-2,3-dihydro-1H-pyrrolo[2,1-c][1,4]- benzothiazin-4-yl)ethanone (4d)
Yield: 6.1 mmol (61%), 7.7 mmol (77%) (“one-pot” method); cream coloured solid.
IR (KBr): 3077, 2954, 2885, 1659, 1587, 1525, 1472, 1360, 1338, 1283, 1261, 1239, 1201, 1136, 1100, 995, 954, 933, 759, 593, 558 cm−1.
1H NMR (200 MHz DMSO-d6): δ = 2.07 - 2.22 (m, 2 H, CH2), 2.52 (s, 3 H, COCH3), 3.34 (t, J = 7.8 Hz, 2 H, CH2), 4.15 (t, J = 7.3 Hz, 2 H, CH2), 7.45 - 7.53 (m, 2 H, CH), 7.60 (t, J = 7.9 Hz, 1 H, CH), 8.01 (d, J = 7.8 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 20.22, 30.78, 35.10, 51.74, 110.13, 117.29, 122.88, 125.26, 127.10, 133.22, 134.48, 159.63, 189.73.
LC/MS: m/z [M + H]+ calcd for C13H13NO3S: 263.32; found: 263.0.
5) 1-(5,5-Dioxido-7,8,9,10-tetrahydropyrido[2,1-c][1,4]- benzothiazin-6-yl)ethanone (4e)
Yield: 2.5 mmol (25%), 4.2 mmol (42%) (“one-pot” method); cream coloured solid.
IR (KBr): 2968, 1665, 1582, 1520, 1467, 1404, 1356, 1274, 1155, 1137, 1110, 1004, 911, 767, 625, 595, 564, 511 cm−1.
1H NMR (200 MHz DMSO-d6): δ = 1.70 - 1.89 (m, 4 H, CH2), 2.52 (s, 3 H, COCH3), 3.04 (t, J = 7.0 Hz, 2 H, CH2), 4.10 (t, J = 5.7 Hz, 2 H, CH2), 7.45 - 7.53 (m, 1 H, CH), 7.70 - 7.80 (m, 2 H, CH), 7.94 (d, J = 7.5 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 17.35, 21.23, 26.33, 32.15, 46.15, 113.10, 117.76, 121.72, 125.11, 126.91, 132.86, 138.97, 158.53, 191.56.
LC/MS: m/z [M + H]+ calcd for C14H15NO3S: 277.34; found: 277.1.
6) Methyl 2,3-dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazine-4-carboxylate 5,5-dioxide (4f)
Yield: 6.8 mmol (68%), 7.3 mmol (73%) (“one-pot” method); cream coloured solid.
IR (KBr): 3087, 3017, 2954, 1696, 1590, 1542, 1482, 1282,1242, 1113, 765, 595, 555, 502 cm−1.
1H NMR (200 MHz DMSO-d6): δ = 2.09 - 2.24 (m, 2 H, CH2), 3.36 (t, J = 7.8, 2 H, CH2), 3.75 (s, 3 H, COOCH3), 4.16 (t, J = 7.3 Hz, 2 H, CH2), 7.42 - 7.49 (m, 2 H, CH), 7.73 (m, 1 H, CH), 7.95 (d, J = 7.4 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 20.02, 34.78, 51.72, 52.03, 100.92, 117.17, 123.07, 125.13, 127.43, 132.96, 134.25, 159.67, 162.77.
LC/MS: m/z [M + H]+ calcd for C13H13NO4S: 279.32; found: 279.1.
7) Methyl 7,8,9,10-tetrahydropyrido[2,1-c][1,4]benzothiazine-6-carboxylate 5,5-dioxide (4g)
Yield: 3.3 mmol (33%), 5.3 mmol (53%) (“one-pot” method); cream coloured solid.
IR (KBr): 3073, 2955, 2877, 1696, 1585, 1521, 1467, 1293, 1250, 1124, 976, 911, 867, 772, 584, 569, 517 cm−1.
1H NMR (200 MHz DMSO-d6): δ = 1.69 - 1.97 (m, 4 H, CH2), 3.08 (t, J = 6.9, 2 H, CH2), 3.75 (s, 3 H, COOCH3), 4.08 (t, J = 5.8 Hz, 2 H, CH2), 7.43 - 7.51 (m, 1 H, CH), 7.68 - 7.79 (m, 2 H, CH), 7.92 (d, J = 7.5 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 17.37, 21.28, 26.69, 46.19, 52.12, 104.47, 117.44, 121.94, 124.98, 127.04, 132.70, 138.91, 157.68, 162.39.
