 Vol.2, No.4, 117-122 (2013) Journal of Agricultural Chemistry and Environment http://dx.doi.org/10.4236/jacen.2013.24017 Cultivation characteristics and flavonoid contents of wormwood (Artemisia montana Pamp.) Yong Joo Kim1, Jeong-Hoon Lee2*, Sun-Ju Kim1* 1Department of Bio-Environmental Chemistry, Chungnam National University, Daejeon, South Korea; 2Department of Herbal Crop Research NIHHS, RDA, Eumseong, South Korea *Corresponding Authors: kimsunju@cnu.ac.kr, artemisia@korea.kr Received 21 October 2013; revised 22 November 2013; accepted 30 November 2013 Copyright © 2013 Yong Joo Kim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The aim of this study was to establish the opti- mum harvesting time and the content of flavon- oids in the leaves, stems, and roots of Artemisia montana Pamp. A. montana was monitored from June to October in 2012. The yield of A. montana at high density (30 × 10 cm) was higher than that of A. montana at low density (30 × 20 and 30 cm). Yield in terms of dry weight was increased with an extended growth period and development stage. High yield achieved at 2580 and 2757 kg·10 a−1 in September and October, respectively. Among the leaves, stems, and underground plant organs, jaceosidin and eupatilin were mainly detected in the leaves, and the highest levels were observed in June, at values of 66.6 and 158.2 mg·100 g−1, respectively. In contrast, api- genin was the major compound detected in the underground plant organs, with levels ranging from 21.2 to 29.5 mg·100 g−1 until September. Therefore, optimal harvest times were between September and October, generating a high yield and adding economic value although a higher level of total flavonoids was observed in crops harvested in June. Keywords: Artemisia montana; Flavonoids; Harvest Time; Plant Density 1. INTRODUCTION Medicinal plants have long been recognized as natural herbs that have minimal to negligible side effects. As the culture of enhancing human well-being has gained popularity, scientists have engaged in extensive studies of medicinal plants, including studies on natural drugs, herbal cosmetics, and natural pigments [1-3]. In Korea, wormwood has been used as an herb. Ar- temisia spp. belongs to Compositae, and taxonomic es- timates have indicated that 200 to 400 species exist worldwide [4]. Approximately 40 species of Artemisia are distributed in Korea, of which 26 species are re- corded in the Color Illustrated Book about the Plants in Korea [5]. In Korean traditional medicine, Artemisia spp. is further classified as Chung-ho, Ae-yeop, In-jin, and Am-ryeo. According to the herbal pharmacopoeia, the Ae-yeop pertains to dried medicinal leaves and young stems of Artemisia argyi Lev., A. princeps var. orientalis (Pamp.) Hara., and A. montana Pamp. Ae-yeop has been used as a medicinal herb [6]; it imparts warmth to the body and controls blood circulation, body temperature, bleeding, and pregnancy. It has also been used as a rem- edy for abdominal pain due to complications, diarrhea, chronic diarrhea, hematemesis, epistaxis, melena, and amenorrhea [7]. Ae-yeop contains various compounds such as flavonoids, steroids, phenylpropanoids, terpe- noids, peptides, sesquiterpenoids, monoterpenoids, and diterpenoids [8,9]. Among these, flavonoids are known to possess excellent antioxidant activity that effectively eliminates reactive oxygen species, as well as a variety of other biological activities, including anti-cancer and anti-inflammatory activities [10]. The major flavonoids in Ae-yeop include eupatilin, jaceosidin, apigenin, and eupafolin [9,11]. Its pharmacological activities include anti-cancer, anti-inflammatory, anti-diabetic, and anti- allergic activities [12-15]. Eupatilin is known to have strong inhibitory effects on gastric ulcers and has been used as the main raw material for the