 Open Journal of Obstetrics and Gynecology, 2013, 3, 51-60 OJOG http://dx.doi.org/10.4236/ojog.2013.39A007 Published Online November 2013 (http://www.scirp.org/journal/ojog/) Ex Utero intrapartum treatment (EXIT) Srinivas Pentyala1,2,3,4*, Aleef Rahman1,4, Pooja Mysore1, Sahana Pentyala1, Kyle Urbanczyk1, Thomas Tumillo1, John Muller1, Yimei Miao2, Sardar Khan2 1Department of Anesthesiology, Stony Brook Medical Center, Stony Brook, USA 2Department of Urology, Stony Brook Medical Center, Stony Brook, USA 3Department of Health Sciences, Stony Brook Medical Center, Stony Brook, USA 4Department of Physiology & Biophysics, Stony Brook Medical Center, Stony Brook, USA Email: *srinivas.pentyala@stonybrook.edu Received 25 September 2013; revised 22 October 2013; accepted 30 October 2013 Copyright © 2013 Srinivas Pentyala et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The anes t hes ia ex u t er o intrapartum treatment (EXIT) procedure is a specialized surgical procedure used to deliver babies who have airway compression due to cystic adenomatoid malformation, bronchopulmon- ary sequestration, cervical teratomas, or other con- genital conditions. EXIT is erroneously known as a routine cesarean section (CS), but is rather an exten- sion of CS with discernible differences. The proce- dure creates an opening in the anesthetized abdomen of the mother and uterus. Once EXIT is complete, the remainder of the CS proceeds. EXIT is much more complex than a routine CS, as it requires coordina- tion between the mother and a multidisciplinary team of surgical and neonatal personnel. This review high- lights current anesthetic concepts during the EXIT procedure. Keywords: Caesarean Section; Airway; Vaginal Birth; Anesthesia; Ex Utero Intrapartum Treatment; EXIT 1. INTRODUCTION As scheduled cesarean sections (CS) become safer, there has been a movement to perform CS upon maternal re- quest [1,2]. Vaginal birth after caesarean (VBAC) is not associated with increased risk of maternal or neonatal mortality and has contributed to the increase in CS pro- cedures in recent years [3-7]. However, a mother may refuse to undergo a CS in most countries. In the CS pro- cedure, laparotomy occurs through a surgical incision made in the abdomen followed by a similar hysterotomy for the uterus. A hysterectomy consists of a CS followed by the removal of the uterus. There are several ways to perform CS. The traditional method involves a midline longitudinal incision. However, it is rarely performed to- day, as it is more prone to complications. Instead, the lower uterine segment section (USS) is used through a transverse cut just above the edge of the bladder, which results in fewer complications. USS may be done in cases of extreme blood loss or when the placenta is in- separable from the uterus. Repeat CS can occur and is typically performed through the old scar. Regional anes- thesia is frequently delivered and general anesthesia is reserved for high risk cases or emergencies. However, the overall risks of general anesthesia for mother and baby are still extremely small. Recent studies did not link epidural anesthesia with any type of labor failure leading to CS, but the general medical practice is to use labor induction drugs after anesthesia to counteract sedative effects [8]. In terms of proper ex utero intrapartum treat- ment (EXIT) procedure, the infant is delivered attached to the umbilical cord and the placenta, while a surgeon establishes the airway to allow the infant to breathe. Once EXIT comes to completion, the umbilical cord