International Journal of Organic Chemistry
Vol. 2 No. 3 (2012) , Article ID: 22744 , 8 pages DOI:10.4236/ijoc.2012.23029
Chemospecific and Regioselective Ethereal Methyl-Oxygen Bond Cleavage Behavior of Aroylated Dimethoxynaphthalenes by Combined Action of AlCl3 and Aroyl Group
Departmentof Organic and Polymer Materials Chemistry, Tokyo University of Agriculture and Technology, Koganei, Japan
Email: aokamoto@cc.tuat.ac.jp
Received July 24, 2012; revised August 26, 2012; accepted September 3, 2012
Keywords: Aroylated 2,7-Dialkoxynaphthalene; Chemospecific and Regioselective Scission of Ethereal Alkyl-Oxygen Bond; Combined Action of AlCl3 and Aroyl Group; Neighboring Group Effect
ABSTRACT
AlCl3-mediated cleavage of ethereal methyl-oxygen bond in aroylated 2,7-dimethoxy naphthalene compounds proceeds chemospecifically and regioselectively. The ethereal bond at the β(2)-position of 1-monoaroylated 2,7-dimeth-oxynaphthalene is cleaved readily and predominantly against the β(7)-position, whereas scission of β-ethereal bonds of 1,8-diaroylated 2,7-dimethoxynaphthalene hardly undergoes like the non-aroylated mother frame compound of 2,7- dimethoxynaphthalene.
1. Introduction
Congested molecular units having non-coplanar alignment of aromatic rings such as biphenyl, binaphthyl, and other poly (aromatic rings) compounds have been in the limelight for building block of functional molecules and polymers [1-4]. Recently, the authors’ group has found that 2,7-dialkoxynaphthalenes readily undertake acidmediated diaroylation with high peri-regioselectivity to give 1-aroyl and 1,8-diaroyl-2,7-dialkoxynaphthalenes in satisfactory yields [5,6]. In crystal, the aroyl group of the resulting molecules attaches to the naphthalene ring as nearly perpendicular manner and for diaroylated derivatives two aroyl groups are situated in opposite directions (anti-orientation) [7-9]. The authors’ group has integrated the naphthalene-1,8-bis(carbonylarylene) unit into poly(arylene ether ketone) backbone and reported unique solubility tendency to organic solvents and characteristic thermal behavior of the resulting polymers with interpretation in relation to the spatial organization of the repeating unit [10]. In addition, some curious reactions of the 2,7-dimethoxynaphthalene and its derivatives such as reversible aroylation depending on Brønsted acid strength [5] and dual aroylation mediated by Lewis acid [6] are also revealed during the studies on aroylation of naphthalene derivatives.
In this article, the authors introduce Methyl-oxygen bond cleavage behavior of β-ethereal substituent in aroylated dimethoxynaphthalenes by the combined action of AlCl3 and aroyl group and discuss the regioselectivity and the chemospecificity based on the comparison of the corresponding reaction behaviors among the homologous and analogous dialkoxynaphthalene molecules.
As well known methyl aryl ether generally resists acid-mediated Methyl-oxygen bond cleavage. So deprotection of methyl group from methoxyarenes needs specific reagents such as iodotrimethylsilane with Lewis acids, or BBr3 in place of AlCl3, which has almost no potential for this purpose [11-14]. Furthermore, the BBr3-mediated cleavage often suffers from low regioselectivity. Therefore, effective and regioselective cleavage of methyl aryl ether by AlCl3 is of interest.
2. Results and Discussion
Table 1 shows the results of AlCl3-mediated ether-cleavage reaction of 1-monoaroyl-2,7-dimethoxynaphthalene (1aa) in refluxing CH2Cl2 solutions. The methyl-oxygen bond in 2-methoxy group of 1-monoaroylnaphthalene 1aa was cleaved in the presence of three equimolar amounts of AlCl3 or more (Entries 1 - 6). Using of five equimolar amounts of AlCl3 quantitatively yielded 2-hydroxy-7-methoxy product 2ba (Entry 5). The exclusive scission of 2-methoxy moiety leaving the other
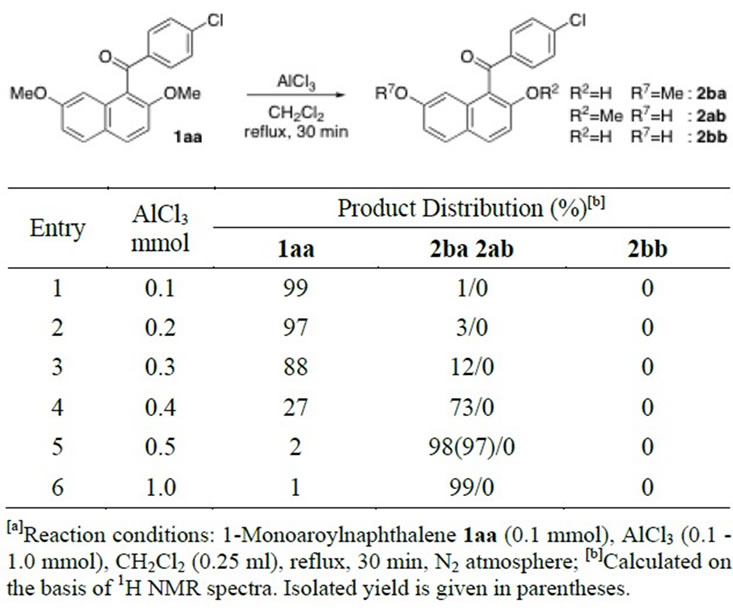
Table 1. Ethereal Methyl-oxygen bond cleavage reaction of 1-monoaroylnaphthalene 1aa by AlCl3[a].
