Journal of Analytical Sciences, Methods and Instrumentation
Vol.3 No.1(2013), Article ID:29149,13 pages DOI:10.4236/jasmi.2013.31004
Exhaled Breath Condensates as a Source for Biomarkers for Characterization of Inflammatory Lung Diseases

1Department of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Section of Allergy and Immunology, Milton S. Hershey Medical Center, The Pennsylvania State University, Hershey, USA; 2Department of Biochemistry and Molecular Biology, College of Medicine, The Pennsylvania State University, Hershey, USA.
Email: fishmael@hmc.psu.edu
Received December 28th, 2012; revised January 28th, 2013; accepted February 8th, 2013
Keywords: Exhaled Breath Condensate, Inflammation; Biomarkers; Hydrogen Peroxide; Ph; Micrornas; Nitric Oxide; Leukotrienes; Prostaglandins; Metabolomics
ABSTRACT
Inflammatory lung diseases such as asthma and chronic obstructive pulmonary disease are common and difficult to diagnose and characterize. This is due in large part to difficulty in obtaining samples directly from the inflamed lung. The collection of lung secretions by traditional methods including bronchoalveolar lavage and induced sputum collection are limited by their invasive nature. Exhaled breath condensate (EBC) is a simple and non-invasive technique of collecting fluid samples, which are representative of airway lining fluid. Advances in collection methods and evolving molecular techniques have led to development of more sensitive assays for existing biomarkers and identification of new biomarkers, which can be potentially useful in monitoring lung inflammation. In this review, we present the current understanding of various biomarkers including small molecules (H2O2, pH and nitric oxide related biomarkers), lipid mediators (8-isprostane, leukotrienes and prostaglandins), small proteins (cytokines and chemokines) and nucleic acids (DNA and microRNAs). We also discuss the differential profile of biomarkers in recognizing different patterns of lung inflammation. As the sensitivity of methods of EBC improves, this biofluid will play an increasing role in diagnosis and monitoring of lung diseases.
1. Introduction
Diseases of the lung such as asthma and chronic obstructive lung disease (COPD) are characterized by airway inflammation. These are common diseases with a US prevalence of 8.4% for asthma [1] and between 3% - 7% for COPD [2]. However, these diseases are difficult to diagnose, distinguish from each other, and characterize phenotypically. Inflammation in the airway involves an interplay between environmental stimuli, airway epithetlial cells, and leukocytes (Figure 1). Stimuli such as infections, allergens, pollutants, oxidants, or irritants directly damage the airway cells or induce them to produce inflammatory mediators such as cytokines, reactive oxygen species (ROS), or acidic products. These mediators subsequently recruit various leukocytes to the lung, which can produce additional products, including cytokines, ROS, nitrates, and lipid mediators that contribute to the chronic inflammatory state. These mediators are found in airway lining fluid (ALF) and their measurement may serve as biomarkers to identify different inflammatory patterns in lung diseases. Their role in the diagnoses and management of variety of lung diseases is now being recognized.
However, collection of ALF has always posed methodological challenges. The most reliable method is collection of fluid by bronchoalveolar lavage (BAL), an invasive technique that requires bronchoscopy to collect samples directly from the lower lung. Other methods, such as induced sputum, may be less invasive. However, it is difficult to reproducibly isolate high quality samples, salivary contamination is common, and it is a difficult and uncomfortable procedure for the subjects.
An emerging non-invasive technique to isolate ALF is exhaled breath condensates (EBC). Physiologically, the exhaled breath is constituted predominately by water vapor and aerosolized particles, generated by ALF. By cooling breath vapor, EBC can be collected and its biochemical composition has been found to be very similar to ALF [3]. One of the main advantages of EBC is the non-invasive collection technique, which can be conveniently performed by the patients in most age groups. The ease of collection of samples, combined with increasing
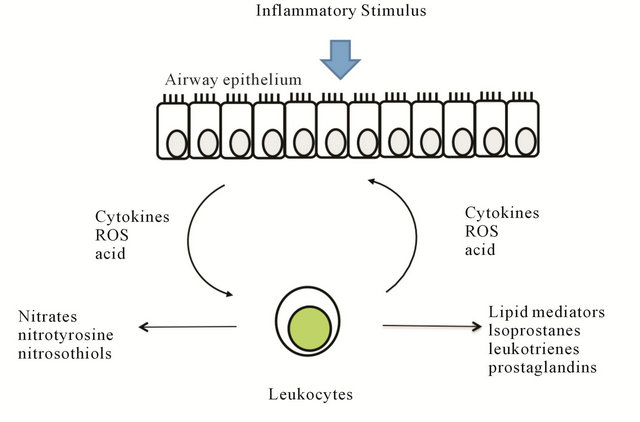
Figure 1. Inflammatory mediators produced in airways. Stimulation ofairway cells by inflammatory stimuli leads to production of inflammatory mediators such as cytokines, reactive oxygen species (ROS), or acidic products. These mediators subsequently recruit various leukocytes to the lung, which can produce additional products, including cytokines, ROS, nitrates, and lipid mediators that contribute to the chronic inflammatory state.
sensitivity of various biomarker detection makes EBC a novel and potentially important diagnostic tool.
2. Collection of EBC
The principle of collecting EBC involves cooling the exhaled air below the dew point by transferring the heat to a chilled condenser surface (Figure 2). This leads to condensation of water vapor content in the breadth over aerosolized ALF particles, leading to formation of enlarged EBC droplets on condenser wall [3]. The volume of EBC collected depends on multiple factors including amount of expired air, material and temperature of condenser etc. There are two commercial devices available for collection of EBC at this point-ECoScreen (VIASYS Healthcare, Hoechberg, Germany) and RTube (Respiratory Research Inc., VA) [3]. The ECoScreen uses an electric cooler, while the Rtube utilizes a metal tube that has been pre-cooled and then placed around the collection vessel. Studies have shown that level of biomarkers obtained from different collection devices varies and hence they cannot be compared directly [4]. Each has advantages, such as greater potential sensitivity of the EcoScreen versus enhanced portability and simplicity of the Rtube [5]. According to recent American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines, the subject should breath orally for around 10 minutes through the collection device, in a tidal pattern and this should allow collection of 1 - 3 ml of condensate [6]. The apparatus should have a salivary trap to minimize the salivary contamination of the condensate and contamination can further be evaluated by checking the amylase level in the collected sample [7].
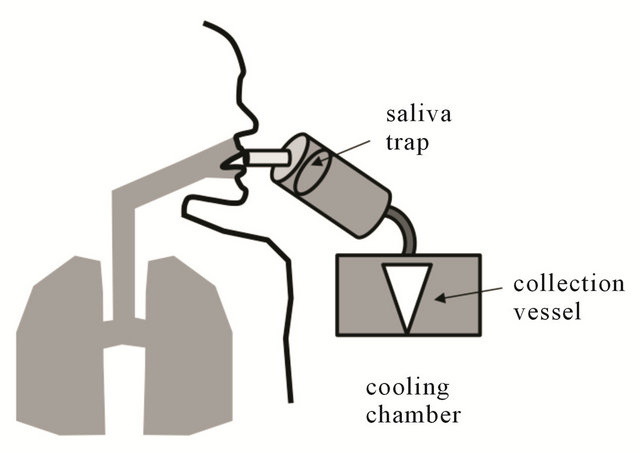
Figure 2. Schematic of EBC collection apparatus. The collection system consists of a salivary trap, a cooling mechanism, and collection vessel.