LC/MS: m/z [M + H]+ calcd for C14H15NO4S: 293.34; found: 293.2.
8) Methyl 8,9,10,11-tetrahydro-7H-azepino[2,1-c]- [1,4]benzothiazine-6-carboxylate 5,5-dioxide (4h)
Yield: 6.8 mmol (68%), 6.4 mmol (64%) (“one-pot” method); cream coloured solid.
IR (KBr): 3072, 2941, 2861, 1698, 1583, 1528, 1463, 1465, 1403, 1298, 1238, 1162, 1120, 986, 959, 905, 849, 774, 691, 589, 564, 546, 509, 464 cm−1.
1H NMR (200 MHz DMSO-d6): δ = 1.65 (br s, 4 H, CH2), 1.84 (br s, 2 H, CH2), 3.06 (br s, 2 H, CH2), 3.76 (s, 3 H, COOCH3), 4.25 - 4.29 (m, 2 H, CH2), 7.44 - 7.51 (m, 1 H, CH), 7.65 - 7.79 (m, 2 H, CH), 7.90 (d, J = 7.7 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 25.27, 26.31, 26.85, 31.68, 49.32, 52.42, 105.65, 117.58, 121.91, 124.81, 126.92, 132.86, 139.24, 159.65, 162.56.
LC/MS: m/z [M + H]+ calcd for C15H17NO4S: 307.37; found: 307.2.
9) 7-Methyl-2,3-dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazine-4-carbonitrile 5,5-dioxide (4i)
Yield: 8.8 mmol (88%), 9.3 mmol (93%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 2.14 - 2.30 (m, 2 H, CH2), 2.42 (s, 3 H, CH3), 3.20 (t, J = 7.9 Hz, 2 H, CH2), 4.23 (t, J = 7.2 Hz, 2 H, CH2), 7.44 (d, J = 8.7 Hz, 1 H, CH), 7.63 (d, J = 8.3 Hz, 1 H, CH), 7.82 (s, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 19.56, 20.33, 33.69, 53.37, 81.55, 112.76, 117.84, 121.97, 125.08, 132.10, 134.54, 136.10, 160.82.
LC/MS: m/z [M + H]+ calcd for C13H12N2O2S: 260.31; found: 260.1.
10) 7-Fluoro-2,3-dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazine-4-carbonitrile 5,5-dioxide (4j)
Yield: 6.7 mmol (67%), 7.2 mmol (72%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 2.16 - 2.31 (m, 2 H, CH2), 3.22 (t, J = 7.8 Hz, 2 H, CH2), 4.26 (t, J = 7.2 Hz, 2 H, CH2), 7.60 - 7.79 (m, 2 H, CH), 7.93 (dd, J(1) = 7.7 Hz, J(2) = 2.6 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 19.43, 33.66, 53.73, 80.85, 108.75, 109.01, 112.36, 120.87, 120.95, 121.40, 121.63, 125.92, 125.99, 131.13, 157.45, 159.91, 161.21.
LC/MS: m/z [M + H]+ calcd for C12H9FN2O2S: 264.28; found: 264.3.
11) 7-Chloro-2,3-dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazine-4-carbonitrile 5,5-dioxide (4k)
Yield: 8.3 mmol (83%), 8.7 mmol (87%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 2.16 - 2.31 (m, 2 H, CH2), 3.22 (t, J = 7.8 Hz, 2 H, CH2), 4.25 (t, J = 7.3 Hz, 2 H, CH2), 7.59 (d, J = 9.2 Hz, 1 H, CH), 7.89 (dd, J(1) = 9.0 Hz, J(2) = 2.4 Hz, 1 H, CH), 8.65 (d, J = 2.5 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 19.58, 33.81, 53.74, 82.18, 112.28, 120.42, 121.91, 126.21, 129.89, 133.30, 133.68, 161.61.
LC/MS: m/z [M + H]+ calcd for C12H9ClN2O2S: 280.73; found: 280.3.