β-ethereal substituent of 7-methoxy group unchanged was achieved even when ten equimolar amounts of AlCl3 were employed against substrate 1aa (Entry 6).
Table 2 presents the results of treatment of non-aroylated mother frame molecule of 2,7-dimethoxynaphthalene (3aa) and the analogous molecule of 3-monoaroyl- 2,7-dimethoxynaphthalene 5aa with AlCl3. Dimethoxynaphthalene (3aa) essentially gave none of alkyl ethercleaved products (Entries 1 and 2). On the other hand, Methyl-oxygen bond in 3-monoaroyl-2,7-dimetoxy-naphthalene 5aa was readily cleaved at the 2-position leaving β(7)-methoxy group unchanged as well as 1-monoaroyl- 2,7-dimethoxynaphthalene 1aa (Entries 3 and 4).
The results of the reaction of monoaroyl-2,7-dimethoxynaphthalenes (1aa and 5aa) and the ketonic-carbonyl-free analogue, 2,7-dimethoxynaphthalene (3aa), obviously indicate that the ether cleavage reaction of the monoaroyl-2,7-dimethoxynaphthalenes proceeds chemospecifically and regioselectively. Regioselective methyloxygen bond cleavage at 2-position of monoaroyl-2,7- dimethoxynaphthalenes 1aa and 5aa apparently demonstrates the presence of the neighboring effect of the aroyl group.
Contrarily, no methyl-oxygen bond cleavage proceeded for 1,8-diaroyl-2,7-dimethoxynaphthalene (7aa) under the similar reaction conditions (Table 3, Entry 1). In refluxing CH2ClCH2Cl solution, only a trace amount of halfly ether-cleaved product 8ba was detected (Entry 2). The ether cleavage reaction proceeded still scarcely even when ten equimolar amounts of AlCl3 were employed (Entry 3). In refluxing toluene solution, 1,8-diaroyl-2,7-dimethoxynaphthalene 7aa gave unidentified compounds with trace amounts of halfly and dually methyl ether-cleaved products 8ba and 8bb (Entry 4).

Table 2. Ethereal Methyl-oxygen bond cleavage reactionof dimethoxynaphthalene analogues 3aa/5aa by AlCl3[a].

Table 3. Ethereal Methyl-oxygen bond cleavagereaction of 1,8-diaroylnaphthalene 7aa by AlCl3[a].
The distinct behavior that contrary to complete cleavage of β(2)-methyl ether bond of 1-monoaroyl-2,7-dimethoxynaphthalene 1aa, the β-methyl ether bonds of 1,8-diaroyl-2,7-dimethoxynaphth alene 7aa were essentially unchanged indicates the plausible origination of the chemospecificity from the facility of formation of the required conformation for methyl ether bond cleavage. The required conformation still remains indeterminable, however, the probable situation of AlCl3 between ketonic carbonyl oxygen atom and ether oxygen atom might promote the scission of methyl-oxygen bond. In the case of 1,8-diaroyl-2,7-dimethoxynaphthalene 7aa, the formation of the required conformation is presumably obstructed sterically compared to 1- and 3-monoaroylated naphthalene derivatives.
The ethyl-oxygen bond cleavage reactions carried out against the corresponding diethoxyna phthalene homologues (Table 4-6) manifest the essentially similar reaction behaviors to those of 2,7-dimethoxynaphthalene
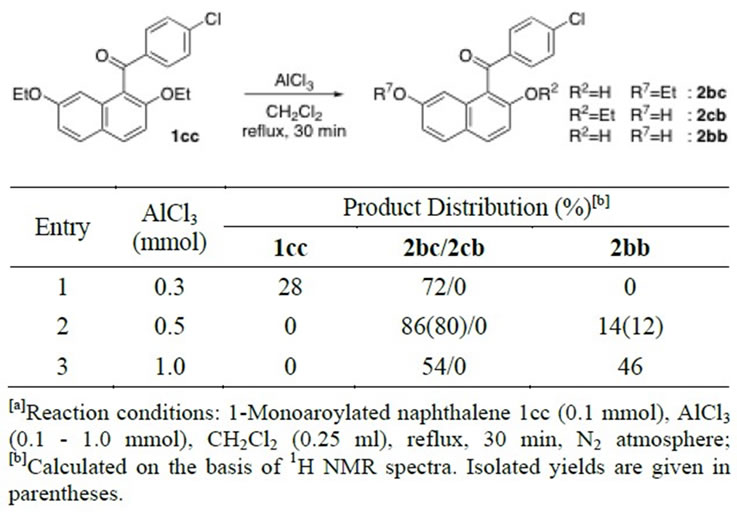
Table 4. Ethereal ethyl-oxygen bond cleavage reaction of 1-monoaroylated naphthalene 1cc by AlCl3[a].