3. Components of EBC and Their Role as Bio-Markers
Expired air, saturated with water vapor deposits in cooling chamber as distilled water and forms the main constituent of EBC. In addition, water-soluble volatile organic compounds (VOCs) present in gaseous phase in exhaled breath dissolve into the condensate. The third component is derived from aerosolized micro-particles which originate directly from ALF in the lower airways and contain both non-volatile constituents and dissolved VOCs [8]. Though the exact source of aerosolized particle in exhaled breath is not very clear studies have indicated a predominantly lower airway origin [9,10]. Detection of VOCs depends on multiple factors including water solubility, gas-liquid partition coefficient and temperature of condenser. The non-volatile compounds form a broad range of molecules ranging from inorganic ions like sodium, to macromolecules such as small protein molecules like cytokines or nucleic acids.
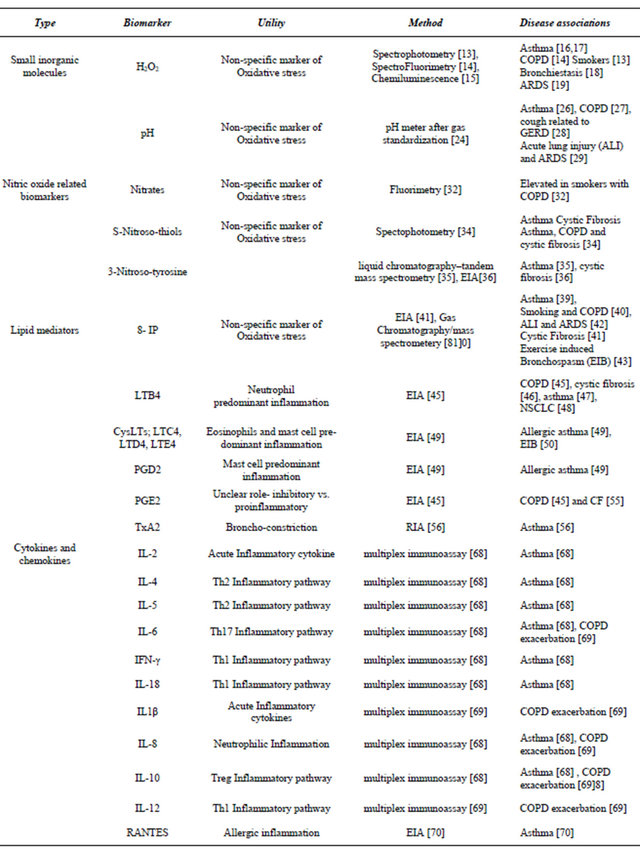
EBC has been recognized as a source of multitude of organic and inorganic compounds including small inorganic molecules (H2O2, pH and nitric oxide related biomarkers), lipid mediators (8-isprostane, leukotrienes and prostaglandins), small proteins (cytokines and chemokines) and nucleic acid derivatives [11]. These biomarkers can reflect the underlying state of pulmonary inflammation and can be altered in various pulmonary diseases like asthma, COPD, bronchiectasis, infections, and lung cancer (Table 1).
4. Small Inorganic Molecules in EBC
Hydrogen peroxide (H2O2) is a reactive oxygen speciesgenerated in inflammatory cells from metabolism of superoxide anion (O2−) by superoxide dismutase. Increased oxidative stress during inflammation induces respiratory bursts resulting in marked production of O2− which leads to increased production of H2O2 [12]. Its levels in EBC have been measured by spectrofluorimetry [13], spectrophotometry [14], and chemiluminescence [15]. As compared to healthy nonsmokers, H2O2 have been reported to be elevated in smokers [13], asthmatics [16] [17], COPD patients [14], bronchiectasis [18] and Acute respiratory distress syndrome (ARDS) [19]. Levels of H2O2 have been shown significantly elevated in acute worsening of asthma [11], COPD exacerbations [20], pulmonary infection related exacerbations of cystic fibrosis [21] and may have potential role in guiding therapy in these situations.
Despite recognition of H2O2, as an indicator of oxidative stress, it has not been established as a reliable EBC biomarker due to several limitations. The mean levels of H2O2 have shown a wide variability in EBC of healthy nonsmoking adults (0.01 - 0.45 micromoles) [13] and healthy children between 8 - 13 years (median H2O2 levels was reported as 0.13 μM, with reference range of <0.01 - 0.48 μM) [22]. Additionally, variability of levels were found on different days of measurement on same subjects and at different times of the day [13]. The concentrations of H2O2 in EBC was also found to be related to expiratory flow rate, suggesting a predominantly bronchial origin of H2O2, as compared to alveolarorigin, further limiting its usefulness as a pulmonary inflammatory marker [23].
EBC pH reflects the acid/base balance of the airways. The major source of acidification of EBC includes intrinsic pulmonary inflammatory burden but there could be additional contribution from gastroesophageal reflux disease [24]. EBC pH from samples collected from oral breathing were found to be similar to endotracheal samples but different from salivary pH, supporting a predominantly lower airway origin of EBC pH [25]. Standard pH analysis is performed after de-aeration of carbon dioxide (CO2) with an inert gas like argon to minimize artifacts [24]. One of the main advantage of EBC pH is the fact that it has been found to be an extremely reproducible and robust parameter and does not show deviation with type of ventilation, gender, time of day collected, volume of EBC collected, duration of EBC collection, degree of hyperventilation [24] or temperature or duration of sample storage [24,25]. Normal values of gas-standardized (CO2-free) EBC pH have been reported between 7.5 and 8.1 [24,25]. Decrease in EBC pH has been reported in both stable and unstable asthma patients as compared to healthy controls, with a greater decrease seen in unstable asthmatics treated with inhaled corticosteroids [26]. Similarly, EBC pH levels have been reported to be lower in patients with COPD compared to age and gender matched controls and correlate with disease severity, as expressed by GOLD stages, especially in ex-smokers [27]. EBC pH may have potential role in diagnosis of upper respiratory symptoms like chronic cough due to underlying acid reflux [28]. A system for continuous monitoring of EBC pH in intubated patients on mechanical ventilation has also been developed to monitor lung health in critically ill patients [24,29].
5. Nitric Oxide Related Biomarkers
Nitric Oxide (NO) is an important bioactive mediator that plays important roles in multiple cellular functions. The levels of NO increase during inflammatory conditions due to activation of inducible NO synthase in response to proinflammatory cytokines [30]. NO can be measured in exhaled breath in form of Fractional excretion of NO (FeNO) and has been shown to correlate with active lower airway inflammation in asthmatics [31]. Nitrates and nitrites are products of NO metabolism that can be detected in EBC and their levels have been reported to be elevated in smokers with COPD as compared to healthy smokers [32]. NO can combine with reactive oxygen molecules to form highly reactive nitrogen species (RNS) which further covalently interact with various biomolecules to form nitrosoand nitro-molecules [33]. S-Nitrosothiols are formed by interaction of RNS with low molecular thiols and have reported to be elevated in severe asthma, COPD and cystic fibrosis [34]. 3-Nitrosotyrosine is another covalent adduct molecule generated by interaction of RNS with tyrosine with in the proteins [33] and has been shown to be elevated in patients with asthma [35] and cystic fibrosis [36]. NO and related molecules are non-specific markers of oxidative stress and may be useful in monitoring various pro-inflammatory pathologies of lower airways.
Table 1. Biomarkers in EBC.
Continued
6. Lipid Mediators
Lipid mediators are produced in leukocytes from the enzymatic metabolism of phospholipids (Figure 3) and have numerous physiologic and pathologic functions. Levels may be altered in a variety or lung diseases underscoring their potential as biomarkers. Isoprostanes are prostaglandin like substances produced in vivo by free radical-induced peroxidation of arachidonic acid, independent of cyclooxygenase (COX) enzymes [37]. They are chemically stable molecules and their levels in body fluids have been reported as a reliable indicator of oxidative stress [38]. Elevated levels of 8-Isoprostane (8-IP) have been reported in asthma [39], healthy smokers and smokers with COPD [40], cystic fibrosis [41], acute lung injury and ARDS [42], and exercise induced bronchospasm [43].