12) 8-Methyl-2,3-dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazine-4-carbonitrile 5,5-dioxide (4l)
Yield: 9.5 mmol (95%), 9.7 mmol (97%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 2.15 - 2.30 (m, 2 H, CH2), 2.45 (s, 3 H, CH3), 3.21 (t, J = 7.8 Hz, 2 H, CH2), 4.23 (t, J = 7.3 Hz, 2 H, CH2), 7.37 (d, J = 8.3 Hz, 1 H, CH), 7.38 (s, 1 H, CH), 7.89 (d, J = 8.6 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 19.58, 21.35, 33.70, 53.33, 82.05, 112.71, 117.70, 122.54, 122.73, 126.89, 134.29, 144.46, 161.15.
LC/MS: m/z [M + H]+ calcd for C13H12N2O2S: 260.31; found: 260.1.
13) 8-Fluoro-2,3-dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazine-4-carbonitrile 5,5-dioxide (4m)
Yield: 8.8 mmol (88%), 9.3 mmol (93%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 2.16 - 2.31 (m, 2 H, CH2), 3.23 (t, J = 7.8 Hz, 2 H, CH2), 4.22 (t, J = 7.2 Hz, 2 H, CH2), 7.36 - 7.55 (m, 2 H, CH), 8.11 (dd, J(1) = 8.9 Hz, J(2) = 5.8 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 19.61, 33.83, 53.78, 82.87, 105.03, 105.39, 112.30, 113.70, 114.01, 121.77, 121.81, 125.90, 126.05, 136.48, 136.64, 161.83, 162.64, 165.97.
LC/MS: m/z [M + H]+ calcd for C12H9FN2O2S: 264.28; found: 264.5.
14) 8-Chloro-2,3-dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazine-4-carbonitrile 5,5-dioxide (4n)
Yield: 8.8 mmol (88%), 9.1 mmol (91%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 2.15 - 2.30 (m, 2 H, CH2), 3.23 (t, J = 7.8 Hz, 2 H, CH2), 4.25 (t, J = 7.3 Hz, 2 H, CH2), 7.60 (d, J = 8.6 Hz, 1 H, CH), 7.69 (s, 1 H, CH), 8.05 (d, J = 8.6 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 19.51, 33.72, 53.60, 82.66, 112.14, 117.67, 123.69, 124.62, 125.91, 135.49, 138.28, 161.76.
LC/MS: m/z [M + H]+ calcd for C12H9ClN2O2S: 280.73; found: 280.3.
15) 3-Methyl-7,8,9,10-tetrahydropyrido[2,1-c][1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4o)
Yield: 7.7 mmol (77%), 8.4 mmol (84%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.70 - 1.99 (m, 4 H, CH2), 2.42 (s, 3 H, CH3), 3.00 (t, J = 6.7 Hz, 2 H, CH2), 4.05 (t, J = 5.8 Hz, 2 H, CH2), 7.64 (d, J = 9.3 Hz, 1 H, CH), 7.73 (d, J = 9.2 Hz, 1 H, CH), 7.81 (s, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 17.45, 20.15, 21.59, 29.80, 48.06, 66.44, 84.17, 112.82, 117.92, 121.15, 126.00, 134.32, 135.96, 136.50, 159.31.
LC/MS: m/z [M + H]+ calcd for C14H14N2O2S: 274.34; found: 274.2.
16) 3-Fluoro-7,8,9,10-tetrahydropyrido[2,1-c][1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4p)
Yield: 6.7 mmol (67%), 7.8 mmol (78%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.72 - 1.99 (m, 4 H, CH2), 3.02 (t, J = 6.7 Hz, 2 H, CH2), 4.08 (t, J = 5.7 Hz, 2 H, CH2), 7.69 - 7.79 (m, 1 H, CH), 7.86 - 7.97 (m, 2 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 17.29, 21.42, 29.73, 48.45, 83.48, 107.73, 107.99, 112.41, 120.99, 121.26, 127.04, 134.94, 157.66, 159.88, 160.13.
LC/MS: m/z [M + H]+ calcd for C13H11FN2O2S: 278.30; found: 278.3.
17) 3-Chloro-7,8,9,10-tetrahydropyrido[2,1-c][1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4q)
Yield: 6.8 mmol (68%), 8.0 mmol (80%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.73 - 2.01 (m, 4 H, CH2), 3.02 (t, J = 6.6 Hz, 2 H, CH2), 4.08 (t, J = 5.8 Hz, 2 H, CH2), 7.88 - 7.89 (m, 2 H, CH), 8.01 - 8.03 (m, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 17.25, 21.35, 29.77, 48.31, 84.60, 112.25, 120.52, 120.87, 126.94, 130.29, 133.25, 136.97, 160.13.