Table 5. Ethereal ethyl-oxygen bond cleavage reaction of diethoxynaphthalene analogues 3cc/5cc by AlCl3[a].
analogues (1aa, 3aa, 5aa, and 7aa). Ethyl-oxygen bonds in 1-mono, 3-mono, and 1,8-diaroylnaphthalenes were somewhat easily cleaved than the methyl-oxygen bonds in the corresponding homologous molecules. According to the reaction conditions, halfly ether-cleaved products were obtained quantitatively or mixtures with dihydroxy derivatives were yielded. For example, 1-monoaroylated 2-hydroxy-7-ethoxynaphthalene (2bc) was solely formed by use of a smaller amount of AlCl3 (Table 4, Entry 1 vs. 2). However, half ethyl ether cleavage of 1,8-diaroylated 2,7-diethoxynaphthalene (7cc) is rather difficult even under mild conditions (Table 6, Entries 2 and 6). The results of the cleavage reaction of unsymmetrically dialkoxylated molecule of 1,8-diaroyl-2-ethoxy-7-methoxy naphthalene (7ca) suggest that the methyl-oxygen bond cleavage is promoted after the cleavage of ethoxy group has completed (Table 6). In refluxing CH2ClCH2Cl solution, the ethyl-oxygen bond of 1,8-diaroyl-2-ethoxy-7-methoxynaphthalene (7ca) was cleaved in preference to the
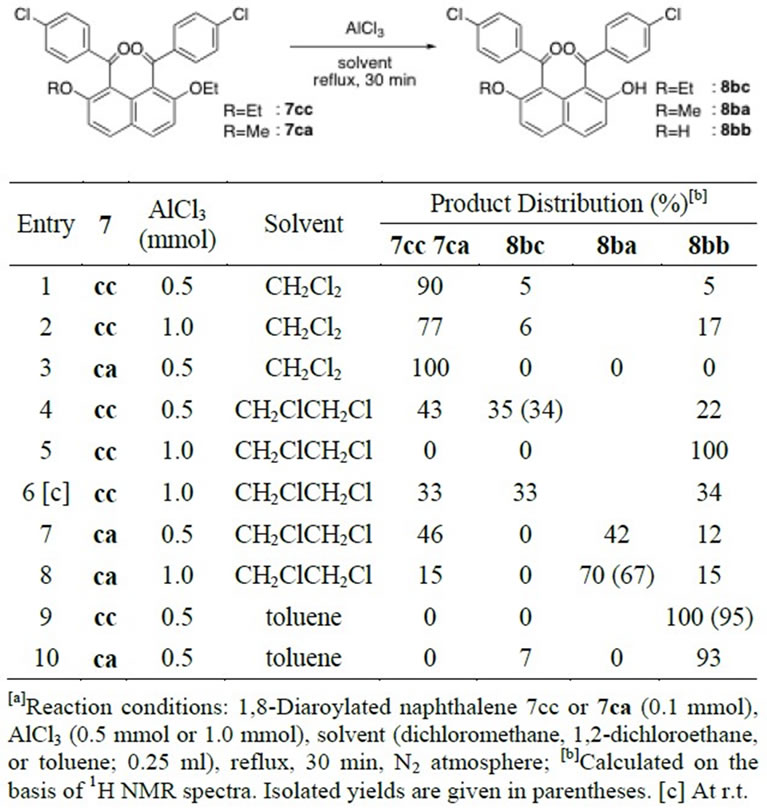
Table 6. Alkyl ether cleavage reaction of 1,8-diaroylated naphthalene 7cc/7ca by AlCl3[a].
methyl-oxygen bond (Entries 7 and 8). In refluxing toluene solution, the two kinds of alkyl-oxygen bonds were thoroughly cleaved (Entry 10).
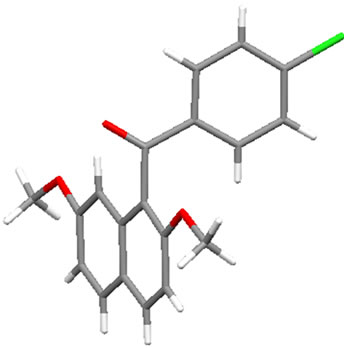
Figure 1 displays the crystal structures of aroylated 2,7-dimethoxynaphthalenes (1aa and 7aa) [15,16] and aroylated 2-hydroxy-7-methoxynaphthalenes (2ba and 8ba) [17,18]. In 1-monoaroyl-2,7-dimethoxynaphthalene 1aa, the aroyl group non-coplanarly attaches to the naphthalene ring. On the other hand, the ketonic carbonyl moiety and hydroxy group in 1-monoaroyl-2-hydroxy-7- methoxynaphthalene 2ba make a coplanar configuration with intramolecular hydrogen bond. The same type of intramolecular hydrogen bond between ketonic carbonyl moiety and hydroxy group is observed in X-ray crystal structure of 1,8-diaroyl-2-hydroxy-7-methoxynaphthalene 8ba. However, in contrast to anti-orientation of 1,8-diaroylnaphthalene 7aa, 1,8-diaroyl-2-hydroxy-7-methoxy naphthalene 8ba has syn-orientation, i.e., two aroyl groups are oriented in same directions.1-Monoaroylnaphthalene 1aa and 1,8-diaroylnaphthalene 7aa have apparently same non-coplanar alignment around the aroyl connections. However, the steric hindrance around the ketonic carbonyl group might be meaningfully different to affect the rotational capability. The aroyl groups of 1,8-diaroylnaphthalene 7aa are deviated out of the naphthalene ring plane more largely than 1-monoaroylnaphthalene 1aa. The angles between C(carbonyl)-C(naphthalene) bond and naphthalene plane are 13.07˚and 11.71˚ for 7aa and 3.21˚ for 1aa.