Leukotrienes are derivatives of arachidonic acid through the action of 5-Lipoxigenase (5-LO) [44]. Leukotriene (LT) B4, formed from hydrolysis of LTA4 has been shown to be a major neutrophil chemoattractant. Elevated LTB4 levels in EBC have been reported in neutrophilic predominant inflammatory lung pathologies including COPD [45] and cystic fibrosis [46]. Its levels have been reported to be significantly elevated in moderate to severely uncontrolled asthma patients whereas in mild episodic asthmatics, its role is not clear [47]. A recent study has also reported elevated LTB4 levels in patients with Non-Small Cell Lung Cancer (NSCLC), indicating presence of neutrophilic inflammation in airways of these patients [48].
Cysteinyl-leukotrienes (CysLTs; LTC4, LTD4, and LTE4), are produced in mast cells and eosinophils and their levels have been shown to be elevated in EBC from patients with asthma induced by allergic inflammation [49]. Another study reported elevation of CysLTs in asthma patients with exercise induced bronchospasm (EIB), with levels correlating with decrease in FEV1 [50], whereas no difference was seen in levels of LTB4 in EIB patients. In a recent study, multiple eicosanoids were measured in EBC samples of aspirin sensitive and aspirin tolerant asthma patients using gas chromatography/mass spectrometry and high-performance liquid chromatography [51]. The authors did not find significant difference in concentration of leukotrienes, prostaglandins, and thromboxanes at baseline or after aspirin challenge in aspirin sensitive asthma patients as compared to aspirin tolerant asthma patients. The eicosanoids that were found to be elevated baseline in aspirin sensitive patients were 5 and 15-hydroxyeicosatetraenoic acid (HETE). Another study reported significant increase in the level ofprostaglandin D2 and E2 metabolites and 5-, 15-HETE in aspirin intolerant asthma patients by usingcomplementary high performance liquid chromatography (HPLC) and gas chromatography-massspectrometry (GC/MS) [52]. The authors proposed that highly sensitive eicosanoid profiling in EBC can be used to identify aspirin intolerant asthma phenotypes.
Prostaglandins are lipid mediators derived from metabolism of arachidonic acid by action of cyclooxygenase (COX) enzyme. Prostaglandin D2 (PGD2) is predominantly produced by mast cells and its levels were reported to increase in EBC after allergen-induced bronchoconstriction in asthmatic patients [49]. The levels were quantified using enzyme immunoassays (EIA) and increase in levels correlated with degree of bronchoconstriction. The role of Prostaglandin E2 (PGE2) is not very clear. Earlier studies reported a predominantly inhibitory role in lung inflammation [53] but more recent studies have postulated that it may also have pro-inflammatory action [54]. A significant increase of PGE2 was reported in COPD patients, both steroid naive and steroid treated, as compared to healthy controls [45]. PGE2 levels were also reported to be elevated in patients with stable and unstable cystic fibrosis [55]. The levels of thromboxane A2 (TxA2), were reported to be increased in asthmatics as compared to healthy controls [56]. These authors also reported an improved TxA2 detection rate with radioimmunoassay (RIA) as compared to standard EIA.
7. Cytokines and Chemokines
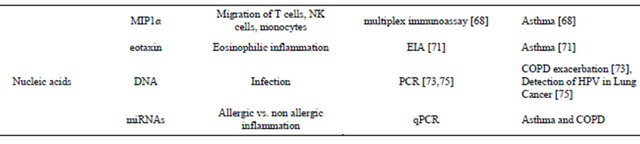
Cytokines and chemokines are small protein molecules involved in intercellular signaling and play an important in inflammatory pathways in lungs and other organs. Elevated levels of cytokines have been reported in BAL specimens of patients with acute lung injury and ARDS
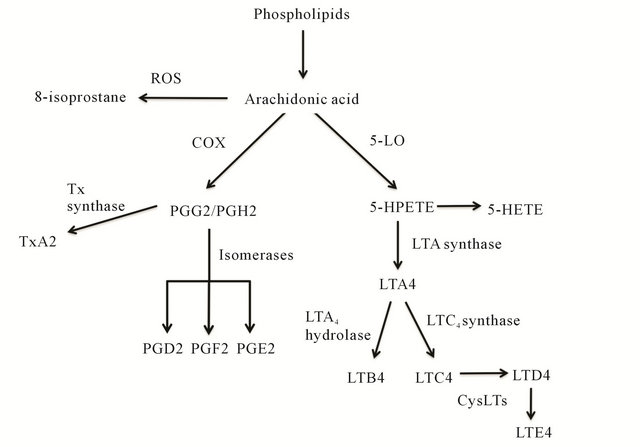
Figure 3. Lipid Mediators. Prostaglandins (PGD2, PGE2 and PGF2) and Thromboxane A2 (TxA2) are derived from arachidonic acid by COX mediated pathway. 8-Isprostane (8-IP) is formed by free radical peroxidation of arachidonic acid, independent of cyclooxygenase (COX) enzymes. Leukotrienes (LTB4 and Cysteinyl-leukotrienes-LTC4, LTD4 and LTE4) are produced by action of 5-lipoxigenase (5-LO).
[57,58], asthma [59], COPD [60] and cystic fibrosis [61]. Elevated cytokines levels have also been reported in induced sputum samples in patients with COPD [62] and asthma [63]. EBC provides a non-invasive alternative for obtaining ALF samples and has been increasingly studied as a source for monitoring cytokines. Unlike other nonspecific markers of oxidative stress, cytokines signatures can be useful in defining type of inflammation and etiology. Interaction of naïve T-cells (Th0) with other inflammatory cells and molecules leads to their differential into either T helper (Th)1 T-cells which are involved in cell mediated immunity or Th2 T-cells, which are involved with antibody mediated immunity and allergic inflammation. Increased levels of IL-4 (Th2 cytokine) and decreased interferon-γ (Th1 cytokine) have been reported in EBC samples of asthmatic children indicating a Th2 predominant inflammation [64]. Other authors reported extremely low concentration of cytokines when collected by standard collecting apparatus and using EIA based analysis [65]. Improved detection of cytokines was reported with use of flow cytometry but this group did not find any difference in cytokine profile between asthma and COPD patients [66]. More recently, the use of glass condenser system has been reported to improve the yield of cytokines and other biomarkers in EBC samples [67]. The use of this system along with use of a more sensitive multiplex immunoassay has been reported to significantly improve the detection of cytokines in EBC [68]. This group reported increased levels of IL-2IL-4, IL-5, IL-6, IFN-γ, IL-18, MIP1α, RANTES, IL10, and IL-8 in EBC samples from asthmatic patients as compared to controls. Elevated levels of cytokines IL1β, IL-6, IL-8, IL-10, IL-12p70 have also been in reported in patients with acute COPD exacerbation as compared to stable COPD, healthy smokers and healthy nonsmokers [69]. More recent studies have also reported increased levels of eosinophil specific chemokines-RANTES [70] and eotaxin [71] in EBC of asthma patients. Another potential use for cytokine monitoring can be in diagnosing airway inflammation in preschool children with wheezing [72].
8. Nucleic Acids
One of the main limitations of EBC is the low concentration of biomolecules, which pushes the detection limits of many assays. An emerging use of EBC is the measurement of nucleic acids, which can be amplified and detected in low numbers by PCR techniques. A few reports have demonstrated the presence of microbacterial DNA in EBC, and the impact on this a methodology for diagnosis of infection is emerging [73-76].