LC/MS: m/z [M + H]+ calcd for C13H11ClN2O2S: 294.75; found: 294.3.
18) 2-Methyl-7,8,9,10-tetrahydropyrido[2,1-c][1,4]- benzothiazine-6-carbonitrile 5,5-dioxide (4r)
Yield: 9.0 mmol (90%), 9.7 mmol (97%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.73 - 2.00 (m, 4 H, CH2), 2.47 (s, 3 H, CH3), 3.01 (t, J = 6.7 Hz, 2 H, CH2), 4.07 (t, J = 6.0 Hz, 2 H, CH2), 7.41 (d, J = 8.3 Hz, 1 H, CH), 7.68 (s, 1 H, CH), 7.88 (d, J = 8.1, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 17.31, 21.42, 29.71, 47.86, 84.66, 112.65, 117.76, 121.60, 123.52, 127.08, 138.04, 144.09, 159.60.
LC/MS: m/z [M + H]+ calcd for C14H14N2O2S: 274.34; found: 274.2.
19) 2-Fluoro-7,8,9,10-tetrahydropyrido[2,1-c][1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4s)
Yield: 7.8 mmol (78%), 8.5 mmol (85%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.72 - 2.00 (m, 4 H, CH2), 3.02 (t, J = 6.7 Hz, 2 H, CH2), 4.03 (t, J = 6.0 Hz, 2 H, CH2), 7.42 - 7.52 (m, 1 H, CH), 7.78 (dd, J(1) = 12.1 Hz, J(2) = 2.2 Hz, 1 H, CH), 8.06 - 8.13 (m, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 17.22, 21.28, 29.76, 48.26, 85.53, 105.35, 105.63, 112.25, 113.97, 114.21, 122.47, 124.88, 124.99, 140.14, 140.25, 160.26, 162.97, 165.45.
LC/MS: m/z [M + H]+ calcd for C13H11FN2O2S: 278.30; found: 278.3.
20) 2-Chloro-7,8,9,10-tetrahydropyrido[2,1-c][1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4t)
Yield: 8.9 mmol (89%), 9.4 mmol (94%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.72 - 1.99 (m, 4 H, CH2), 3.02 (t, J = 6.7 Hz, 2 H, CH2), 4.08 (t, J = 5.9 Hz, 2 H, CH2), 7.66 (dd, J(1) = 8.6 Hz, J(2) = 1.7 Hz, 1 H, CH), 7.96 (d, J = 1.6 Hz, 1 H, CH), 8.03 (d, J = 8.6 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 17.22, 21.28, 29.80, 49.22, 85.38, 112.20, 117.98, 123.74, 124.50, 126.31, 138.24, 139.24, 160.36.
LC/MS: m/z [M + H]+ calcd for C13H11ClN2O2S: 294.75; found: 294.6.
21) 3-Methyl-8,9,10,11-tetrahydro-7H-azepino[2,1-c]- [1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4u)
Yield: 6.9 mmol (69%), 7.2 mmol (72%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.71 (br s, 4 H, CH2), 1.81 (br s, 2 H, CH2), 2.42 (s, 3 H, CH3), 3.16 (br s, 2 H, CH2), 4.28 - 4.32 (m, 2 H, CH2), 7.60 - 7.70 (m, 2 H, CH), 7.78 (s, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 20.02, 24.87, 26.01, 27.34, 34.14, 50.60, 85.91, 112.81, 118.13, 120.86, 125.47, 134.43, 136.07, 136.30, 163.94.
LC/MS: m/z [M + H]+ calcd for C15H16N2O2S: 288.37; found: 288.1.
22) 3-Fluoro-8,9,10,11-tetrahydro-7H-azepino[2,1-c][1,4]- benzothiazine-6-carbonitrile 5,5-dioxide (4v)
Yield: 6.6 mmol (66%), 6.1 mmol (61%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.71 (br s, 4 H, CH2), 1.82 (br s, 2 H, CH2), 3.18 (br s, 2 H, CH2), 4.31 - 4.35 (m, 2 H, CH2), 7.68 - 7.78 (m, 1 H, CH), 7.82 - 7.90 (m, 2 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 24.72, 25.90, 27.33, 34.22, 51.08, 85.57, 107.62, 107.87, 112.51, 121.23, 121.45, 126.61, 126.68, 135.36, 157.45, 159.92, 164.47.