preparation of natural drugs [16,17]. In addition, the size of A. montana commonly used as Ae-yeop is larger than the size of other wormwood species. It has also been proven to have antioxidant and anti-diabetic effects because of compo- nents such as caffeic acid, caffeoylquinic acid, catechol, *These corresponding authors contributed equally to this work. Copyright © 2013 SciRes. OPEN ACCESS  Y. J. Kim et al. / Journal of Agricultural Chemistry and Environment 2 (2013) 117-122 118 hyperoside, and protocatechuic acid making this herb a potential drug resource [18,19]. However, agronomic evaluation of this crop has not been conducted properly because of difficulties in its morphological classification among similar species. To establish “Good Agricultural Practices” (GAP), plant growth characteristics and yields of wormwood (Artemisia montana Pamp.) were evalu- ated on the basis of plant density and harvest times. Consequently, this study examined changes in the fla- vonoid (apigenin, jaceosidin, and eupatilin) (Figure 1) contents on the basis of cultivation characteristics and harvest times of A. montana, to develop an economically significant crop. 2. MATERIALS AND METHODS 2.1. Plant Materials A. montana was collected from Gyeongsangbukdo Ulleung-gun and planted in a test package at the De- partment of Herbal Crop Research of the Korean Na- tional Institute of Horticultural & Herbal Science on May 10, 2012. The plants were stored at KMRH under the Voucher number: MPS0002514 (Figure 2). The plants were grown in seedling trays containing 200 holes in a greenhouse at the beginning of March 2012. After plant- ing, the leaves were collected on the 10th day of each month from July to October to investigate the crop char- acteristics. Samples were harvested five times every month from June to October for analysis of flavonoids. The test package was prepared using 2000 kg·10 a−1 base manure and covered with black plastic bags. A random- ized complete block design was used in triplicate. For planting density, spacing between furrows and rows was 90 and 30 cm, respectively. The planting intervals were 10, 20, and 30 cm. After planting, 20 specimens were evaluated three times at 30-day intervals. For extraction, block sampling was used. The quantity was converted to the number per 10 a after harvesting in one m3 test envi- ronment. 2.2. Seed Characteristics To determine the seed characteristics of A. montana, 20 seeds were randomly selected in triplicate. The shape, size, and color of the seeds were evaluated. For 1000- seed weight, the average value of 10 measurements was calculated. For germination, seeds with uniform size and color, as well as devoid of pest contamination, were se- lected using a caliper and microscope. The selected seeds were placed in disposable petri dishes and maintained in constant-temperature incubators set at 15, 20, 25, and 30˚C. The petri dishes were lined with filter paper soaked with distilled water. Germination was defined as the emergence of young leaves and roots of approximately 1 mm in length through the seed coat. The first germina- tion time, bud burst period and germination rate were monitored. 2.3. Equipment and Reagents Flavonoid standards, jaceosidin, and eupatilin were purchased from Chengdu Biopurify Phytochemicals Ltd. (Chendu, Sichuan, China) and apigenin from Sigma- Aldrich (St. Louis, MO, USA). Seed characteristics were microscopically evaluated (Olympus SZ61; Olympus Co. Tokyo, Japan). Seed germination was monitored in a constant temperature incubator (Multi-Room Incubator, Wisecube, Wonju-si, Korea). Flavonoid analysis based on growth stage was performed using the Agilent 1100 series HPLC system (Agilent Technologies, CA, USA). 