The bond lengths between ketonic carbonyl group and
 (a)
(a)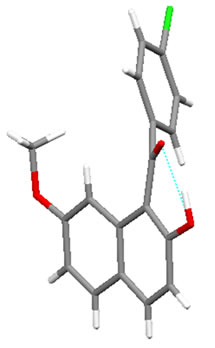 (b)
(b)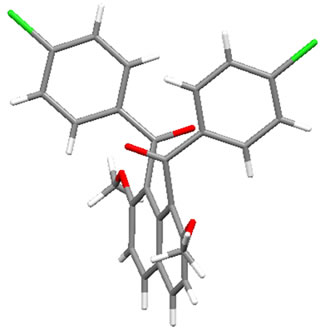 (c)
(c)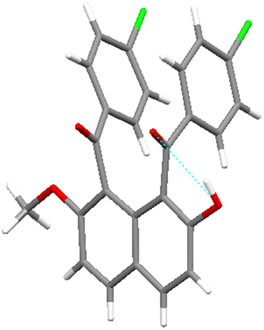 (d)
(d)
Figure 1. X-ray crystal structures of aroylnaphthalenes: (a) molecule 1aa; (b) molecule 2ba; (c) molecule 7aa; (d) molecule 8ba (For clarity, an ethanol molecule is removed from the figure).
naphthalene ring of 1,8-diaroylnaphthalene 7aa are longer than that of 1-monoaroylnaphthalene 1aa (1.516 Å and 1.520 Å for 7aa; 1.506 Å for 1aa). About the 8-aroylgroup adjacent to methoxy group in 1,8-diaroyl-2-hydroxy-7-methoxynaphthalene 8ba, the corresponding angle and bond length are 9.13˚ and 1.509 Å, respecttively.
These data indicate that steric hindrance around ketonic carbonyl group increases in the order of monoaroyldimethoxy derivative 1aa 3. Conclusion Conclusively, the AlCl3-mediated scission behavior of alkyl-oxygen linkage of β-alkoxy groups in non-aroylated, 1-mono-, and 1,8-diaroyl-2,7-dimethoxynaphthalenes (3aa, 1aa, and 7aa) shows distinct chemospecificity. Methyl-oxygen bond cleavage of 1-monoaroyl- 2,7-dimethoxynaphthalene (1aa) smoothly and regioselectively proceeds at the 2-position, however, that of 1,8-diaroylnaphthalene (7aa) is apparently deactivated like the inertness of 2,7-dimethoxynaphthalene (3aa). The single aroyl group promotes the ether cleavage of the adjacent methoxy group, whereas the two adjacent aroyl groups situated at the peri-position disturb AlCl3 mediated scission of the neighboring β-methyl ether group. Replacement of one or two methoxy groups with ethoxy ones a little blunts the chemospecificity and the regioselectivity of aroylated naphthalenes. The observed specificity in the alkyl ether-cleavage reaction affords some hitherto-unknown aspects in structures and chemical properties relationship of these congested non-coplanarly aromatic-rings-accumulated molecules. 4. Experimental All reagents were of commercial quality and were used as received. Solvents were dried and purified using standard techniques. 4.1. Measurement 1H NMR spectra were recorded on a JEOL JNM-AL300 spectrometer (300 MHz) and a JEOL ECX400 spectrometer (400 MHz). Chemical shifts are expressed in ppm relative to internal standard of Me4Si (δ 0.00). 13C NMR spectra were recorded on a JEOL JNM-AL300 spectrometer (75 MHz) and a JEOL ECX400 spectrometer (100 MHz). Chemical shifts are expressed in ppm relative to internal standard of CDCl3 (δ 77.0). IR spectra were recorded on a JASCO FT/IR-4100 spectrometer. Elemental analyses were performed on a Yanaco CHN CORDER MT-5 analyzer. High-resolution FAB mass spectra were recorded on a JEOL MStation (MS700) ion trap mass spectrometer in positive ion mode. 4.2. Typical Procedure of Methyl-OxygenBond Cleavage Reaction Mediated by AlCl3 To a solution of 1-(4-chorobenzoyl)-2,7-dimetoxynaphthalene (1aa, 0.1 mmol, 32.7 mg) and in dichloromethane (0.25 ml), AlCl3 (0.5 mmol, 66.7 mg) was added by portions at ambient temperature under nitrogen atmosphere. After the reaction mixture was stirred in the refluxing solution for 30 min, it was poured into iced water (20 ml) and the mixture was extracted with CHCl3 (15 ml × 3). The combined extracts were washed with sat. NaCl aq. and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure to give solid. The crude product was purified by recrystallization (2ca, hexane, isolated yield 75%). Other ether cleavage reactions were undertaken by essentially the same procedure as above. 