LC/MS: m/z [M + H]+ calcd for C14H13FN2O2S: 292.33; found: 292.3.
23) 3-Chloro-8,9,10,11-tetrahydro-7H-azepino[2,1-c]- [1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4w)
Yield: 4.6 mmol (46%), 4.8 mmol (48%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.70 (br s, 4 H, CH2), 1.77 - 1.81 (m, 2 H, CH2), 3.18 (br s, 2 H, CH2), 4.30 - 4.34 (m, 2 H, CH2), 7.78 - 8.01 (m, 3 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 24.7, 25.82, 27.30, 34.28, 50.96, 86.66, 112.36, 120.81, 126.60, 129.92, 133.45, 137.45, 164.67.
LC/MS: m/z [M + H]+ calcd for C14H13ClN2O2S: 308.78; found: 308.3.
24) 2-Methyl-8,9,10,11-tetrahydro-7H-azepino[2,1-c]- [1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4x)
Yield: 7.3 mmol (73%), 8.9 mmol (89%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.71 (br s, 4 H, CH2), 1.83 (br s, 2 H, CH2), 2.48 (s, 3 H, CH3), 3.17 (br s, 2 H, CH2), 4.29 - 4.33 (m, 2 H, CH2), 7.40 (d, J = 8.0 Hz, 1 H, CH), 7.58 (s, 1 H, CH), 7.86 (d, J = 7.9 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 21.45, 24.91, 26.10, 27.35, 34.25, 50.57, 86.56, 112.73, 117.96, 121.48, 123.21, 126.81, 138.53, 144.39, 164.19.
LC/MS: m/z [M + H]+ calcd for C15H16N2O2S: 288.37; found: 288.3.
25) 2-Fluoro-8,9,10,11-tetrahydro-7H-azepino[2,1-c]- [1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4y)
Yield: 7.3 mmol (73%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.71 (br s, 4 H, CH2), 1.78 - 1.81 (m, 2 H, CH2), 3.18 (br s, 2 H, CH2), 4.28 - 4.32 (m, 2 H, CH2), 7.41 - 7.50 (m, 1 H, CH), 7.69 (dd, J(1) = 11.7 Hz, J(2) = 2.2 Hz, 1 H, CH), 8.03 - 8.11 (m, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 24.92, 25.91, 27.39, 34.43, 51.03, 87.65, 105.59, 105.96, 112.46, 113.80, 114.11, 122.26, 124.89, 125.04, 140.62, 140.78, 162.89, 164.81, 166.21.
LC/MS: m/z [M + H]+ calcd for C14H13FN2O2S: 292.33; found: 292.4.
26) 2-Chloro-8,9,10,11-tetrahydro-7H-azepino[2,1-c]- [1,4]benzothiazine-6-carbonitrile 5,5-dioxide (4z)
Yield: 5.3 mmol (53%) (“one-pot” method); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 1.71 (br s, 4 H, CH2), 1.80 - 1.82 (m, 2 H, CH2), 3.16 - 3.19 (m, 2 H, CH2), 4.31 - 4.35 (m, 2 H, CH2), 7.63 (dd, J(1) = 8.4 Hz, J(2) = 1.6 Hz, 1 H, CH), 7.84 (d, J = 1.5 Hz, 1 H, CH), 8.00 (d, J = 8.6 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 24.73, 25.80, 27.32, 34.36, 50.84, 87.36, 112.32, 118.10, 123.66, 124.17, 126.03, 138.43, 139.65, 164.83.
LC/MS: m/z [M + H]+ calcd for C14H13ClN2O2S: 308.78; found: 308.3.
2-Methyl-1,4-benzoxathiine 4,4-dioxide (5).
The mixture of 10 mmol [(2-fluorophenyl)sulfonyl] acetone 1b, 10 mmol DBU and 2 mL of 1,4-dioxane were heated 80˚C - 90˚C for 3 - 4 hours (monitored by TLC, eluent—CHCl3). The reaction mixture was diluted with 2-propanol after cooling. The precipitate formed was filtered and crystallized from 2-propanol-DMF mixture.
Yield: 5.5 mmol (55%); cream coloured solid.
1H NMR (200 MHz DMSO-d6): δ = 2.23 (s, 3 H, CH3), 6.82 (s, 1 H, CH), 7.43 - 7.53 (m, 2 H, CH2), 7.68 - 7.77 (m, 1 H, CH), 7.93 (d, J = 7.9 Hz, 1 H, CH).