2.4. Extraction and Analysis of Flavonoids Approximately 10 g of powder was extracted from each plant organ of A. montana by using methanol (MeOH), thus generating 2.4 g from the leaves, 1.7 g from the stems, and 1.8 g from the roots. Each extract (10 mg) was placed in a 2 mL Eppendorf tube and mixed with 1 mL of MeOH. After 5 min of ultrasonic extraction, the extracts were centrifuged at 3,000 rpm at 4˚C for 5 min. The supernatant was filtered using a 0.45 µm PTFE hydrophilic syringe filter (i.d., 13 mm) and collected in a vial for HPLC. For the analysis of flavonoids, the Agilent 1100 Series HPLC system (Agilent Technologies, CA, USA) equipped with u-Bondapak TM C18 (10 µm, 3.9 × 300 mm, Wa- ters, MA, USA) was used. Detection was conducted at a wavelength of 354 nm, the flow rate was 1 mL·min−1, and the column oven temperature was set at 30˚C. Ap- proximately 20 µL of the sample was injected using an auto sampler. The mobile phase solvents used were sol- vent A [Water: H2PO4 (99.6: 0.4, v/v)] and solvent B [acetonitrile]. The gradient program used as follows: 0 - 30 min, 30% → 70% solvent B; 30 - 40 min, 70% → Figure 1. Chemical structure of flavonoids in A. montana. (a) apigenin; (b) jaceosidin; (c) eupatilin. Copyright © 2013 SciRes. OPEN ACCESS  Y. J. Kim et al. / Journal of Agr icultural Chemistry and Environment 2 (2013) 117-122 119 Figure 2. Specimen of A. montana. Horti- cultural traits: A. montana is a perennial plant that belongs to Asteraceae and has creeping roots and upright stems. The cau- line leaves with hairs are alternately ar- ranged. The size of A. montana is larger, compared to the closely related taxa. 100% solvent B; 40 - 50 min, 100% → 30% solvent B; 50 - 55 min, keep 30% solvent B. A stock solution of each flavonoid standards (apigenin, jaceosidin, and eu- patilin) was made with 1 mL of MeOH and diluted with MeOH to make 50, 100, 200, 250, and 500 µg·mL−1 for standard solutions. After taking 20 μL of each standard solution, HPLC chromatography was conducted to quan- tify each component. A calibration curve was created using the concentration of the standard solution as the variable. The linear regression equation of the calibration curve of each component was apigenin, y = 7.2412x + 8.8393; jaceosidin, y = 9.6193x + 8.8391; and eupatilin, y = 8.5583x + 6.1677. The coefficient of determination was R2 = 0.9999. By substituting the HPLC peak area analyzed in each sample for the calibration curve regres- sion equation, the amount of each compound (μg·mL−1) was calculated. By calculating the yield, the extracts were quantified (mg·100 g−1 of MeOH extracts). 2.5. Statistical Analysis Data were analyzed by the application of the Duncan’s multiple range test (DMRT, n = 3) at p ≤ 0.05 using the SAS statistical program (SAS 9.3, SAS Institute Inc., Cary, NC, USA). The F-value is the ratio of the mean square due to regression to the mean square due to error and indicates the influence (significance) of each con- trolled factor on the tested model. 3. RESULTS AND DISCUSSION 3.1. Seed Characteristics A. montana seeds were oblong in shape, and the hair- less achene was wrapped in a white fruit coat. The length, width, and 1000-seed weight were 1.37 mm, 0.52 mm, and 0.110 g, respectively (Figure 3). The first germina- tion time was 2 days at 20˚C - 30˚C. However, the first germination time was 3 days at 15˚C. The bud burst pe- riod was observed at 20˚C - 30˚C and at 15˚C were 2 days and 5 days, respectively. This trend was similar to that of A. capillaris, which is a closely related species [20]. Germination rates at 15˚C, 20˚C, 25˚C, and 30˚C were 84.7%, 90.0%, 92.7%, and 87.3%, respectively, which were slightly higher. The germination rate was the high- est at 25˚C (Figure 3). The germination rate of A. mon- tana increased up to a temperature of 30˚C, and, there- after, decreased with higher temperature. Thus, 30˚C was considered favorable for the initial germination of A. montana. Our results were consistent with the findings of Thompson [21]. Meanwhile, seed germination was close- ly related to environmental conditions, such as genetic differences, seed maturity, temperature, moisture, oxygen, and sunlight [22]. The temperature has been reported to have the greatest effect on germination rate [23,24]. Thus, when considering the seed characteristics of A. montana in this experiment, the optimum germination temperature was 25˚C. This condition can influence the distribution and seeding time of this species. 