Another useful biomarker may be microRNAs (miRNAs), which are small 20 - 25 nucleotide, noncoding RNAs. MiRNAs are involved in post-transcripttional regulation of gene expression and are emerging as regulatory molecules of inflammation [77]. Differential expression of specific miRNAs in sputum of non-small cell lung cancer patients [78], especially in adenocarcinoma [79] can potentially serve as biomarkers for early detection of lung cancers and highlights their potential role in disease diagnosis.
We recently demonstrated that miRNAs are expressed in EBCs, and they can be reproducibly isolated and quantified by real time PCR (Figure 4(a)). In this methodology, an adapter is added to the 3’ end of miRNA, and specific miRNA are amplified by using a forward primer specific to miRNAs of interest and a universal reverse primer to the adapter. After real time PCR analysis, the miRNAs were subjected to gel electrophoresis which confirmed the presence of one species at the expected size of the miRNA plus adapter sequence.
MiRNAs in can be secreted into bodily fluids from cells in exosomes, vesicles that contain miRNA, mRNA, and proteins. Exosomes can be isolated in the pellets of serum or saliva subjected to ultracentrifugation. We found that in contrast to saliva, exosomes are not present in EBC, and miRNAs exist free in solution (Figures 4(b) and (c)). We profiled miRNAs from EBC samples of asthmatics (n = 10), COPD patients (n = 10) and healthy controls (n = 14), and found that miRNAs were differenttially expressed between the groups (Figure 4(d)). For instance, in asthmatics, miR-1248 and miR1291 expression were lower compared to COPD patients and healthy controls. These miRNAs are predicted to regulate allergic mediators, such as IL-13, IL-5, GATA3, and the high affinity IgE receptor. Thus, miRNAs may not only be used as biomarkers to differentiate between types of lung disease, but may also offer insight into the pathogenesis of disease.
We also found that small nucleolar RNAs such as SNORD44 is expressed in EBC. These RNAs have served as “housekeeping” genes to normalize expression of miRNAs between samples. Methods of normalization between subjects are essential components of EBC biomarker measurement. Additionally, since as little as 50 - 100 molecules/ml of miRNA can be reproducibly quantified using qPCR, this may be an especially promising technique.
9. Limitations and Future Directions
EBC is a promising technology that allows insight into pulmonary pathophysiology in a non-invasive way. The concentration of biomolecules in EBC is highly diluted (up to 2000 to 10,000 fold) by condensed water vapor [6],
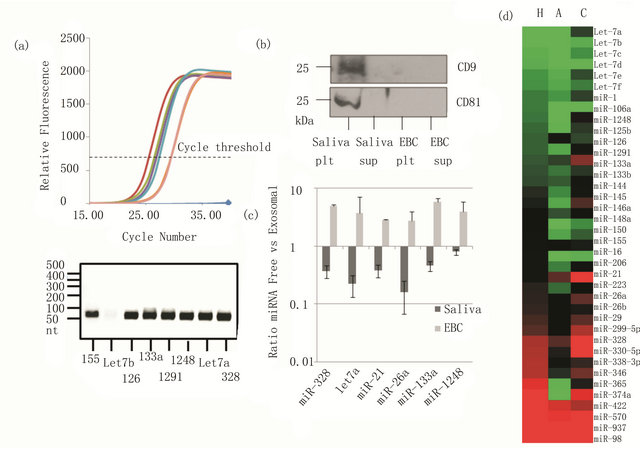
Figure 4. MiRNA expression in EBC: (a) real time PCR analysis of miRNAs and analysis of the products by gel electrophoresis; (b) Saliva or EBC were subjected to ultracentrifugation to pellet exosomes. Western blot for the exosome-specific proteins CD81 and CD9 showed their presence in the ultracentrifugation pellet (plt) of the saliva but not pellet of EBC or the supernatant (sup) or EBC or saliva; (c) Real time PCR analysis of miRNA expression demonstrates that they are present in the exosomal fraction of saliva but in the supernatant of EBC; (d) Heat map showing average expression of miRNA in healthy subjects (H), asthmatics (A), or COPD patients (C).
barely at lower detection limit by standard immunoassay techniques. The role of dilution indicators including conductivity of sample [80], urea and electrolytes [10] have been proposed for standardization of EBC samples but have not yet been established. In the absence of standardization, the wide variability of biomolecules concentration has become one of the main limitations of this technology. Using ratios of related biomarkers instead of absolute values, or normalization of data to housekeeping genes in the case of miRNA, may be useful as standardization measures.
With the development of newer analytical methods like liquid chromatography/mass spectrometry (LC/MS) [81], GC/MS, HPCL, radioimmunoassay (RIA) [56] and multiplex immunoassays [68] and use of glass condenser system for EBC collection [68], there have been significant improvement in the yield and detection threshold of various biomarkers. Another important development is application of metabolomics, which is study of measuring biomolecules in metabolic pathway in body fluids. In a recent study [82], nuclear magnetic resonance technique (NMR) spectroscopy was applied to analyze metabolomics in EBC samples from healthy subjects, COPD patients and patients with laryngectomy and significant difference in their metabolic fingerprints were reported in-between study groups.
In summary, EBC is an exciting technology, allowing an insight into biomolecule milieu of airway lining fluid. It is an important source of biomarkers, both non-specific markers of oxidative stress and specific biomarkers indicating different aspects of pulmonary inflammation. As the ability to comprehensively measure many markers increase via high-throughput methods, EBC will likely play a prominent role in disease diagnosis and characterization. At present, it is primarily used in research, but is emerging as a novel tool in diagnosing and managing inflammatory lung conditions and developing personalized pharmacological strategies.
REFERENCES
- Centers for Disease Control and Prevention, “Vital Signs: Asthma Prevalence, Disease Characteristics, and SelfManagement Education—United States, 2001-2009,” 2011. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6017a4.htm
- Centers for Disease Control and Prevention, “Chronic Obstructive Pulmonary Disease among Adults—United States, 2011,” 2012. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6146a2.htm
- J. Hunt, “Exhaled Breath Condensate: An Evolving Tool for Noninvasive Evaluation of Lung Disease,” The Journal of Allergy and Clinical Immunology, Vol. 110, No. 1, 2002, pp. 28-34. doi:10.1067/mai.2002.124966
- J. Liu, D. H. Conrad, S. Chow, V. H. Tran, D. H. Yates and P. S. Thomas, “Collection Devices Influence the Constituents of Exhaled Breath Condensate,” European Respiratory Journal, Vol. 30, No. 4, 2007, pp. 807-808. doi:10.1183/09031936.00080207
- O. U. Soyer, E. A. Dizdar, O. Keskin, C. Lilly and O. Kalayci, “Comparison of Two Methods for Exhaled Breath Condensate Collection,” Allergy, Vol. 61, No. 8, 2006, pp. 1016-1018. doi:10.1111/j.1398-9995.2006.01064.x
- I. Horváth, J. Hunt, P. J. Barnes, K. Alving, A. Antczak, E. Baraldi, G. Becher, W. J. C. van Beurden, M. Corradi, R. Dekhuijzen, R. A. Dweik, T. Dwyer, R. Effros, S. Erzurum, B. Gaston, C. Gessner, A. Greening, L. P. Ho, J. Hohlfeld, Q. Jöbsis, D. Laskowski, S. Loukides, D. Marlin, P. Montuschi, A. C. Olin, A. E. Redington, P. Reinhold, E. L. J. van Rensen, I. Rubinstein, P. Silkoff, K. Toren, G. Vass, C. Vogelberg and H. Wirtz, “Exhaled Breath Condensate: Methodological Recommendations and Unresolved Questions,” European Respiratory Journal, Vol. 26, No. 3, 2005, pp. 523-548. doi:10.1183/09031936.05.00029705
- R. M. Effros, M. B. Dunning 3rd, J. Biller and R. Shaker, “The Promise and Perils of Exhaled Breath Condensates,” American Journal of Physiology Lung Cellular and Molecular Physiology, Vol. 287, No. 6, 2004, pp. L1073- L1080. doi:10.1152/ajplung.00069.2004
- M. D. Davis, A. Montpetit and J. Hunt, “Exhaled Breath Condensate: An Overview,” Immunology and Allergy Clinics of North America, Vol. 32, No. 3, 2012, pp. 363- 375. doi:10.1016/j.iac.2012.06.014
- G. I. Sidorenko, E. I. Zborovskiĭ and D. I. Levina, “Surface-Active Properties of the Exhaled Air Condensate (a New Method of Studying Lung Function),” Terapevticheskiî Arkhiv, Vol. 52, No. 3, 1980, pp. 65-68.