13C NMR (75 MHz DMSO-d6): δ = 20.37, 103.44, 118.73, 122.43, 124.54, 126.09, 133.96, 149.90, 158.08.
LC/MS: m/z [M + H]+ calcd for C9H8O3S: 196.22; found: 196.3.
(2E,Z)-2-{[(2-fluorophenyl)sulfonyl]methylidene}az-epane (6).
The mixture of 1-[(2-fluorophenyl)sulfonyl]acetone 1b (10 mmol) with S-methyllactim 2f (15 mmol) was heated at 90˚C for 6 to 8 hours (monitored by TLC, eluent— CHCl3). The reaction mixture was diluted with 2-propanol after cooling. The precipitate formed was filtered and crystallized from 2-propanol-DMF mixture.
Yield: 2.5 mmol (25%); cream coloured solid.
LC/MS: m/z [M + H]+ calcd for C9H8O3S: 269.34; found: 269.5.
3. Conclusion
The reaction of different methylene active (2-fluorophenyl)sulfones with Oand S-methylactims has been studied. A facile one-pot method for synthesis of 2,3- dihydro-1H-pyrrolo[2,1-c][1,4]benzothiazines and their homologues by interaction of methylenactive (2-fluorophenyl)sulfones with homologues of either 5-methoxy- 3,4-dihydro-2H-pyrrole or 5-(methylthio)-3,4-dihydro- 2H-pyrrole has been developed.
REFERENCES
- F. Varano, D. Catarzi, V. Colotta, G. Filacchioni, L. Cecchi, A. Galli and C. Costagli, “Synthesis of 2-Sub-stituted-6,8-dichloro-3,4-dihydro-3-oxo-2H-1,4-benzothiazine-1,1-dioxides and -1-Oxides as Glycine-NMDA Receptor Antagonists,” Il Farmaco, Vol. 53, No. 12, 1998, pp. 752-757. doi:10.1016/S0014-827X(98)00097-4
- P. Rafferty, D. Calderwood, L. D. Arnold, B. Gonzalez Pascual, J. L. Ortego Matinez, M. J. Perez de Vega and I. F. Fernandez, “Benzothiazinone and Benzoxazinone Compounds,” PCT Int. Appl. WO 2000075139, 2000.
- J. F. Blake, J. B.Fell, J. P. Fischer, R. T. Hendricks, J. E. Robinson, S. R. Spencer and P. J. Stengel, “Heterocyclic Antiviral Compounds,” US Patent No. 2006040927, 2006.
- H. C. Hansen, T. M. Tagmose and J. B. Hansen, “Fused 1,4-Thiazine-2-carbonitrile Derivatives, Their Preparation and Use,” PCT Int. Appl. WO 2000055147, 2000.
- T. Yamamoto, I. Watanabe, K. Harada and S. Ikeda, “Benzo[1,4]thiazine Derivatives and Drugs Comprising the Same,” PCT Int. Appl. WO 9813357, 1998.
- I. Iijima, S. Nomura, K. Okumura, K. Takashima and K. Suzuki, Jpn. Kokai Tokkyo Koho JP 04041483,1992.
- H. Kano and S. Takahashi, “Shionogi Kenkyusho Nenpo,” Shionogi Kenkyusho Nempo, Vol. 11, No. 1, 1961, pp. 1-3.
- G. Fengler, D. Arlt, K. Grohe, H. J. Zeiler and K. Metzger, “4H-1,4-Benzothiazin-Derivate,” Ger. Offen. DE 3229125, 1984.
- T. Kachhee, V. Gupta, D. C. Gautam and R. Gupta, “Synthesis of 4H-1,4-Benzothiazine-1,1-dioxides (Sulfones) and Phenothiazine-5,5-dioxides (Sulfones),” Phosphorus, Sulfur, and Silicon and the Related Elements, Vol. 180, No. 10, 2005, pp. 2225-2234. doi:10.1080/104265090917790
- G. Kumar, V. Gupta, D. Gautam and R. Gupta, “Synthesis of Sulfones OF 4H-1,4-Benzothiazines and Phenothiazines,” Phosphorus, Sulfur, and Silicon and the Related Elements, Vol. 179, No. 10, 2004, pp. 1941-1948. doi:10.1080/10426500490466931
- V. Molteni, X. He, J. Nabakka, K. Yang, A. Kreusch, P. Gordon, B. Bursulaya, I. Warner, T. Shin, T. Biorac, N. S. Ryder, R. Goldberg, J. Doughty and Y. He, “Identification of Novel Potent Bicyclic Peptide Deformylase Inhibitors,” Bioorganic & Medicinal Chemistry Letters, Vol. 14, No. 6, 2004, pp. 1477-1481. doi:10.1016/j.bmcl.2004.01.014
- K. Gupta, B. S. Rathore, R. Gupta, V. Gupta and M. Kumar, “Synthesis and Spectral Studies of 4H-1,4-Benzothiazine S,S-Dioxides (Sulfones),” Heterocyclic Communications, Vol. 9, No. 4, 2003, p. 381.