3.2. Growth Characteristics by Planting Density The growth characteristics and yields of A. montana on the basis of planting density are shown in Table 1. Plant height ranged from 168.3 to 176 cm. It appeared that a higher density was often associated with a smaller height. Such findings were contrary to the results of a few studies [3,25,26], suggesting a higher planting den- sity in Achyranthes japonica, Asparagus cochinchinensis, and Ligusticum chuanxiong. Results indicated that the heights of the plants were comparatively higher because of the competition among the species and decrease in light intensity. The results showed a similar tendency to that of Song et al. [27], who reported that a higher plant- ing density in P. sonchifolia and W. japonica led to a lesser height because of competition between species [1]. Leaf dry weight ranged from 32 to 79.3 g. A lower plant density was associated with a higher leaf dry weight. The dry weight of the aerial plant organs per 10 a was the highest in the 30 × 10 cm plots. However, no significant differences were observed between the result and plant- ing distance of 30 × 20 cm. A higher number of aerial plant organs of A. montana were associated with a Copyright © 2013 SciRes. OPEN ACCESS 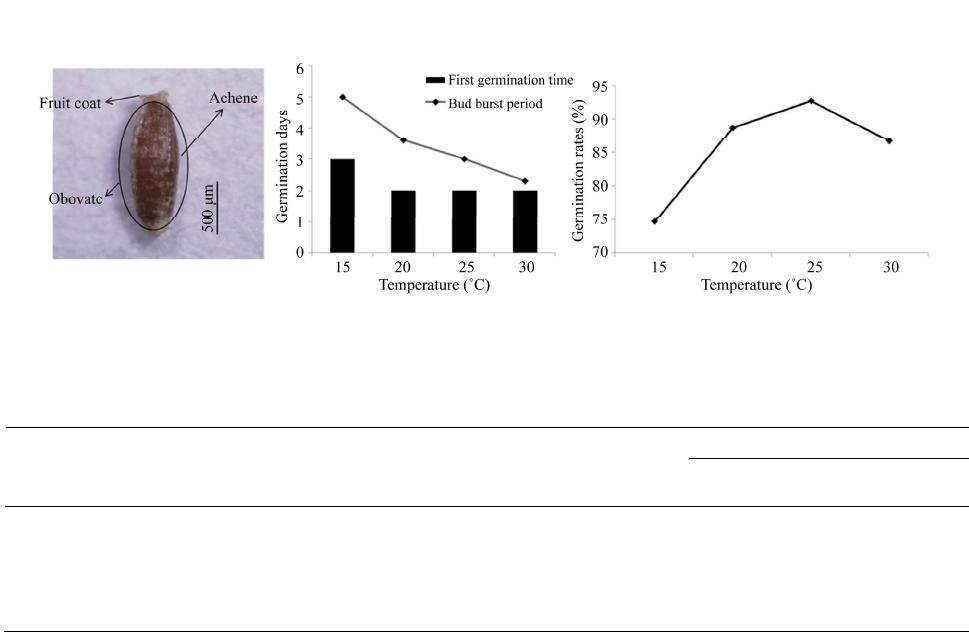 Y. J. Kim et al. / Journal of Agricultural Chemistry and Environment 2 (2013) 117-122 120 (a) (b) (c) Figure 3. Plant growth characteristics of A. montana according to different temperatures. (a) seed characteristics; (b) first germination time and bud burst period (days); (c) germination rates (%). Table 1. Plant growth characteristics and yields of A. montana according to plant density in September. Yield (kg·10 a−1, dry weight) Plant density (cm) No. of plant (ea·10 a−1) Plant height (cm) Stem diameter (mm) Leaf weight (g, dry weight) Dry weight ratio (%) Aerial part organa) Underground part organ 30 × 10 30,000 168.3 ± 4.10a 8.7 ± 1.40a 32.0 ± 1.50c 55.3a 2580 ± 224.0a 480.0 ± 91.24a 30 × 20 15,000 171.5 ± 6.17a 9.9 ± 1.87a 53.2 ± 8.14b 58.4a 2330 ± 91.65a 532.5 ± 68.74a 30 × 30 9,000 176.0 ± 6.42a 10.2 ± 170a 79.3 ± 4.25a 47.9b 1847 ± 69.18b 559.5 ± 53.31a F-value 1.41NS 0.70NS 233.53*** 12.60** 19.75** 0.93NS Within each column, values followed by the same letters in a column are not significantly different at p ≤ 0.05 (n = 3). Significance level about F-value is rep- resented at *p < 0.05; **p < 0.01; ***p < 0.001; NSnot significant. a)Aerial plant organ indicated above-ground parts including stems and leaves. greater planting density. These results were similar to that of other medicinal plants such as A. japonica, A. cochinchinensis, and L. chuanxiong [3,25,26]. 3.3. Growth Characteristics by Harvest Time Growth characteristics and yields of A. montana on the basis of harvest time were investigated using 30 × 10 cm plots (Table 2). The height sharply increased from 122.87 to 169.4 cm during the period of July-August, without showing considerable differences after August. The rainy season in July may have affected the growth of A. montana because of the sufficient water and sunlight. After the flowering period in August, growth stopped. The stem diameter was the greatest in August when the growth rate was the highest. Significant differences were observed between August