4.3. Synthetic Procedure of Aroylnaphthalenes 1-(4-Chlorobenzoyl)-2,7-dimethoxynaphthalene (1aa). To a solution of 2,7-dimethoxynaphthalene (0.200 mmol, 68.2 mg) and 4-chlorobenzoyl chloride (0.22 mmol, 38.5 mg) in dichloromethane (0.5 ml), AlCl3 (0.22 mmol, 29.3 mg) was added by portions at 0˚C under nitrogen atmosphere. After the reaction mixture was stirred at r.t. for 3 h, it was poured into iced water (20 ml) and the mixture was extracted with CHCl3 (15 ml × 3). The combined extracts were washed with 2 M NaOH aq., sat. NaCl aq. and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to give powdery product. The crude product was purified by recrystallization (hexane, isolated yield 78%). 3-(4-Chlorobenzoyl)-2,7-dimethoxynaphthalene (5aa). The title compound was prepared by treatment of a mixture of 2,7-dimethoxynaphthalene (10 mmol; 1.88 g) and 4-chlorobenzoic acid (11 mmol 1.72 g) with phosphorus pentoxide–methanesulfonic acid mixture (P2O5-MsOH [1/10 w/w]; 10 ml) at 60˚C for 8 hours. After the reaction mixture was stirred at 353 K for 8 h, the mixture was extracted with CHCl3 (10 ml × 3). The combined extracts were washed with 2 M aqueous NaOH followed by washing with brine. The organic layers thus obtained were dried over anhydrous MgSO4. The solvent was removed under reduced pressure to give cake. The crude product was purified by recrystallization (ethanol, isolated yield 56%). 1,8-Bis(4-chlorobenzoyl)-2,7-dimethoxynaphthalene(7aa). To a solution of 2,7-dimethoxynaphthalene (0.200 mmol, 68.2 mg) and 4-chlorobenzoyl chloride (0.66 mmol, 116 mg) in dichloromethane (0.5 ml), TiCl4 (1.8 mmol, 341 mg) was added by portions at r.t. under nitrogen atmosphere. After the reaction mixture was stirred at r.t. for 3 h, it was poured into iced water (20 ml) and the mixture was extracted with CHCl3 (15 ml × 3). The combined extracts were washed with 2 M NaOH aq., sat. NaCl aq. and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to give powdery product. The crude product was purified by recrystallization (ethanol, isolated yield 88%). 1,8-Bis(4-chlorobenzoyl)-2,7-diethoxynaphthalene (7cc). To a solution of TiCl4 (180 mmol, 34.2 g) and 4- chlorobenzoyl chloride (60 mmol, 10.5 g) in dichloromethane (25 ml), 2.7-diethoxynaphthalene/dichloromethane (20 mmol, 4.33 g/25 ml) was added by portions at r.t. under nitrogen atmosphere. After the reaction mixture was stirred at r.t. for 3 h, it was poured into iced water (200 ml) and the mixture was extracted with CHCl3 (40 ml × 3). The combined extracts were washed with 2 M NaOH aq., sat. NaCl aq. and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to give powdery pale white product (94% yield). The crude product was purified by recrystallization (chloroform/ethanol, isolated yield 62%). 1,8-Bis(4-chlorobenzoyl)-2-ethoxy-7-methoxynaphthalene (7ca). To a solution of 1-(4-chlorobenzoyl)-2- ethoxy-7-methoxynaphthalene (0.200 mmol, 68.2 mg) and 4-chlorobenzoyl chloride (0.44 mmol, 77.0 mg) in dichloromethane (0.5 ml), TiCl4 (1.32 mmol, 248 mg) was added by portions at r.t. under nitrogen atmosphere. After the reaction mixture was stirred at r.t. for 3 h, it was poured into iced water (20 ml) and the mixture was extracted with CHCl3(15 ml × 3). The combined extracts were washed with 2 M NaOH aq., sat. NaCl aq. and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to give powdery product. The crude product was purified by silicagel column chromatography (hexane : AcOEt = 1 : 1, isolated yield 87%). 