- R. M. Effros, B. Peterson, R. Casaburi, J. Su, M. Dunning, J. Torday, J. Biller and R. Shaker, “Epithelial Lining Fluid Solute Concentrations in Chronic Obstructive Lung Disease Patients and Normal Subjects,” Journal of Applied Physiology, Vol. 99, No. 4, 2005, pp. 1286-1292. doi:10.1152/japplphysiol.00362.2005
- C. Caffarelli, E. Calcinai, L. Rinaldi, C. Povesi Dascola, L. Terracciano and M. Corradi, “Hydrogen peroxide in exhaled breath condensate in asthmatic children during acute exacerbation and after treatment,” Respiration, Vol. 84, No. 4, 2012, pp. 291-298. doi:10.1159/000341969
- A. B. Tonnel and B. Wallaert, “Oxidants and Bronchial Inflammatory Processes,” The European Respiratory Journal, Vol. 3, No. 9, 1990, pp. 987-988.
- D. Nowak, S. Kalucka, P. Białasiewicz and M. Król, “Exhalation of H2O2 and Thiobarbituric Acid Reactive Substances (TBARs) by Healthy Subjects,” Free Radical Biology and Medicine, Vol. 30, No. 2, 2001, pp. 178-186. doi:10.1016/S0891-5849(00)00457-3
- F. De Benedetto, A. Aceto, B. Dragani, A. Spacone, S. Formisano, R. Cocco and C. M. Sanguinetti, “Validation of a New Technique to Assess Exhaled Hydrogen Peroxide: Results from Normals and COPD Patients,” Monaldi Archives for Chest Disease, Vol. 55, No. 3, 2000, pp. 185-188.
- Y. Hu, Z. Zhang and C. Yang, “A Sensitive Chemiluminescence Method for the Determination of H2O2 in Exhaled Breath Condensate,” Analytical Science, Vol. 24, No. 2, 2008, pp. 201-205. doi:10.2116/analsci.24.201
- A. H. Al-Obaidy and A.-G. M. Al-Samarai, “Exhaled Breath Condensate pH and Hydrogen Peroxide as NonInvasive Markers for Asthma,” Saudi Medical Journal, Vol. 28, No. 12, 2007, pp. 1860-1863.
- A. Emelyanov, G. Fedoseev, A. Abulimity, K. Rudinski, A. Fedoulov, A. Karabanov and P. J. Barnes, “Elevated Concentrations of Exhaled Hydrogen Peroxide in Asthmatic Patients,” Chest, Vol. 120, No. 4, 2001, pp. 1136- 1139. doi:10.1378/chest.120.4.1136
- S. Loukides, I. Horvath, T. Wodehouse, P. J. Cole and P. J. Barnes, “Elevated Levels of Expired Breath Hydrogen Peroxide in Bronchiectasis,” American Journal of Respiratory and Critical Care Medicine, Vol. 158, No. 3, 1998, pp. 991-994.
- D. Kietzmann, R. Kahl, M. Müller, H. Burchardi and D. Kettler, “Hydrogen Peroxide in Expired Breath Condensate of Patients with Acute Respiratory Failure and with ARDS,” Intensive Care Medicine, Vol. 19, No. 2, 1993, pp. 78-81. doi:10.1007/BF01708366
- E.-J. D. Oudijk, W. B. M. Gerritsen, E. H. J. Nijhuis, D. Kanters, B. L. P. Maesen, J.-W. J. Lammers and L. Koenderman, “Expression of Priming-Associated Cellular Markers on Neutrophils during an Exacerbation of COPD,” Respiratory Medicine, Vol. 100, No. 10, 2006, pp. 1791- 1799. doi:10.1016/j.rmed.2006.01.022
- Q. Jöbsis, H. C. Raatgeep, S. L. Schellekens, A. Kroesbergen, W. C. Hop and J. C. de Jongste, “Hydrogen Peroxide and Nitric Oxide in Exhaled Air of Children with Cystic Fibrosis during Antibiotic Treatment,” European Respiratory Journal, Vol. 16, No. 1, 2000, pp. 95-100. doi:10.1034/j.1399-3003.2000.16a17.x
- Q. Jöbsis, H. C. Raatgeep, S. L. Schellekens, W. C. Hop, P. W. Hermans and J. C. de Jongste, “Hydrogen Peroxide in Exhaled Air of Healthy Children: Reference Values,” European Respiratory Journal, Vol. 12, No. 2, 1998, pp. 483-485. doi:10.1183/09031936.98.12020483
- M. B. Schleiss, O. Holz, M. Behnke, K. Richter, H. Magnussen and R. A. Jorres, “The Concentration of Hydrogen Peroxide in Exhaled Air Depends on Expiratory Flow Rate,” European Respiratory Journal, Vol. 16, No. 6, 2000, pp. 1115-1118. doi:10.1034/j.1399-3003.2000.16f16.x
- M. D. Davis and J. Hunt, “Exhaled Breath Condensate pH Assays,” Immunology and Allergy Clinics of North America, Vol. 32, No. 3, 2012, pp. 377-386. doi:10.1016/j.iac.2012.06.003
- J. Vaughan, L. Ngamtrakulpanit, T. N. Pajewski, R. Turner, T. A. Nguyen, A. Smith, P. Urban, S. Hom, B. Gaston and J. Hunt, “Exhaled Breath Condensate pH is a Robust and Reproducible Assay of Airway Acidity,” European Respiratory Journal, Vol. 22, No. 6, 2003, pp. 889-894. doi:10.1183/09031936.03.00038803
- M. M. Tomasiak-Lozowska, Z. Zietkowski, K. Przeslaw, M. Tomasiak, R. Skiepko and A. Bodzenta-Lukaszyk, “Inflammatory Markers and Acid-Base Equilibrium in Exhaled Breath Condensate of Stable and Unstable Asthma Patients,” International Archives of Allergy and Immunology, Vol. 159, No. 2, 2012, pp. 121-129. doi:10.1159/000335674
- A. I. Papaioannou, S. Loukides, M. Minas, K. Kontogianni, P. Bakakos, K. I. Gourgoulianis, M. Alchanatis, S. Papiris and K. Kostikas, “Exhaled Breath Condensate pH as a Biomarker of COPD Severity in Ex-Smokers,” Respiratory Research, Vol. 12, 2011, p. 67. doi:10.1186/1465-9921-12-67
- J. Hunt, Y. Yu, J. Burns, B. Gaston, L. Ngamtrakulpanit, D. Bunyan, B. K. Walsh, A. Smith and S. Hom, “Identification of Acid Reflux Cough Using Serial Assays of Exhaled Breath Condensate pH,” Cough, Vol. 2, 2006, p. 3. doi:10.1186/1745-9974-2-3
- B. K. Walsh, D. J. Mackey, T. Pajewski, Y. Yu, B. M. Gaston and J. F. Hunt, “Exhaled-Breath Condensate pH Can Be Safely and Continuously Monitored in Mechanically Ventilated Patients,” Respiratory Care, Vol. 51, No. 10, 2006, pp. 1125-1131.