can be clamped and the infant is delivered. The remainder of the CS proceeds, as EXIT requires coordination between the mother and specialists operation. The difficulty lies in preserving enough blood flow through the umbilical cord and protecting the placenta to avoid contractions of the uterus. The basic principles of EXIT were developed for the initial purpose of reverse tracheal occlusion of the infant, especially for cases of severe congenital diaphragmatic hernia (CDH). EXIT provides the advantage of uteropla- cental gas exchange but on placental support. Through the early development of EXIT, additional principles were gathered, which have improved outcomes, most no- tably in cases of airway obstruction. The prodroms of EXIT have expanded and now include giant fetal neck masses, lung or mediastinal tumors, congenital high air- way obstruction syndrome, and EXIT to extracorporeal *Corresponding author. OPEN ACCESS  S. Pentyala et al. / Open Journal of Obstetrics and Gynecology 3 (2013) 51-60 52 membrane oxygenation (ECMO) [9]. The EXIT procedure encompasses situations in which obstruction is already anticipated. Not only is EXIT use- ful in CDH with intrauterine tracheal occlusion, but ad- ditional indicators have been proposed. Reports of cases, which utilized the EXIT procedure, varied, but stress the importance of combining fetal ultrasound (US) and mag- netic resonance imaging (fMRI) in the characterization of cervical masses and usefulness in programming the procedure with a multidisciplinary team. For instance, anesthesia of the mother can be induced with thiopental, succinylcholine, and fentanyl followed with intubation, and maintained with isoflurane and nitrous oxide [10]. Any abdominal midline incision should be followed with a low transverse incision of the uterus. Immediate laryn- goscopy is a main indicator of an administered tracheo- stomy. Surfactant can be given after ventilation to facili- tate compliant delivery [11]. After reducing the concen- tration of anesthetic, administration of oxytocin can help with uterine contractility establishment and avoidance of uterine atony in the postoperative period. Case reports indicated the procedure of EXIT to ECMO for a fetus with CDH and cardiac defect, con- genital high airway obstruction syndrome, resection of congenital cystic adenomatoid malformation of the lung on uteroplacental bypass, unilateral pulmonary agenesis, and thoracoomphalopagus conjoined twins. The average duration of uteroplacental bypass was 14.7-min to 30.3- min, during which hemodynamic instability is not re- corded by fetal heart rate, pulse oximeter, or fetal echo- cardiography, except in rare cases. Blood loss through the mother is on average 574.1-mL to 848.3-mL [12]. In selected groups of infants with CDH, tracheal occlusion is still recommended to obstruct the normal flow of lung fluid and to stimulate lung expansion and growth. The solution is to arrange delivery to allow the occlusion to be removed and the airway secured, if the uterus is to be kept relaxed and the uteroplacental blood flow intact. The technique of tracheal occlusion remains under study in clinical trials [13]. Recent treatment of CDH suggests onset within 24 hrs of life and has likewise been a main concern. However, the use of modalities is dependent on the situation of each institution. Permissive hypercapnea respiration aims to avoid barotraumas in aspiration and has reportedly improved outcomes [14]. In terms of EXIT to ECMO, infants usually pass ventilation trials, but require ECMO within 