4.4. Identification of the Products 1-(4-Chlorobenzoyl)-2,7-dimethoxynaphthalene (1aa). Colorless needle (hexane), Mp 121.5˚C - 122˚C; IR (KBr): 1667, 1628, 1586, 1512 cm–1; 1H NMR δ (300 MHz, CDCl3): 3.73 (3H, s), 3.79 (3H, s), 6.78 (1H, d, J = 2.4 Hz), 7.02 (1H, dd, J = 2.4, 9.0 Hz), 7.16 (1H, d, J = 9.0 Hz), 7.39 (2H, d, J = 8.4 Hz), 7.72 (1H, d, J = 9.0 Hz), 7.78 (2H, d, J = 8.4 Hz), 7.87 (1H, d, J = 9.0 Hz) ppm; 13C NMR δ (75 MHz, CDCl3): 55.168, 56.239, 101.88, 110.05, 117.15, 121.06, 124.34, 128.86, 129.72, 130.87, 131.28, 132.94, 136.45, 139.71, 155.02, 158.96, 196.81 ppm; Calcd for C19H15O3Cl: C, 69.83%; H, 4.63%; Found: C, 69.61%; H, 4.74%. 1-(4-Chlorobenzoyl)-2-hydroxy-7-methoxynaphthalene (2ba). Yellow platelet (hexane), Mp 118˚C - 118.5˚C; IR (KBr): 3434, 1623, 1583, 1513, 1214, 843 cm–1; 1H NMR δ (300 MHz, CDCl3): 3.37 (s, 3H), 6.58 (d, 1H, J = 2.4 Hz), 6.91 (dd, 1H, J = 2.4, 9.0 Hz), 7.07 (d, 1H, J = 9.0 Hz), 7.40 (d, 2H, J = 8.7 Hz), 7.58 (d, 2H, J = 8.7 Hz), 7.63 (d, 1H, J = 9.0 Hz), 7.85 (d, 1H, J = 9.0 Hz), 11.35 (s, 1H) ppm; 13C NMR δ (75 MHz, CDCl3): 54.5, 106.5, 113.4, 115.8, 116.4, 123.7, 128.9, 130.2, 130.7, 133.8, 136.5, 138.7, 138.8, 158.2, 162.6, 199.1 ppm; Anal. Calcd for C18H13ClO3: C 69.13, H 4.19. Found: C 69.11, H 4.09. 1-(4-Chlorobenzoyl)-2,7-dihydroxynaphthalene (2bb). Yellow oil; IR (KBr): 3398, 1653, 1625, 1586, 1515, 1240, 1215 cm–1; 1H NMR δ (400 MHz, CDCl3): 5.18 (1H, s), 6.61 (1H, d, J = 2.2 Hz), 6.89 (1H, dd, J = 2.2, 8.6 Hz), 7.07 (1H, d, J = 8.8 Hz), 7.39 (2H, d, J = 8.2 Hz), 7.60 (2H, d, J = 8.2 Hz), 7.66 (1H, d, J = 8.8 Hz), 7.85 (1H, d, J = 8.8 Hz), 11.14 (1H, s) ppm; 13C NMR δ (100 MHz, CDCl3): 109.62, 113.16, 115.07, 116.55, 123.75, 128.95, 130.76, 130.97, 133.92, 136.48, 138.26, 139.07, 154.53, 162.33, 198.71 ppm; HRMS (FAB; m-nitrobenzyl alcohol [m-NBA]) m/z: [M + H]+; Calcd for C17H12O3Cl: 299.0475; Found: 299.0502. 3-(4-Chlorobenzoyl)-2,7-dimethoxynaphthalene (5aa). Yellow needle (EtOH), Mp 152˚C; IR (KBr): 1657, 1622, 1588, 1503 cm–1; 1H NMR δ (300MHz, CDCl3): 3.82 (3H, s), 3.94 (3H, s), 7.05 (1H, dd, J = 2.4, 8.9 Hz), 7.10 (1H, d, J = 2.4 Hz), 7.13 (1H, s), 7.40 (2H, d, J = 8.7 Hz), 7.69 (1H, d, J = 8.7 Hz), 7.77 (2H, d, J = 8.7 Hz), 7.79 (1H, s) ppm; 13C NMR δ (75 MHz, CDCl3): 55.349, 55.522, 105.03, 105.44, 117.17, 123.20, 127.41, 128.50, 130.08, 130.24, 131.22, 137.28, 138.83, 139.20, 155.67, 159.47, 194.81 ppm; Calcd for C19H15O3Cl: C, 69.83%; H, 4.63%. Found: C, 69.75%; H, 4.83%. 3-(4-Chlorobenzoyl)-2-hydroxy-7-methoxynaphthalene (6ba). Yellow needle (AcOEt + hexane); Mp 177˚C; IR (KBr): 3470, 1643, 1594, 1561, 1511 cm–1; 1H NMR δ (300 MHz, CDCl3): 3.93 (3H, s), 6.94 (1H, br), 6.96 (1H, d, J = 8.4, 2.4 Hz), 7.23 (1H, s), 7.52 (2H, d, J = 8.9 Hz), 7.57 (1H, d, J = 8.4 Hz), 7.69 (2H, d, J = 9.0 Hz), 7.99 (1H, s), 11.26 (1H, s) ppm; 13C NMR δ (75 MHz, CDCl3): 55.383, 103.68, 111.25, 117.80, 118.50, 122.24, 128.72, 130.78, 131.24, 136.09, 136.36, 138.43, 140.07, 158.17, 161.09, 200.01 ppm; HRMS (FAB; m-NBA) m/z: [M + H]+; Calcd for C18H14O3Cl: 313.0631. Found: 313.0624. 3-(4-Chlorobenzoyl)-2,7-dihydroxynaphthalene (6bb). Yellow powder (AcOEt + hexane), Mp 148˚C - 148.5˚C; IR (KBr): 3422, 1648, 1593, 1561, 1524 cm–1; 1H NMR δ (400 MHz, CDCl3): 6.01 (1H, s), 6.94 (1H, dd, J = 8.6, 2.4 Hz), 6.99 (1H, d, J = 2.4 Hz), 7.17 (1H, s), 7.53 (2H, d, J = 8.4 Hz), 7.63 (1H, d, J = 9.2 Hz), 7.69 (2H, d, J = 8.8 Hz), 8.02 (1H, s), 11.22 (1H, s) ppm; 13C NMR δ (100 MHz, CDCl3): 107.64, 110.70, 116.84, 118.76, 122.33, 128.79, 130.81, 132.00, 136.38, 136.50, 138.55, 140.02, 157.43, 158.03, 200.13 ppm; HRMS (FAB; m-NBA) m/z: [M + H]+; Calcd for C17H12O3Cl: 299.0475; Found: 299.0501. 