- J. P. Eiserich, R. P. Patel and V. B. O’Donnell, “Pathophysiology of Nitric Oxide and Related Species: Free Radical Reactions and Modification of Biomolecules,” Molecular Aspects of Medicine, Vol. 19, No. 4-5, 1998, pp. 221-357. doi:10.1016/S0098-2997(99)00002-3
- R. C. Strunk, S. J. Szefler, B. R. Phillips, R. S. Zeiger, V. M. Chinchilli, G. Larsen, K. Hodgdon, W. Morgan, C. A. Sorkness and R. F. Lemanske Jr., “Relationship of Exhaled Nitric Oxide to Clinical and Inflammatory Markers of Persistent Asthma in Children,” The Journal of Allergy and Clinical Immunology, Vol. 112, No. 5, 2003, pp. 883- 892. doi:10.1016/j.jaci.2003.08.014
- J. Liu, A. Sandrini, M. C. Thurston, D. H. Yates and P. S. Thomas, “Nitric Oxide and Exhaled Breath Nitrite/Nitrates in Chronic Obstructive Pulmonary Disease Patients,” Respiration, Vol. 74, No. 6, 2007, pp. 617-623. doi:10.1159/000106379
- A. van der Vliet, J. P. Eiserich, M. K. Shigenaga and C. E. Cross, “Reactive Nitrogen Species and Tyrosine Nitration in the Respiratory Tract: Epiphenomena or a Pathobiologic Mechanism of Disease?” American Journal of Respiratory and Critical Care Medicine, Vol. 160, No. 1, 1999, pp. 1-9.
- M. Corradi, P. Montuschi, L. E. Donnelly, A. Pesci, S. A. Kharitonov and P. J. Barnes, “Increased Nitrosothiols in Exhaled Breath Condensate in Inflammatory Airway Diseases,” American Journal of Respiratory and Critical Care Medicine, Vol. 163, No. 4, 2001, pp. 854-858.
- E. Baraldi, G. Giordano, M. F. Pasquale, S. Carraro, A. Mardegan, G. Bonetto, C. Bastardo, F. Zacchello and S. Zanconato, “3-Nitrotyrosine, a Marker of Nitrosative Stress, Is Increased in Breath Condensate of Allergic Asthmatic Children,” Allergy, Vol. 61, No. 1, 2006, pp. 90-96. doi:10.1111/j.1398-9995.2006.00996.x
- B. Balint, S. A. Kharitonov, T. Hanazawa, L. E. Donnelly, P. L. Shah, M. E. Hodson and P. J. Barnes, “Increased Nitrotyrosine in Exhaled Breath Condensate in Cystic Fibrosis,” European Respiratory Journal, Vol. 17, No. 6, 2001, pp. 1201-1207. doi:10.1183/09031936.01.00072501
- J. D. Morrow, K. E. Hill, R. F. Burk, T. M. Nammour, K. F. Badr and L. J. Roberts 2nd, “A Series of Prostaglandin F2-Like Compounds are Produced in Vivo in Humans by a Non-Cyclooxygenase, Free Radical-Catalyzed Mechanism,” Proceedings of the National Academy Sciences of the USA, Vol. 87, No. 23, 1990, pp. 9383-9387. doi:10.1073/pnas.87.23.9383
- P. Montuschi, P. J. Barnes and L. J. Roberts 2nd, “Isoprostanes: Markers and Mediators of Oxidative Stress,” FASEB Journal, Vol. 18, No. 15, 2004, pp. 1791-1800. doi:10.1096/fj.04-2330rev
- P. Montuschi, M. Corradi, G. Ciabattoni, J. Nightingale, S. A. Kharitonov and P. J. Barnes, “Increased 8-Isoprostane, a Marker of Oxidative Stress, in Exhaled Condensate of Asthma Patients,” American Journal of Respiratory and Critical Care Medicine, Vol. 160, No. 1, 1999, pp. 216-220.
- P. Montuschi, J. V. Collins, G. Ciabattoni, N. Lazzeri, M. Corradi, S. A. Kharitonov and P. J. Barnes, “Exhaled 8-Isoprostane as An in Vivo Biomarker of Lung Oxidative Stress in Patients with COPD and Healthy Smokers,” American Journal of Respiratory and Critical Care Medicine, Vol. 162, No. 3, 2000, pp. 1175-1177.
- P. Montuschi, S. A. Kharitonov, G. Ciabattoni, M. Corradi, L. van Rensen, D. M. Geddes, M. E. Hodson and P. J. Barnes, “Exhaled 8-Isoprostane as a New Non-Invasive Biomarker of Oxidative Stress in Cystic Fibrosis,” Thorax, Vol. 55, No. 3, 2000, pp. 205-209. doi:10.1136/thorax.55.3.205
- C. T. Carpenter, P. V. Price and B. W. Christman, “Exhaled Breath Condensate Isoprostanes Are Elevated in Patients with Acute Lung Injury or ARDS,” Chest, Vol. 114, No. 6, 1998, pp. 1653-1659. doi:10.1378/chest.114.6.1653
- M. Barreto, M. P. Villa, C. Olita, S. Martella, G. Ciabattoni and P. Montuschi, “8-Isoprostane in Exhaled Breath Condensate and Exercise-Induced Bronchoconstriction in Asthmatic Children and Adolescents,” Chest, Vol. 135, No. 1, 2009, pp. 66-73. doi:10.1378/chest.08-0722
- B. Samuelsson, “Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation,” Science, Vol. 220, No. 4597, 1983, pp. 568-575. doi:10.1126/science.6301011
- P. Montuschi, S. A. Kharitonov, G. Ciabattoni and P. J. Barnes, “Exhaled Leukotrienes and Prostaglandins in COPD,” Thorax, Vol. 58, No. 7, 2003, pp. 585-588. doi:10.1136/thorax.58.7.585
- A. Bodini, C. D’Orazio, D. Peroni, M. Corradi, G. Folesani, E. Baraldi, B. M. Assael, A. Boner and G. L. Piacentini, “Biomarkers of Neutrophilic Inflammation in Exhaled Air of Cystic Fibrosis Children with Bacterial Airway Infections,” Pediatric Pulmonology, Vol. 40, No. 6, 2005, pp. 494-499. doi:10.1002/ppul.20336
- S. Caballero Balanzá, A. Martorell Aragonés, J. C. Cerdá Mir, J. Belda Ramírez, J. B. Ramírez, R. Navarro Iváñez, A. Navarro Soriano, R. Félix Toledo and A. Escribano Montaner, “Leukotriene B4 and 8-Isoprostane in Exhaled Breath Condensate of Children with Episodic and Persistent Asthma,” Journal of Investigational Allergology and Clinical Immunology, Vol. 20, No. 3, 2010, pp. 237-243.
- G. E. Carpagnano, G. P. Palladino, D. Lacedonia, A. Koutelou, S. Orlando and M. P. Foschino-Barbaro, “Neutrophilic Airways Inflammation in Lung Cancer: The Role of Exhaled LTB-4 and IL-8,” BMC Cancer, Vol. 11, 2011, p. 226. doi:10.1186/1471-2407-11-226
- E. Ono, H. Mita, M. Taniguchi, N. Higashi, T. Tsuburai, M. Hasegawa, E. Miyazaki, T. Kumamoto and K. Akiyama, “Increase in Inflammatory Mediator Concentrations in Exhaled Breath Condensate after Allergen Inhalation,” Journal of Allergy and Clinical Immunology, Vol. 122, No. 4, 2008, pp. 768-773.