- T. S. Yokum, J. Alsina and G. Barany, “Solid-Phase Syntheses of Heterocycles Containing the 2-Aminothiophenol Moiety,” Journal of Combinatorial Chemistry, Vol. 2, No. 3, 2000, pp. 282-292. doi:10.1021/cc9900854
- S. C. Schou, H. C. Hansen, T. M. Tagmose, H. C. M. Boonen, A. Worsaae, M. Drabowski, P. Wahl, P. O. G. Arkhammar, T. Bodvarsdottir, M.-H. Antoine, P. Lebrun and J. B. Hansen, “Synthesis and Pharmacological Evaluation of 4H-1,4-Benzothiazine-2-carbonitrile 1,1-dioxide and N-(2-Cyanomethylsulfonylphenyl)acylamide Derivatives as Potential Activators of ATP Sensitive Potassium Channels,” Bioorganic & Medicinal Chemistry Letters, Vol. 13, No. 1, 2005, pp. 141-155. doi:10.1016/j.bmc.2004.09.051
- A. I. Gerasyuto, S. G. Zlotin and V. V. Semenov, “Synthesis of 2,3-Dihydrobenzothiazol-1,1-dioxide and 2,3- Dihydro-1,4-benzothiazin-3-one Nitroderivatives from 2,4-Diand 2,4,6-Trinitrobenzamides,” Synthesis, Vol. 2, 2001, pp. 300-304. doi:10.1055/s-2001-10820
- F. Babudri, S. Florio, A. M. Vitrani and L. Di Nunno, “Synthesis of 4H-1,4-Benzothiazines via Lithiation Alpha to Sulphur of 2-Acylaminophenyl Alkyl Sulphides, Sulphoxides, and Sulphones,” Journal of the Chemical Society, Perkin Transactions 1, Vol. 8, 1984, pp. 1899-1903. doi:10.1039/p19840001899
- Y. Ishikawa, Y. Terao, K. Suzuki, N. Shikano and M. Sekiya, “Cyclization of α- and β-Alkylthio-Substituted Amines Possessing Positively Charged Carbon at the Nitrogen. A New Synthetic Method for Thiazolidines, Thiomorpholines and Dihydro-1,4-benzothiazines,” Chemical & Pharmaceutical Bulletin, Vol. 32, No. 2, 1984, pp. 438-446. doi:10.1248/cpb.32.438
- G. Pagani, “Ricerche sui Solfoni Ciclici. Nota V. 4H- 1,4-Benzothiazine-1,1-diossido,” Gazzetta Chimica Italiana, Vol. 97, 1967, pp. 1804-1807.
- A. S. Angeloni and G. Pappalardo, “Benzothiazinone Diossidi e Loro Derivati,” Gazzetta Chimica Italiana, Vol. 91, 1961, pp. 633-635.
- G. Fengler, D. Arlt and K. Grohe, “4H-1,4-Benzothiazin-derivate,” Ger. Offen. DE 3229124, 1984.