and other times. Leaf dry weight was the highest in October. A longer growth pe- riod was associated with a higher yield. Dry weight ratio during the growth period decreased by 44.7% in Sep- tember and by 71.9% in July. It may be possible that af- ter the rainy season of July to August, the high moisture content caused the higher dry weight ratio; in contrast, in September the hot and dry weather caused the lower lev- els of moisture content and dry weight ratio. Since Sep- tember, the dry weight ratio has remained almost con- stant. Apparently, the reason was that the change in cli- mate after September was not significant. The dry weight of the aerial plant organs by harvest time was 2757 kg·10 a−1, which was the highest in October. No significant differences were observed between the results collected in October, 2757 kg·10 a−1, and the dry weight of the aerial plant organs harvested in September, 2580 kg·10 a−1. Thus, considering leaf dry weights and the yields, the optimal harvesting time for A. montana was the period between mid-September and early October. 3.4. Flavonoid Analysis Jaceosidin and eupatilin were detected only in the leaves, whereas apigenin was detected in the roots (Ta- ble 3). Contents of jaceosidin and eupatilin with respect to harvest time showed a similar pattern, and the contents in the leaves harvested in June were the highest levels (66.6 and 158.2 mg·100 g−1, respectively). The contents of jaceosidin and eupatilin significantly decreased in July. The contents of jaceosidin and eupatilin in the leaves of A. princeps collected in May were the highest (38.6 and 211.4 mg 100 g−1, respectively) [28]. The levels of monoterpene in A. princeps were documented the highest level in May 8, and they decreased rapidly after mid- May [29]. Our results were similar to those of previous studies. The level of apigenin in the roots ranged from 21.2 to 29.5 mg 100 g−1. The content increased from June to August and thereafter decreased. These results have also been observed in other medicinal plants such as A. Copyright © 2013 SciRes. OPEN ACCESS  Y. J. Kim et al. / Journal of Agr icultural Chemistry and Environment 2 (2013) 117-122 121 Table 2. Growth characteristics and yields of A. montana in different harvest times. Harvest times Plant height (cm) Stem diameter (mm) Leaf weight (g, dry weight) Dry weight ratio (%) Ratio of leaf weight (%) Aerial part organa) (kg·10 a−1, dry weight) July 122.8 ± 0.23b 8.9 ± 0.59b 13.5 ± 0.68c 28.1c 43.3a 937.0 ± 15.10c August 169.4 ± 0.42a 11.7 ± 0.47a 26.0 ± 2.53b 35.1b 31.7b 2,459 ± 62.45b September 168.3 ± 0.10a 8.7 ± 1.40b 32.0 ± 1.50ab 55.3a 37.2b 2,580 ± 224.0ab October 165.4 ± 1.27a 9.4 ± 1.04b 39.3 ± 7.66a 51.6a 42.8a 2,757 ± 137.2a F-value 187.31*** 6.15* 21.92*** 109.72*** 10.46** 115.73** Within each column, values followed by the same letters in a column are not significantly different at p ≤ 0.05 (n = 3). Significance level about F-value is rep- resented at *p < 0.05; **p < 0.01; ***p < 0.001; NSnot significant. a)Aerial plant organ indicated above-ground parts including stems and leaves. Table 3. Flavonoid contents (mg·100 g−1, n = 3) in MeOH extracts of A. montana harvested at different development stages from June to October. Harvest times Parts Apigenin Jaceosidin Eupatilin Roots 21.2 ± 0.40 NDa) ND Stems tr.b) ND ND June Leaves ND 66.6 ± 2.18 158.2 ± 15.5 Roots 24.8 ± 0.97 ND ND Stems TR ND ND July Leaves ND 4.5 ± 0.32 14.6 ± 0.26 Roots 29.5 ± 0.24 ND ND Stems TR ND ND August Leaves ND 17.6 ± 0.32 5.3 ± 0.11 Roots 25.7 ± 0.18 ND ND Stems TR ND ND September Leaves ND 0.9 ± 0.05 8.8 ± 0.10 Roots 11.2 ± 0.12 ND ND Stems TR ND ND October Leaves ND 2.1 ± 0.06 11.0 ± 0.10 a)ND, not detected. b)tr., trace amounts. capillaris. Capillarisin content in A. capillaris increased until flowering time and thereafter decreased [30]. After August, which was the flowering time of A. montana, certain ingredients in the underground plant organs de- creased. The levels of active ingredients are influenced by cli- matic conditions, including precipitation and temperature. For A. princeps, whose Ae-yeop was used as herbal drugs, samples collected from April to May showed excellent antioxidant effects. The samples collected from August to September showed high antimicrobial activity [31]. Thus, according to the results of this study, A. montana had the potential of serving as natural drugs, and farm- ers may thus consider wormwood as an economically beneficial crop. REFERENCES [1] Byeon, H.S., Heo, S.J., Lim, S.J. and Seo, J.S. (2004) Effect of planting density on growth and yield of Wasabia japonica Matsum. Korean Journal of Medicinal Crop Science, 12, 300-303. [2] Hwang, T.E. (2009) Changes in antioxidant activity dur- ing growth of Artemisia iwayomogi. Korean Journal of Medicinal Crop Science, 17, 286-292. [3] Kim, D.H., Park, C.B. and Kim, J.Y. (2010) Effect of propagation method, planting density, amount of nitrogen fertilizer and cropping years on growth and yield of As- paragus cochinchinensis (Lour.) Merr. Korean Journal of Medicinal Crop Science, 18, 93-97. [4] Lee, J.H., Park, C.B., Park, C.G. and Moon, S.G. (2010) A phylogenetic analysis of Korean artemisia L. based on ITS sequences. The Plant Resources Society of Korea, 23, 293-302. [5] Lee, C.B. (2003) Color illustrated book about the plants (Second). Hyangmunsa, Seoul, 373-374. [6] Korea Food & Drug Administration (2013) National stan- dard of traditional medicinal (herbal and botanical) mate- rials. Shinilbooks, 125-126. [7] Jung, B.S. and Sin, M.G. (1990) Herb medicine diction- ary (plant). Yeongrimsa, Jeonju, 1014-1015. Copyright © 2013 SciRes. OPEN ACCESS  Y. J. Kim et al. / Journal of Agricultural Chemistry and Environment 2 (2013) 117-122 122 [8] Ryu, S.N. (2008) Bioactive constituents and utilities of Ganghwayakssuk (Artemisia princeps Pamp.). The Ko- rean Society International Agriculture, 20, 308-314. [9] Ganghwagun (2007) Artemisia princeps in Ganghwa. Academy, Incheon, 51-56. [10] Kim, E.J., Choi, J.Y., Yu, M.R., Kim, M.Y., Lee, S.H. and Lee, B.H. (2012) Total polyphenols, total flavonoid con- tents, and antioxidant activity of Korean natural and me- dicinal plants. Korean Journal of Food Science and Te- chnology, 44, 337-342. http://dx.doi.org/10.9721/KJFST.2012.44.3.337 [11] Bang, M.H., Kim, D.H., Yoo, J.S., Lee, D.Y., Song, M.C., Yang, H.J., Jeong, T.S., Lee, K.T., Choi, M.S., Chung, H.C. and Baek, N.I. (2005) Development of biologically active compounds from edible plant sources XIV. Isola- tion and identification of flavonoids from the aerial parts of sajabalssuk (Artemisia herba). The Korean Society for Applied Biological Chemistry, 48, 418-420. [12] Kim, E.Y. and Kim, A.K. (2011) Enhancement of TRAIL- induced apoptosis in human hepatocellular carcinoma cells by apigenin. Yakhak Hoeji, 55, 49-55. [13] Jeong, M.A., Lee, K.W., Yoon, D.Y. and Lee, H.J. (2007) Jaceosidin, a phamacologically active flavone derives from Artemisia argyi, inhibits phorbol-ester-induced up- regulation of COX-2 and MMP-9 by blocking phospho- rylation of ERK-1 and -1 in cultured human mammary epithelial cells. New York Academy of Science, 1095, 458- 466. [14] Kang, Y.J., Jung, U.J., Lee, M.K., Kim, H.J., Jeon, S.M., Park, Y.B., Chung, H.G., Baek, N.I., Lee, K.T., Jeong, T.S. and Choi, M.S. (2008) Eupatilin, isolated from Artemisia princeps Pampanini, enhances hepatic glucose metabo- lism and pancreatic β-cell function in type 2 diabetic mice. Diabetes Research and Clinical Practice, 82, 25-32. http://dx.doi.org/10.1016/j.diabres.2008.06.012 [15] Lee, S.H., Bae, E.A., Park, E.K., Shin, Y.W., Baek, N.I., Han, E.J., Chung, H.G. and