- S. Carraro, M. Corradi, S. Zanconato, R. Alinovi, M. F. Pasquale, F. Zacchello and E. Baraldi, “Exhaled Breath Condensate Cysteinyl Leukotrienes Are Increased in Children with Exercise-Induced Bronchoconstriction,” Journal of Allergy and Clinical Immunology, Vol. 115, No. 4, 2005, pp. 764-770. doi:10.1016/j.jaci.2004.10.043
- L. Mastalerz, M. Sanak, J. Kumik, A. Gawlewicz-Mroczka, N. Celejewska-Wójcik, A. Cmiel and A. Szczeklik, “Exhaled Eicosanoids Following Bronchial Aspirin Challenge in Asthma Patients with and without Aspirin Hypersensitivity: The Pilot Study,” Journal of Allergy (Cairo), Vol. 2012, 2012, Article ID: 696792. doi:10.1155/2012/696792
- M. Sanak, A. Gielicz, G. Bochenek, M. Kaszuba, E. Niżankowska-Mogilnicka and A. Szczeklik, “Targeted Eicosanoid Lipidomics of Exhaled Breath Condensate Provide a Distinct Pattern in the Aspirin-Intolerant Asthma Phenotype,” Journal of Allergy and Clinical Immunology, Vol. 127, No. 5, 2011, pp. 1141-1147.
- I. D. Pavord and A. E. Tattersfield, “Bronchoprotective Role for Endogenous Prostaglandin E2,” Lancet, Vol. 345, No. 8947, 1995, pp. 436-438. doi:10.1016/S0140-6736(95)90409-3
- R. J. Church, L. A. Jania and B. H. Koller, “Prostaglandin E(2) Produced by the Lung Augments the Effector Phase of Allergic Inflammation,” Journal of Immunology, Vol. 188, No. 8, 2012, pp. 4093-4102. doi:10.4049/jimmunol.1101873
- V. Lucidi, G. Ciabattoni, S. Bella, P. J. Barnes and P. Montuschi, “Exhaled 8-Isoprostane and Prostaglandin E2 in Patients with Stable and Unstable Cystic Fibrosis,” Free Radical Biology and Medicine, Vol. 45, No. 6, 2008, pp. 913-919. doi:10.1016/j.freeradbiomed.2008.06.026
- E. Huszár, Z. Szabó, A. Jakab, I. Barta, I. Herjavecz and I. Horváth, “Comparative Measurement of Thromboxane A2 Metabolites in Exhaled Breath Condensate by Different Immunoassays,” Inflammation Research, Vol. 54, No. 8, 2005, pp. 350-355. doi:10.1007/s00011-005-1361-x
- M. G. Kiehl, H. Ostermann, M. Thomas, C. Müller, U. Cassens and J. Kienast, “Inflammatory Mediators in Bronchoalveolar Lavage Fluid and Plasma in Leukocytopenic Patients with Septic Shock-Induced Acute Respiratory Distress Syndrome,” Critical Care Medicine, Vol. 26, No. 7, 1998, pp. 1194-1199. doi:10.1097/00003246-199807000-00019
- H. Schütte, J. Lohmeyer, S. Rosseau, S. Ziegler, C. Siebert, H. Kielisch, H. Pralle, F. Grimminger, H. Morr and W. Seeger, “Bronchoalveolar and Systemic Cytokine Profiles in Patients with ARDS, Severe Pneumonia and Cardiogenic Pulmonary Oedema,” European Respiratory Journal, Vol. 9, No. 9, 1996, pp. 1858-1867. doi:10.1183/09031936.96.09091858
- C. E. Brightling, F. A. Symon, S. S. Birring, P. Bradding, I. D. Pavord and A. J. Wardlaw, “TH2 Cytokine Expression in Bronchoalveolar Lavage fluid T Lymphocytes and Bronchial Submucosa Is a Feature of Asthma and Eosinophilic Bronchitis,” Journal of Allergy and Clinical Immunology, Vol. 110, No. 6, 2002, pp. 899-905. doi:10.1067/mai.2002.129698
- [61] B. Barceló, J. Pons, A. Fuster, J. Sauleda, A. Noguera, J. M. Ferrer and A. G. N. Agustí, “Intracellular Cytokine Profile of T Lymphocytes in Patients with Chronic Obstructive Pulmonary Disease,” Clinical & Experimental Immunology, Vol. 145, No. 3, 2006, pp. 474-479. doi:10.1111/j.1365-2249.2006.03167.x
- [62] T. L. Bonfield, J. R. Panuska, M. W. Konstan, K. A. Hilliard, J. B. Hilliard, H. Ghnaim and M. Berger, “Inflammatory Cytokines in Cystic Fibrosis Lungs,” American Journal of Respiratory and Critical Care Medicine, Vol. 152, No. 6, 1995, pp. 2111-2118.
- [63] S. S. Hacievliyagil, H. Gunen, L. C. Mutlu, A. B. Karabulut and İ. Temel, “Association between Cytokines in Induced Sputum and Severity of Chronic Obstructive Pulmonary Disease,” Respiratory Medicine, Vol. 100, No. 5, 2006, pp. 846-854. doi:10.1016/j.rmed.2005.08.022
- [64] W. A. Neveu, J. L. Allard, D. M. Raymond, L. M. Bourassa, S. M. Burns, J. Y. Bunn, C. G. Irvin, D. A. Kaminsky and M. Rincon, “Elevation of IL-6 in the Allergic Asthmatic Airway Is Independent of Inflammation But Associates with Loss of Central Airway Function,” Respiratory Research, Vol. 11, No. 1, 2010, p. 28. doi:10.1186/1465-9921-11-28
- [65] S. K. Shahid, S. A. Kharitonov, N. M. Wilson, A. Bush and P. J. Barnes, “Increased Interleukin-4 and Decreased Interferon-γ in Exhaled Breath Condensate of Children with Asthma,” American Journal of Respiratory and Critical Care Medicine, Vol. 165, No. 9, 2002, pp. 1290- 1293. doi:10.1164/rccm.2108082
- [66] P. P. R. Rosias, E. Dompeling, M. A. Dentener, H. J. Pennings, H. J. E. Hendriks, M. P. A. Van Iersel and Q. Jöbsis, “Childhood Asthma: Exhaled Markers of Airway Inflammation, Asthma Control Score, and Lung Function Tests,” Pediatric Pulmonology, Vol. 38, No. 2, 2004, pp. 107-114. doi:10.1002/ppul.20056
- [67] C. M. H. H. T. Robroeks, Q. Jöbsis, J. G. M. C. Damoiseaux, P. H. M. Heijmans, P. P. R. Rosias, H. J. E. Hendriks and E. Dompeling, “Cytokines in Exhaled Breath Condensate of Children with Asthma and Cystic Fibrosis,” Annals of Allergy, Asthma & Immunology, Vol. 96, No. 2, 2006, pp. 349-355. doi:10.1016/S1081-1206(10)61247-1
- [68] P. P. Rosias, C. M. Robroeks, A. Kester, G. J. den Hartog, W. K. Wodzig, G. T. Rijkers, L. J. Zimmermann, C. P. van Schayck, Q. Jöbsis and E. Dompeling, “Biomarker Reproducibility in Exhaled Breath Condensate Collected with Different Condensers,” European Respiratory Journal, Vol. 31, No. 5, 2008, pp. 934-942. doi:10.1183/09031936.00073207