- S. E. Lopez, J. Charris, N. Urdaneta and G. Lobo, “Synthesis of N-Aryl Substituted 4H-1,4-Benzothiazine 1,1- Dioxide 2-Carboxylic Acid-Esters,” Phosphorus, Sulfur, and Silicon and the Related Elements, Vol. 143, No. 1, 1998, pp. 53-61. doi:10.1080/10426509808045484
- S. E. Lopez, M. V. Godoy, N. Urdaneta and M. Rosales, “An Improved Procedure for the Preparation of N-Aryl Substituted 4H-1,4-Benzothiazine 1,1-Dioxide Derivatives,” Phosphorus, Sulfur, and Silicon and the Related Elements, Vol. 156, No. 1, 2000, pp. 69-80. doi:10.1080/10426500008044994
- S. E. Lopez, J. Charris, N. Urdaneta, C. E. Canelon, J. Salazar, J. Herrera and J. E. Angel, “Unexpected Desulfonation OF α-Phenylsulfonyl Enaminoacrylates during Their Cyclisation to New N-Aryl 4H-1,4-Benzothiazinel,l-dioxides,” Phosphorus, Sulfur, and Silicon and the Related Elements, Vol. 175, No. 1, 2001, pp. 87-97. doi:10.1080/10426500108040258
- S. E. Lopez, J. Salazar, O. Rebollo and J. Restrepo, “A Microwave Induced Cyclisation of α-Phenylsulfonyl-enaminoacrylates for the Preparation of 4-Aryl-4H-1,4-benzothiazine 1,1-Dioxide Derivatives,” Journal of Heterocyclic Chemistry, Vol. 42, No. 5, 2005, pp. 1007-1010. doi:10.1002/jhet.5570420541
- J. Charris, A. Barazarte, J. Dominguez and N. Gamboa, “Microwave-Assisted Synthesis of Quinolones and 4H- 1,4-Benzo-thiazine 1,1-Dioxides,” Journal of Chemical Research, Vol. 1, No. 2, 2005, pp. 27-28. doi:10.3184/0308234053431158
- T. P. Culbertson, “Synthesis of 4H-1,4-Benzothiazine 1- Oxide and 1,1-Dioxide. Analogs of Quinolone Antibacterial Agents,” Journal of Heterocyclic Chemistry, Vol. 28, No. 7, 1991, p. 1701.
- G. M. Coppola and R. E. Damon, “Novel Heterocycles. 6. The Condensation of Ethyl o-Fluorobenzoyl Acetate with Cyclic Imino Ethers,” Journal of Heterocyclic Chemistry, Vol. 17, No. 8, 1980, pp. 1729-1731. doi:10.1002/jhet.5570170818
- L. V. Ershov and V. G. Granik, “Lactams of Acetals and Acid Amides, 45. Synthesis of Condensed 2-Pyridones from Activated Amides, Lactams, and Lactones,” Chemistry of Heterocyclic Compounds, Vol. 21, No. 7, 1985, pp. 771-774. doi:10.1007/BF00519144
- S.-I. Hirokami, T. Takahashi, M. Nagata and T. Yamazaki, “Rearrangements of Dewar 4-Pyrimidinones and 4-Methoxy-2-azetidinones. Reactions through Azetidinyl and Acyl Cations,” Journal of Organic Chemistry, Vol. 52, No. 12, 1987, pp. 2455-2468. doi:10.1021/jo00388a022
- K. Sukata, “A Simple and Convenient Method for the Synthesis of Sulfones Using Polyethylene Glycols or Their Dialkyl Ethers as Solvents or Catalysts,” Bulletin of the Chemical Society of Japan, Vol. 57, No. 2, 1984, pp. 613-614. doi:10.1246/bcsj.57.613
- H. Techer, M. Lavergne and M. Pesson, “Organic Syntheses without Solvent: Preparation of Sulfones and Dithioacetals,” Synthesis, Vol. 1, 1987, pp. 56-59. doi:10.1055/s-1987-27843
- J. S. Grossert, P. K. Dubey, G. H. Gill, T. S. Cameron and P. A. Gardner, “The Preparation, Spectral Properties, Structures, and Base-Induced Cleavage Reactions of Some α-Halo-β-ketosulfones,” Canadian Journal of Chemistry, Vol. 62, No. 4, 1984, pp. 798-807. doi:10.1139/v84-133
- C. A. Zezza, M. B. Smith, B. A. Ross, A. Arhin and P. L. E. Cronin, “Reaction of Organolithium Reagents with Lactim Ethers: Preparation of Cyclic 2-Alkyl Imines or 2,2- Dialkyl Amines,” Journal of Organic Chemistry, Vol. 49, No. 23, 1984, pp. 4397-4399. doi:10.1021/jo00197a013
- J. P. Célérier, M. G. Richaud and G. Lhommet, “Imidoylation Reactions: A Simple Direct Synthesis of 3-Amino- 2-alkenoic Esters (ß-Enaminoesters),” Synthesis, Vol. 3, 1983, pp. 195-197. doi:10.1055/s-1983-30276
NOTES
*Corresponding author.