Kim, D.H. (2007) Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps in IgE-induced hypersensitivity. International Immunopharmacology, 17, 678-684. [16] Ryu, S.N., Han, S.S., Kim, K.S. and Jeong, H.G. (2006) Chemical component of mugwort (Artemisia princeps Pampanini) leaves collected in Korea. Korean Journal of Crop Science, 51, 220-223. [17] Oh, T.Y., Ahn, B.O., Ko, J.I., Ryu, B.K., Son, M.W., Kim, S.H., Kim, W.B. and Lee, E.B. (1997) Studies on protec- tive effect of DA-9601 an Artimisiae herba extract, against ethanol-induced gastric mucosal damage and its mecha- nism. The Korean Society of Applied Pharmacology, 5, 202-210. [18] Lee, G.D., Kim, J.S., Bae, J.O. and Yoon, H.S. (1992) Antioxidative effectiveness of water extract and ether ex- tract in wormwood (Artemisia montana Pampan.). The Korean Society of Food Science and Nutrition, 21, 17-22. [19] Jung, H.A., Islam, M.D.N., Kwon, Y.S., Jin, S.E., Son, Y.K., Park, J.J., Sohn, H.S. and Choi, J.S. (2011) Extrac- tion and identification of three major aldose reductase in- hibitors from Artemisia montana. Food and Chemical Toxicology, 49, 376-384. http://dx.doi.org/10.1016/j.fct.2010.11.012 [20] Lim, J.R., Choo, B.K., Park, C.B., Kim, D.H., Choi, J.S. and Choi, Y.G. (2004) Germination and growth character- istics in different sowing date of Artemisia capillaris Thunb. Korean Journal of Medicinal Crop Science, 12, 295-299. [21] Thompson, P.A. (1970) Characterization of the germina- tion response to temperature of dpecies and ecotypes. Nature, 225, 827-831. http://dx.doi.org/10.1038/225827a0 [22] Hwang, I.S., Yoo, J.H., Seong, E.S., Lee, J.G., Kim, H.Y., Kim, N.J., Lee, J.D., Ham, J.K., Ahn, Y.S., Kim, N.Y. and Yu, C.Y. (2012) The effect of temperature and seed soak- ing on germination in Cynanchum wilfordii (Maxim.) Hemsl. Korean Journal of Medicinal Crop Science, 20, 136-139. http://dx.doi.org/10.7783/KJMCS.2012.20.2.136 [23] Heydecker, W. (1977) Stress and seed germination: An agronomic view. In: Elsevier, A.K., Ed., The Physiology and Biochemistry of Seed Dormancy and Germination, North Holland and Biomedical Press, Amsterdam, 237- 282. [24] Bewley, J.D. and Black, M. (1982) Physiology and bio- chemistry of seeds in relation to germination. 2nd edition, Springer-Verlag press, New York, 365. [25] Kim, G.C., Im, D.J., Yu, H.S. and Lee, S.T. (1994) Effect of planting density on the growth and yield of Ligusticum chuanxiong Hort. Korean Journal of Medicinal Crop Sci- ence, 12, 26-31. [26] Kim, M.S., Chung, B.J., Park, G.C., Park, T.D., Kim, S.C. and Shim, J.H. (1998) Effect of planting density on the growth characteristics and root yield of Achyranthes ja- ponica N. Korean Journal of Medicinal Crop Science, 6, 137-141. [27] Song, I.G., Ho, Q.S., Hwang, S.G., Yun, J.S., Choi, I.S., Lee, C.H. and Lee, J.K. (1997) Effects of planting data and planting density on growth and tuber yield of yacon in the middle region. The Plant Resources Society of Ko- rea, 10, 17-23. [28] Ryu, S.N., Han, S.S., Yang, J.J., Jeong, H.G. and Kang, S.S. Variation of eupatilin and jaceosidin content of mug- wort. The Korean Society of Crop Science, 50, 204-207. [29] Kim, J.H. (1996) Seasonal variation in concentration and composition of monoterpenes from Artemisia princeps var. orientalis. Journal of Ecology and Environment, 19, 321-328. [30] Choi, S.R., Ju, I.O., You, D.H., Song, Y.E., Jang, I. and Ryu, J. (2007) Changes of major components and growth characteristics according to harvesting times of Artemisia capillaris Thunberg. Korean Journal of Medicinal Crop Science, 15, 189-193. [31] Yun, K.W., Jeong, H.J. and Kim, J.H. (2008) The influ- ence of the growth season on the antimicrobial and anti- oxidative activity in Artemisia princeps var. orientalis. Industrial Crops and Products, 27, 69-74. http://dx.doi.org/10.1016/j.indcrop.2007.07.017 Copyright © 2013 SciRes. OPEN ACCESS
|