48-hr before delivery. The overall survival mo- rality of EXIT to ECMO is suggested to be around 64% [15]. With the assumption of severe CDH, EXIT to ECMO is associated with infants expected to have a poor prognosis under conventional techniques. In addition, it is reported through EXIT that postpartum wound com- plications are increased to around 15% with no effect on the rate of endometritis. In addition, there is no differ- ence in hematocrit level change or postpartum hospital stay [16]. The presence of large fetal neck masses is one of the causes of airway obstructions. The relationship of the neck mass to airway structures can be established with US and fMRI. The EXIT procedure can be helpful in such cases and to obtain a fetal airway [17-19]. In par- ticular cases of life-threatening fetal neck masses (con- sider CVR values between 2.1 and 4.5 at maximum size or between 1.9 and 3.6 near term) [20], EXIT with the course of diagnostic accuracy of imaging results, intra- operative complications and outcomes, can lead to poly- hydramnios as a symptom. The possibility of wedging of the lungs is almost always a sign of detectible hyperex- tension. In addition, the chance of the trachea pulled up into the neck may lead to the underestimation of the site of tracheostomy. The occurrence of polyhydramnios is noted as a result of esophageal compression [9]. Fetal fMRI provides the most accurate diagnosis in most cases while ultrasonography can be used as an al- ternative [21]. It is evident for neck areas, especially the upper respiratory tract, that EXIT procedure can be indi- cated in selected cases and include exposure and temporary obstruction of the trachea to reduce the viscera and pre- vent pulmonary hypoplasia in CDH, prenatal tracheot- omy in laryngeal atresia, and intranatal establishment of an airway in airway-obstructing embryonic tumors [21, 22]. It is necessary to utilize fMRI for evaluation of fetal neck masses prior to operation through the EXIT. With diagnosis either through fMRI, US, spin-echo, fast gra- dient-echo, or half-fourier single shot turbo spin-echo, the sequences were able to demonstrate fetal airway rela- tive to the mass. In addition, the sequences were able to give a precise definition of the mass because of larger scopes of view, which would otherwise be obtained with only fMRI as opposed to US. The fast gradient-echo se- quence is known to provide the most definition of a mass due to its decreased motion artifacts. However, fMRI brings the most essential information about the anatomy of the fetal neck masses and the adja- cent airways prior to selection for the EXIT procedure [23]. In general, fetal neck masses can present a major challenge with subsequent risks of hypoxia, brain injury, and death. A multidisciplinary approach combined with accurate imaging sequences is the main precedent of a successful outcome [24]. The EXIT procedure provides up to 1-hr of good uteroplacental support and is still a choice to secure an airway in a large neck mass [21]. Labor after CS is associated with a greater perinatal risk than CS without labor. A factor like prior VBAC is asso- ciated with a high rate of successful labor compared with patients without VBAC. For instance, US measurement of the lower uterine segment thickness is around 3.5-mm and is followed with a negative predictive value for uter- Copyright © 2013 SciRes. OPEN ACCESS  S. Pentyala et al. / Open Journal of Obstetrics and Gynecology 3 (2013) 51-60 53 ine defect risks [7]. In comparison with planned repeat low-transverse