- [69] C. M. H. H. T. Robroeks, G. T. Rijkers, Q. Jöbsis, H. J. E. Hendriks, J. G. M. C. Damoiseaux, L. J. I. Zimmermann, O. P. van Schayck and E. Dompeling, “Increased Cytokines, Chemokines and Soluble Adhesion Molecules in Exhaled Breath Condensate of Asthmatic Children,” Clinical & Experimental Allergy, Vol. 40, No. 1, 2010, pp. 77-84. doi:10.1111/j.1365-2222.2009.03397.x
- [70] C. Gessner, R. Scheibe, M. Wötzel, S. Hammerschmidt, H. Kuhn, L. Engelmann, G. Hoheisel, A. Gillissen, U. Sack and H. Wirtz, “Exhaled Breath Condensate Cytokine Patterns in Chronic Obstructive Pulmonary Disease,” Respiratory Medicine, Vol. 99, No. 10, 2005, pp. 1229- 1240. doi:10.1016/j.rmed.2005.02.041
- [71] Z. Zietkowski, M. M. Tomasiak, R. Skiepko and A. Bodzenta-Lukaszyk, “RANTES in Exhaled Breath Condensate of Stable and Unstable Asthma Patients,” Respiratory Medicine, Vol. 102, No. 8, 2008, pp. 1198-1202. doi:10.1016/j.rmed.2008.03.010
- [72] Z. Zietkowski, R. Skiepko, M. M. Tomasiak-Lozowska, E. Zietkowska and A. Bodzenta-Lukaszyk, “Eotaxin in Exhaled Breath Condensate of Allergic Asthma Patients with Exercise-Induced Bronchoconstriction,” Respiration, Vol. 82, No. 2, 2011, pp. 169-176. doi:10.1159/000323180
- [73] K. D. G. van de Kant, E. M. M. Klaassen, Q. Jöbsis, K. Koers, G. T. Rijkers, C. P. van der Grinten, O. C. P. van Schayck, V. L. Passos and E. Dompeling, “Wheezing in Preschool Children Is Associated with Increased Levels of Cytokines/Chemokines in Exhaled Breath Condensate,” The Journal of Allergy and Clinical Immunology, Vol. 126, No. 3, 2010, pp. 669-671. doi:10.1016/j.jaci.2010.07.013
- [74] T. Zakharkina, A.-R. Koczulla, O. Mardanova, A. Hattesohl and R. Bals, “Detection of Microorganisms in Exhaled Breath Condensate during Acute Exacerbations of COPD,” Respirology, Vol. 16, No. 6, 2011, pp. 932-938. doi:10.1111/j.1440-1843.2011.01977.x
- [75] Z. Xu, F. Shen, X. Li, Y. Wu, Q. Chen, X. Jie and M. Yao, “Molecular and Microscopic Analysis of Bacteria and Viruses in Exhaled Breath Collected Using a Simple Impaction and Condensing Method,” PLoS ONE, Vol. 7, No. 7, 2012, Article ID: e41137. doi:10.1371/journal.pone.0041137
- [76] G. E. Carpagnano, A. Koutelou, M. I. Natalicchio, D. Martinelli, C. Ruggieri, A. Di Taranto, R. Antonetti, F. Carpagnano and M. P. Foschino-Barbaro, “HPV in Exhaled Breath Condensate of Lung Cancer Patients,” British Journal of Cancer, Vol. 105, No. 8, 2011, pp. 1183- 1190. doi:10.1038/bjc.2011.354
- [77] K. Chikasue, M. Kimura, K. Ikeda, T. Ohnishi, S. Kawanishi, T. Iio, M. Kataoka and Y. Arao, “Detection of Torque Teno Virus DNA in Exhaled Breath by Polymerase Chain Reaction,” Acta Medica Okayama, Vol. 66, No. 5, 2012, pp. 387-397.
- [78] E. Sonkoly, M. Ståhle and A. Pivarcsi, “MicroRNAs and Immunity: Novel Players in the Regulation of Normal Immune Function and Inflammation,” Seminars in Cancer Biology, Vol. 18, No. 2, 2008, pp. 131-140. doi:10.1016/j.semcancer.2008.01.005
- [79] Y. Xie, N. W. Todd, Z. Liu, M. Zhan, H. Fang, H. Peng, M. Alattar, J. Deepak, S. A. Stass and F. Jiang, “Altered miRNA Expression in Sputum for Diagnosis of Non-Small Cell Lung Cancer,” Lung Cancer, Vol. 67, No. 2, 2010, pp. 170-176. doi:10.1016/j.lungcan.2009.04.004
- [80] L. Yu, N. W. Todd, L. Xing, Y. Xie, H. Zhang, Z. Liu, H. Fang, J. Zhang, R. L. Katz and F. Jiang, “Early Detection of Lung Adenocarcinoma in Sputum by a Panel of MicroRNA Markers,” International Journal of Cancer, Vol. 127, No. 12, 2010, pp. 2870-2878. doi:10.1002/ijc.25289
- [81] R. M. Effros, J. Biller, B. Foss, K. Hoagland, M. B. Dunning, D. Castillo, M. Bosbous, F. Sun and R. Shaker, “A Simple Method for Estimating Respiratory Solute Dilution in Exhaled Breath Condensates,” American Journal of Respiratory and Critical Care Medicine, Vol. 168, No. 12, 2003, pp. 1500-1505. doi:10.1164/rccm.200307-920OC
- [82] P. Montuschi, “LC/MS/MS Analysis of Leukotriene B4 and Other Eicosanoids in Exhaled Breath Condensate for Assessing Lung Inflammation,” Journal of Chromatography B, Vol. 877, No. 13, 2009, pp. 1272-1280. doi:10.1016/j.jchromb.2009.01.036
- [83] G. de Laurentiis, D. Paris, D. Melck, M. Maniscalco, S. Marsico, G. Corso, A. Motta and M. Sofia, “Metabonomic Analysis of Exhaled Breath Condensate in Adults by Nuclear Magnetic Resonance Spectroscopy,” European Respiratory Journal, Vol. 32, No. 5, 2008, pp. 1175- 1183. doi:10.1183/09031936.00072408
Abbreviations
COPD: Chronic obstructive pulmonary disease EBC: Exhaled breath condensate H2O2: Hydrogen peroxide
DNA: Deoxyribonucleic acid RNA: Ribonucleic acid MiRNAs: microRNAs ROS: Reactive oxygen species ALF: Airway lining fluid BAL: Bronchoalveolar lavage VOCs: Volatile organic compounds
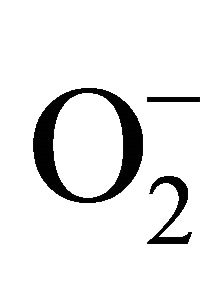 : superoxide anion
: superoxide anion
ARDS: Acute respiratory distress syndrome FeNO: Fractional excretion of NO RNS: Reactive nitrogen species NO: Nitric oxide CO2: Carbon dioxide COX: Cyclooxygenase 8: IP- 8-Isoprostane 5-LO: 5-Lipoxigenase LT: Leukotriene NSCLC: Non-small cell lung cancer CysLTs: Cysteinyl-leukotrienes EIB: Exercise induced bronchospasm HETE: Hydroxyeicosatetraenoic acid PGD2: Prostaglandin D2 EIA: Enzyme immunoassays PGE2: Prostaglandin E2 TxA2: Thromboxane A2 RIA: Radioimmunoassay Th0: Naïve T-cells Th: T helper IL: Interleukin IFN-γ: Interferon-gamma RANTES: Regulated and normal T cell expressed and secreted MIP1α: Macrophage Inflammatory Proteins1α
PCR: Polymerase chain reaction Treg: Regulatory T cells NK cells: Natural killer cells NMR: Nuclear magnetic resonance HPCL: High performance liquid chromatography GC/MS: Gas chromatography/mass spectrometry LC/MS: Liquid chromatography/mass spectrometry