1,8-Bis(4-chlorobenzoyl)-2,7-dimethoxynaphthalene (7aa). Yellow needle (EtOH + AcOEt), Mp 216˚C - 217˚C; IR (KBr): 1665, 1611, 1588, 1512 cm–1; 1H NMR δ (300MHz, CDCl3): 3.70 (6H, s), 7.21 (2H, d, J = 9.0 Hz), 7.33 (4H, d, J = 8.6 Hz), 7.64 (4H, d, J = 8.6 Hz), 7.96 (2H, d, J = 9.0 Hz) ppm; 13C NMR δ (75 MHz, CDCl3): 56.297, 111.07, 120.57, 125.45, 128.37, 129.90, 130.40, 132.43, 137.07, 138.99, 156.39, 195.95 ppm; Calcd for C26H18O4Cl2: C, 67.11%; H, 3.90%. Found: C, 67.10%; H, 4.09%. 1,8-Bis(4-chlorobenzoyl)-2-hydroxy-7-methoxynaph-thalene (8ba). Yellow platelet (EtOH), Mp 232.5˚C - 233.5˚C; IR (KBr): 1643, 1612, 1587, 1510, 1278, 1089, 831 cm–1; 1H NMR δ (300 MHz, CDCl3): 3.60 (s, 3H), 6.91 (d, J = 8.7 Hz, 2H), 7.20 - 7.10 (m, 6H), 7.33 (d, J = 8.7 Hz, 2H), 7.94–7.86 (m, 2H), 9.34 (s, 1H) ppm; 13C NMR δ (75 MHz, CDCl3): 56.0, 110.6, 117.3, 121.3, 124.6, 127.7, 128.5, 130.5, 131.9, 132.6, 133.7, 135.0, 136.3, 136.8, 138.4, 139.7, 157.6, 159.5, 195.1, 196.8 ppm; HRMS (FAB; m-NBA) m/z: [M + H]+;calcd for C25H17O4Cl2, 451.0504; found, 451.0520. Anal. Calcd for C25H16O4Cl2: C 66.53, H 3.57. Found: C 66.31, H 3.76. 1,8-Bis(4-chlorobenzoyl)-2,7-dihydroxynaphthalene (8bb). Yellow powder (AcOEt), Mp 302.2˚C - 307.4˚C; IR (KBr); 3160 (O-H), 1643 (C=O), 1587 (Ar), 1511 (Ar) cm–1; 1H NMR δ (300 MHz, CDCl3): 7.11 - 7.15 (4H, broad), 7.14 (2H, J = 8.7 Hz), 7.25 - 7.28 (4H, m), 7.93 (2H, d, J = 9.0 Hz), 11.13 (2H, s) ppm. 1H NMR δ (300 MHz, DMSO): 7.09 (2H, d, J = 8.7 Hz), 7.49 (4H, d, J = 8.4 Hz), 7.61 (4H, d, J = 8.7 Hz), 7.93 (2H, d, J = 9.0 Hz), 10.12 (2H, s) ppm; 13C NMR δ (75 MHz, DMSO): 115.44, 117.61, 123.76, 128.79, 131.19, 132.73, 137.65, 137.96, 155.04, 196.56 ppm; HRMS (FAB; m-NBA) m/z: [M + H]+, calcd for C24H14Cl2O4Na, 459.0167; found, 459.0132. 1-(4-Chlorobenzoyl)-2,7-diethoxynaphthalene (1cc). Colorless needle (hexane), Mp 124˚C - 125˚C; IR (KBr): 1660, 1625, 1582, 1514, 1242, 1216 cm–1; 1H NMR δ (400 MHz, CDCl3): 1.09 (3H, t, J = 6.8 Hz), 1.36 (3H, t, J = 7.2 Hz), 3.96 (2H, q, J = 10, 14 Hz), 4.04 (2H, q, J = 10, 14 Hz), 6.83 (1H, d, J = 2.4 Hz), 7.01 (1H, dd, J = 2.4, 9.2 Hz), 7.10 (1H, d, J = 8.8 Hz), 7.38 (2H, d, J = 8.6 Hz), 7.70 (1H, d, J = 8.8 Hz), 7.76 (2H, d, J = 8.6 Hz), 7.83 (1H, d, J =9.2 Hz) ppm; 13C NMR δ (100 MHz, CDCl3): 14.577, 14.596, 63.394, 64.805, 102.78, 111.20, 117.33, 121.41, 124.32, 128.72, 129.65, 130.72, 131.30, 133.12, 136.99, 139.40, 154.63, 158.35, 196.97 ppm; HRMS (FAB; m-NBA) m/z: [M + H]+; Calcd for C21H20O3Cl: 355.1101; Found:355.1061. 1-(4-Chlorobenzoyl)-7-ethoxy-2-hydroxynaphthalene (2cb). Glassy yellow solid (oil); IR (KBr): 3239, 1656, 1619, 1594, 1571, 1513, 1203 cm–1; 1H NMR δ (400 MHz, CDCl3): 1.22 (3H, t, J = 7.0 Hz), 3.52 (2H, q, J = 10, 14 Hz), 6.55 (1H, d, J = 2.5 Hz), 6.90 (1H, dd, J = 2.5, 8.8 Hz), 7.05 (1H, d, J = 8.8 Hz), 7.40 (2H, d, J = 8.4 Hz), 7.57 (2H, d, J = 8.4 Hz), 7.61 (1H, d, J = 9.2 Hz), 7.83 (1H, d, J =9.2 Hz), 11.29 (1H, s) ppm; 13C NMR δ (100 MHz, CDCl3): 14.510, 62.898, 107.08, 113.42, 116.25, 116.30, 123.60, 128.93, 130.15, 130.65, 133.86, 136.52, 138.62, 138.78, 138.80, 157.61, 162.48, 199.07 ppm; HRMS (FAB; m-NBA) m/z: [M + H]+; Calcd for C19H16O3Cl: 327.0788; Found: 327.0784. 