CS, VBAC is not shown to increase the risk of maternal or neonatal mortality [4]. In a study, which examined the infant risk associated with VBAC through examination of depressed Apgar score, the Ap- gar scores within 5-min suggested only insignificant dif- ferences between patients who delivered VBAC and those patients who delivered vaginally without CS. In- fants in the VBAC group were more likely to have an umbilical arterial pH of no more than 7.1. VBAC poses a low level of risk to the infant, but the potential damage in fetal acidemia is unknown [6]. In addition, there is an insignificant difference in uterine rupture or bladder in- jury and with VBAC, a risk for composite adverse ma- ternal outcome or transfusion is generally lower. Among almost all VBAC, risk for overall major ma- ternal morbidities and maternal fever is relatively low, so that physicians can make a favorable benefit-risk ratio explicit when counseling [3]. In looking at whether or not women were able to exercise informed choices to explore decisions about the method of delivery and how the choices are interpreted following the birth, expected mothers must have access to non-biased information in order to engage in a collaborative understanding with midwives and obstetricians. For women, psychological and social implications about VBAC may trump any physical concerns [1]. 2. IMAGING AND DIANOSTICS Antenatal ultrasound is commonly used to detect and surgically correct fetal masses, which requires intrapar- tum surgical intervention to save the fetus from future harm during full time birth. Specific anesthetic concepts are needed for ensuring umbilical perfusion [25]. If the diagnosis is accurately made through image sequencing, the EXIT procedure may be life-saving [26-29]. The most important concern of the anesthesiologist is the us- age of deep volatile anesthesia for a prolonged period of time in combination with any necessary intravenous in- fusions. The hemodynamical stability of the mother is the main goal. Normal blood gas values in the umbilical artery means gas exchange is not negatively affected during EXIT [30]. Epidural anesthesia with monitoring allows surgery to take place without complications [31]. In numerous ways anesthetic considerations for EXIT procedures are identical to considerations for non-ob- stetric surgery during pregnancy, including concerns for the mother, avoidance of teratogenic drugs and asphyxia, and prevention of preterm labor. Anesthetic considera- tions also depend on the location of the placenta and dis- tinct from maternal surgery for the EXIT procedure, and the infant is not considered for anesthetic interference [32]. Instead, the infant can be the primary patient along with the mother and complications can be effectively recognized, predicted, and avoided by monitoring. Moni- toring is crucial for assessing the response to corrective maneuvers [33,34]. Occasionally, the bellows may pop- off the valve, even if the gas flow is turned off. This can be due to the ventilator entering the breathing circuit through leaks in the bellows. Therefore, testing the integ- rity of the bellows is suggested to avoid complications [35]. 3. METHODOLOGY OF ANESTHESIA In addition to the usual considerations of anesthesia in obstetrics, the special considerations for the EXIT pro- cedure can be summarized to fetoplacental circulation through profound uterine relaxation and airway manipu- lations and controls [36]. As part of a planned EXIT pro- cedure, a multidisciplinary team (obstetric and surgical personnel) to care for the mother, and neonatal surgical personnel to care for the infant, are equally needed [37]. All cases require the specialist airway skills of the pedi- atric anesthetist. As part of a multidisciplinary team in- volved in EXIT, the anesthetist may be suddenly called upon to secure a compromised airway when no antenatal diagnosis has been made. Still after elective surgical ex- cision, airway compromise may occur and require inter- vention. There are several concerns to be addressed in all the postpartum, surgery, and postoperative stages, and the understanding of the techniques employed to over- come the potential difficulties are key [38]. In specific to the anesthetist, extracorporeal membrane oxygenation (ECMO) has in the past been found to significantly boost survival rates in infants with respiratory collapse cases, but there has been a decrease in the use of ECMO. In- stead, the methods of high frequency oscillatory ventila- tion (HFOV), inhaled nitric oxide (iNO), and surfactant therapy are used [39-41]. The instances of ECMO utili- zation found within the past decades are likely obsolete and unmet for instances today. Moreover, data supports the increasing trend of HFOV, iNO, and surfactant over ECMO [42]. Recent case studies of the anesthetic man- agement in high-risk EXIT cases are presented in Table 1. 4. METHODOLOGY OF FETAL SURGERY There is a misconception that the EXIT procedure is the same as a CS, but the goals of the EXIT and CS differ. For instance, CS intents to maximize the uterine to pre- vent postpartum hemorrhage and minimize transplacental diffusion to avoid neonatal depression. Whereas, EXIT aims to achieve a state of uterine hypotonia to maintain uteroplacental gas exchange, preserve uterine volume, maintain maternal blood pressure, and avoid cardiac de- pression through the appropriated level of anesthesia [9]. Copyright © 2013 SciRes. OPEN ACCESS 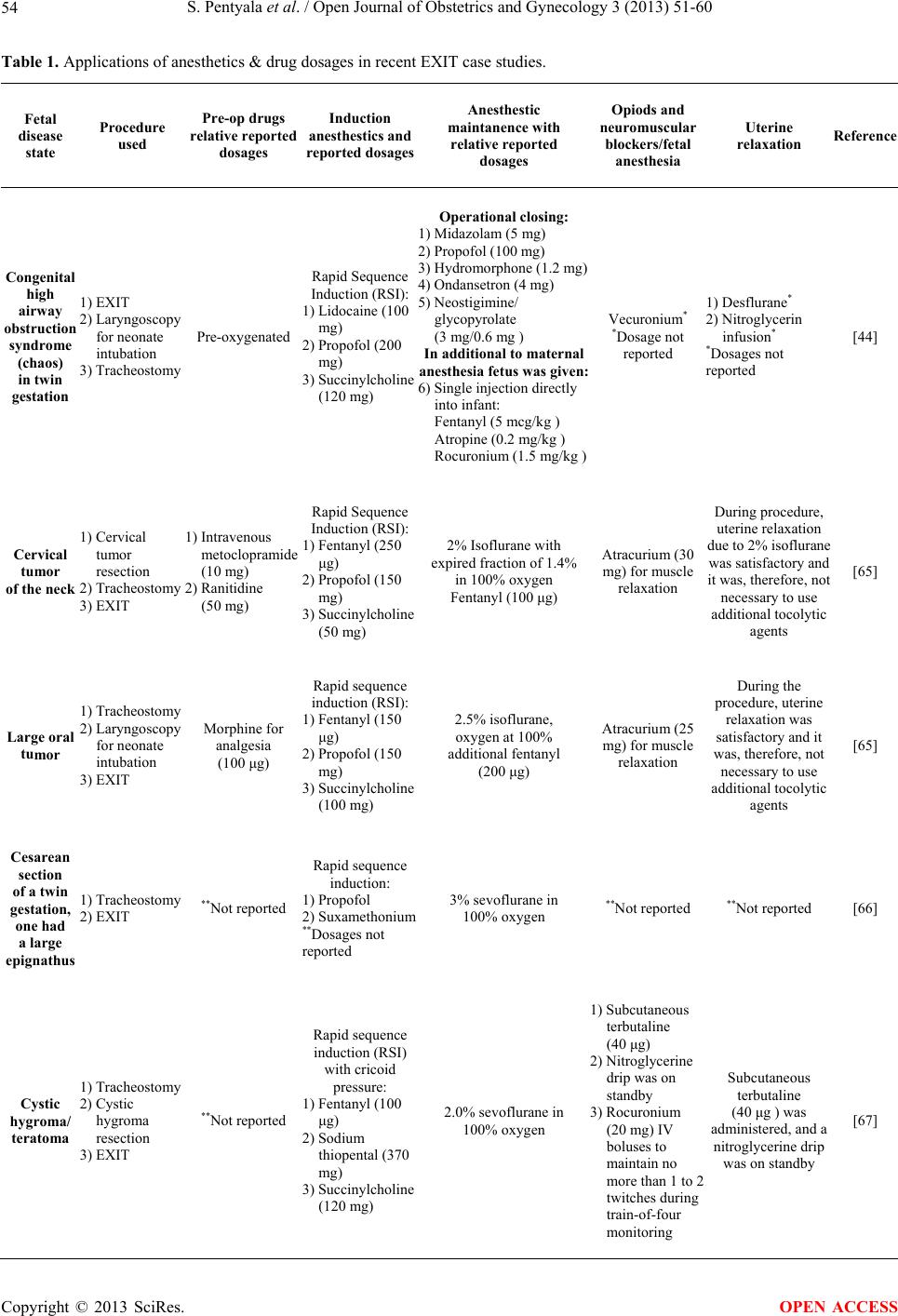 S. Pentyala et al. / Open Journal of Obstetrics and Gynecology 3 (2013) 51-60 Copyright © 2013 SciRes. 54 OPEN ACCESS Table 1. Applications of anesthetics & drug dosages in recent EXIT case studies. Fetal disease state Procedure used Pre-op drugs relative reported dosages Induction anesthestics and reported dosages Anesthestic maintanence with relative reported dosages Opiods and neuromuscular blockers/fetal anesthesia Uterine relaxation Reference Congenital high airway obstruction syndrome (chaos) in twin gestation 1) EXIT 2) Laryngoscopy for neonate intubation 3) Tracheostomy Pre-oxygenated Rapid Sequence Induction (RSI): 1) Lidocaine (100 mg) 2) Propofol (200 mg) 3) Succinylcholine (120 mg) Operational closing: 1) Midazolam (5 mg) 2) Propofol (100 mg) 3) Hydromorphone (1.2 mg) 4) Ondansetron (4 mg) 5) Neostigimine/ glycopyrolate (3 mg/0.6 mg ) In additional to maternal anesthesia fetus was given: 6) Single injection directly into infant: Fentanyl (5 mcg/kg ) Atropine (0.2 mg/kg ) Rocuronium (1.5 mg/kg ) Vecuronium* *Dosage not reported 1) Desflurane* 2) Nitroglycerin infusion* *Dosages not reported [44] Cervical tumor of the neck 1) Cervical tumor resection 2) Tracheostomy 3) EXIT 1) Intravenous metoclopramide (10 mg) 2) Ranitidine (50 mg) Rapid Sequence Induction (RSI): 1) Fentanyl (250 μg) 2) Propofol (150 mg) 3) Succinylcholine (50 mg) 2% Isoflurane with expired fraction of 1.4% in 100% oxygen Fentanyl (100 μg) Atracurium (30 mg) for muscle relaxation During procedure, uterine relaxation due to 2% isoflurane was satisfactory and it was, therefore, not necessary to use additional