3-(4-Chlorobenzoyl)-2,7-diethoxynaphthalene (5cc). Yellow oil (AcOEt+hexane); IR (KBr): 1663, 1626, 1585, 1504, 1211 cm–1; 1H NMR δ (400 MHz, CDCl3): 1.14 (3H, t, J = 6.8 Hz), 1.49 (3H, t, J = 6.8 Hz), 4.04 (2H, q, J = 10, 14 Hz), 4.16 (2H, q, J = 10, 14 Hz), 7.04 (1H, dd, J = 2.4, 8.8 Hz), 7.05 (1H, br), 7.07 (1H, s), 7.40 (2H, d, J = 8.4 Hz), 7.69 (1H, d, J =8.8 Hz), 7.74 (2H, d, J = 8.4 Hz), 7.83 (1H, s) ppm; 13C NMR δ (100 MHz, CDCl3): 14.234, 14.777, 63.528, 63.804, 105.65, 106.06, 106.08, 117.35, 123.20, 127.61, 128.31, 130.14, 130.36, 130.99, 137.06, 137.46, 138.83, 154.95, 158.78, 195.18 ppm; HRMS (FAB; m-NBA) m/z: [M + H]+; Calcd for C21H20O3Cl: 355.1101; Found: 355.1112. 3-(4-Chlorobenzoyl)-7-ethoxy-2-hydroxynaphthalene (6cb). Yellow needle, (CHCl3 + hexane); Mp 151˚C; IR (KBr): 3448, 1638, 1593, 1560, 1509, 1236, 1209 cm–1; 1H NMR δ (300 MHz, CDCl3): 1.49 (3H, t, J = 7.0 Hz), 4.17 (2H, q, J = 11, 14 Hz), 6.95 (1H, brs), 6.95 (1H, dd, J = 8.8, 2.4 Hz), 7.22 (1H, s), 7.52 (2H, d, J = 8.4 Hz), 7.59 (1H, d, J = 8.7 Hz), 7.69 (2H, d, J = 8.4 Hz), 8.00 (1H, s), 11.26 (1H, s) ppm; 13C NMR δ (400 MHz, CDCl3): 14.663, 63.699, 104.40, 111.22, 118.09, 118.52, 122.24, 128.77, 128.92, 130.81, 131.27, 136.15, 138.46, 140.21, 158.19, 160.53, 200.05 ppm; HRMS (FAB; m-NBA) m/z: [M + H]+; Calcd for C19H16O3Cl: 327.0788. Found: 327.0784. 1,8-Bis(4-chlorobenzoyl)-2,7-diethoxynaphthalene (7cc). Colorless needle (Chloroform/EtOH), Mp 214.1˚C - 216.2˚C; IR (KBr): 1660 (C=O), 1610(Ar), 1510 (Ar), 1274 (O-Et) cm–1; 1H NMR δ (300 MHz, CDCl3): 0.96 (6H, t, J = 6.9 Hz), 3.97 (4H, q, J = 7.2 Hz), 7.15 (2H, d, J = 9.0 Hz), 7.34 (4H, d, J = 8.4 Hz), 7.67 (4H, d, J = 8.4 Hz), 7.92 (2H, d, J = 8.7 Hz) ppm; 13C NMR δ (100 MHz, CDCl3); 14.52, 64.48, 112.19, 121.06, 125.53, 128.38, 130.45, 132.52, 137.78, 138.78, 156.10, 196.63 ppm.; HRMS (FAB; m-NBA) m/z: [M + H]+; calcd for C28H23Cl2O4, 493.0973; found, 493.0958. 1,8-Bis(4-chlorobenzoyl)-2-ethoxy-7-methoxynaphthalene (7ca). Pale yellow needle (silicagel column, hexane: AcOEt = 1:1), Mp 209.3˚C - 210.1˚C; IR (KBr): 1663, 1609, 1587, 1510, 1267, 1047, 838 cm–1; 1H NMR δ (300 MHz, CDCl3): 0.96 (t, J = 7.0 Hz, 3H), 3.70 (s, 3H), 3.97 (q, J = 10, 14 Hz, 2H), 7.21 - 7.14 (m, 2H), 7.97 - 7.91 (m, 2H), 7.33 (d, J = 8.7 Hz, 4H), 7.67 - 7.63 (m, 4H) ppm; 13C NMR δ (75 MHz, CDCl3): 14.4, 56.4, 65.0, 111.2, 112.3, 121.1, 121.3, 125.6, 128.3, 128.4, 130.2, 130.4, 130.5, 132.3, 132.4, 137.3, 137.7, 138.8, 139.0, 156.0, 156.5, 196.0, 196.1 ppm; HRMS (FAB; m-NBA)m/z: [M + H]+; calcd for C27H21O4Cl2, 479.0817; found, 479.0822. Anal. Calcd for C27H20O4Cl2: C, 67.65; H, 4.21. Found: C, 67.45; H, 4.10. 1,8-Bis(4-chlorobenzoyl)-2-ethoxy-7-hydroxynaphthalene (8bc). Yellow powder (silicagel column, AcOEt), Mp 200.3˚C - 203.9˚C; IR (KBr): 3378, 1644, 1611, 1589, 1567, 1510, 1208, 1225, 1281, 1090, 803 cm–1; 1H NMR δ (400 MHz, CDCl3): 0.85 (3H, t, J = 6.8 Hz), 3.89 (2H, q, J = 10, 14 Hz), 6.88 (2H, d, J = 8.0 Hz), 7.07 (1H, d, J = 8.8 Hz), 7.09 - 7.16 (3H, m), 7.18 (2H, d, J = 8.4 Hz), 7.31 (2H, d, J = 8.4 Hz), 7.88 (1H, d, J = 8.4 Hz), 7.90 (1H, d, J = 8.8 Hz), 9.53 (1H, s) ppm; 13C NMR δ (100 MHz, CDCl3): 14.043, 69.491, 111.33, 113.98, 117.07, 121.13, 124.39, 127.46, 128.42, 130.31, 131.98, 132.68, 133.70, 135.09, 136.54, 136.73, 138.13, 139.58, 157.08, 159.57, 195.59, 197.12 ppm; HRMS (FAB; m-NBA) m/z: [M + H]+, calcd for C26H19Cl2O4, 465.0660; found 465.0629. 5. Acknowledgements This work was partially supported by the Shorai Foundation for Science and Technology and the Iron and Steel Institute of Japan (ISIJ) Research Promotion Grant. REFERENCES