tocolytic agents [65] Large oral tumor 1) Tracheostomy 2) Laryngoscopy for neonate intubation 3) EXIT Morphine for analgesia (100 μg) Rapid sequence induction (RSI): 1) Fentanyl (150 μg) 2) Propofol (150 mg) 3) Succinylcholine (100 mg) 2.5% isoflurane, oxygen at 100% additional fentanyl (200 μg) Atracurium (25 mg) for muscle relaxation During the procedure, uterine relaxation was satisfactory and it was, therefore, not necessary to use additional tocolytic agents [65] Cesarean section of a twin gestation, one had a large 2) EXIT epignathus 1) Tracheostomy **Not reported Rapid sequence induction: 1) Propofol 2) Suxamethonium **Dosages not reported 3% sevoflurane in 100% oxygen **Not reported **Not reported [66] Cystic hygroma/ teratoma 1) Tracheostomy 2) Cystic hygroma resection 3) EXIT **Not reported Rapid sequence induction (RSI) with cricoid pressure: 1) Fentanyl (100 μg) 2) Sodium thiopental (370 mg) 3) Succinylcholine (120 mg) 2.0% sevoflurane in 100% oxygen 1) Subcutaneous terbutaline (40 μg) 2) Nitroglycerine drip was on standby 3) Rocuronium (20 mg) IV boluses to maintain no more than 1 to 2 twitches during train-of-four monitoring Subcutaneous terbutaline (40 μg ) was administered, and a nitroglycerine drip was on standby [67]  S. Pentyala et al. / Open Journal of Obstetrics and Gynecology 3 (2013) 51-60 55 Continued Micrognathia glossoptosi, abnormal ears with preauricular tags, and ventricular septal defect *Newborn died of sepsis and cardiac failure one month later* 1) Tracheostomy 2) EXIT Intravenous (IV) metoclopramide (10 mg) Rapid sequence induction: 1) Propofol (200 mg) 2) Remifentanil (20 μg) 3) Rocuronium (50 mg) 1) Combination of nitrous oxide (N2O)/oxygen 0.6/0.4 and remifentanil infusion (1.0 mg of remifentanil diluted in 50 mL of 0.9% NaCl) titrated by syringe pump up to 0.8 μg/kg/min 2) Repeated 1.0 mg boluses of midazolam (total 5 mg for the procedure) were used to potentiate the hypnotic and amnestic effects of the remifentanil/N2O combination 3) In addition to maternal anesthesia, fetus was given Fentanyl (30 μg) In additional to maternal anesthesia fetus was given: intramuscular injection of rocuronium (3.0 mg) Nitroglycerin (NTG) infusion was started after induction of anesthesia at 0.06 μg/kg/min and increased to 0.3 μg/kg/min [68] Cervical teratoma 1) Tracheostomy 2) EXIT Pre-oxygenated Rapid sequence induction (RSI): propofol or thiopental, fentanyl, and succinylcholine or rocuronium **Dosages not reported Sevoflurane* *Dosage not reported **Not reported Initial sevoflurane was gradually increased to 5.5% [69] Large goitre 1) Tracheostomy 2) EXIT **Not reported Spinal-epidural (CSE) L3-L4: 1) Bupivacaine (12 mg) 2) Fentanyl (15 μg) 3) Morphine (150 μg) Intrathecally Remifentanil initiated at 0.15 μg·kg−1·min−1 No muscle relaxation or anaesthesia was required for the fetus i.v. bolus of Nitroglycerine (50 μg) followed by an infusion at 50 μg·min−1 [70] Severe arthrogryposis 1) Tracheostomy 2) EXIT **Not reported Spinal-epidural (CSE) L3-L4: 1) Bupivacaine (12 mg) 2) Fentanyl (15 μg) 3) Morphine (150 μg) Intrathecally Remifentanil infusion initiated at 0.1 μg·kg−1·min−1 and titrated up to 0.15 μg·kg−1·min−1 No neuromuscular locking agents or analgesic adjuncts were needed A nitroglycerin bolus of 100 ug i.v. followed by an infusion of 50 - 100 μg·min−1 [70] Arthrogryposis with temporoman- dibular joint involvement and a hyper extended neck 1) Tracheostomy 2) EXIT **Not reported Spinal-epidural (CSE) L3-L4: 1) Bupivacaine (12 mg) 2) Fentanyl (15 μg) 3) Morphine (150 μg) Intrathecally Remifentanil infusion was also started at that time at 0.10 μg·kg−1·min−1 and titrated up to 0.2 μg·kg−1·min−1 No neuromuscular locking agents or analgesic adjuncts were needed Nitroglycerin infusion was initiated (100 μg·min−1) [70] Anterior neck mass and polyhydramnios 1) Teratoma resection from neck 2) Tracheostomy 3) EXIT **Not reported Rapid sequence induction (RSI) 1) Thiopental sodium (3 mg/kg) 2) Succinylcholine (1.5 mg/kg) 1) Sevoflurane 2 - 3 vol% 2) Nitrous oxide in oxygen (50-50) combined with 3) Dose of Remifentanil (0.1 - 0.5 μg/min/kg) and rocuronium (50 mg total) **Not reported Two boluses of intravenous Nitroglycerine (30 μg), followed by an infusion at 0.5 - 1 μg/kg/min [71] Massive obstetric hemorrhage/ polyhydramnios and a fetal cervical teratoma 1) Tracheostomy 2) EXIT 1)Pre-oxygenated 2) Oral nifedipine (20 mg every 4 hr) 3) Subcutaneous terbutaline (0.25 mg) Rapid sequence induction Propofol (180 mg) succinylcholine (100 mg) fentanyl 100 μg i.v. with cricoid pressure Sevoflurane (1.5% - 2%) was administered with 50% oxygen and 50% nitrous oxide Intermittent boluses of vecuronium Intravenous Nitroglycerin (200 μg) [72] Copyright © 2013 SciRes. OPEN ACCESS  S. Pentyala et al. / Open Journal of Obstetrics and Gynecology 3 (2013) 51-60 Copyright © 2013 SciRes. 56 OPEN ACCESS Continued Tumoral mass on the anterior neck (cervical teratoma) 1) Hysterotomy 2) Tracheostomy 3) EXIT 1) Intravenous metoclopramide (10 mg) 2) Ranitidine (50 mg) Rapid sequence induction: oxygenation with 100% under mask 1) Intravenous fentanyl (250 µg) 2) Propofol (140 mg) Isoflurane in 2.5% concentration at 3% through gauged vaporizer and administered in mixture of O2 and N2O (50%) Rocuronium (50 mg) **Not reported [73] Cavernous lymphangioma large septate mass protruding into the hypopharynx and the distal portion of the nasopharynx 1) Tracheostomy 2) EXIT **None reported 1) Thiopental (300 mg ) 2) Succinylcholine (100 mg) 3) Fentanyl (50 ug ) 0.4% - 2.5% sevoflurane in 100% oxygen **None Reported Magnesium sulfate* *Dosage not reported [74] Severe micrognathia and polyhydramnios 1) Tracheostomy 2) EXIT **None reported Rapid sequence induction: general anesthesia (with cricoid pressure) 1) Propofol, (150 mg) 2) Succinylcholine (100 mg) 1) 5% sevoflurane 2) 50% nitrous oxide & oxygen 3) Fentanyl (300 mcg) Vecuronium (5 mg) Nitroglycerin infusion (30 mcg/min) [75] In terms of preoperative considerations, the physiology of pregnancy contributes to a variety of risks. The mother is at risk for aspiration pneumonitis due to decreased pressure of the lower esophageal sphincter, the increased pressure of the gravid uterus on the stomach, and gastric acid production. In addition, the cardiovascular system takes a decrease in preload during supine positioning, and there is an expanded blood volume, lower hematocrit, and increased peripheral venous capacity. The pulmonary function likewise is affected through alterations in func- tional residual capacity, suggesting the increased chance of hypoxia. The anesthetics are used mainly to decrease myometrial tone intraoperatively, and there is an inhala- tion anesthetic regime administered. The first stage in- volves anesthesia maintenance at 0.5-MAC of desflurane, isoflurane or sevoflurane in oxygen, which is decreased to 0.2-MAC before maternal incision, then increased before hysterotomy when needed [43]. The second stage prevents uterine atony and excessive maternal bleeding through measures including decreased anesthetic to 0.5- MAC, followed by 20-U oxytocin in 500-mL saline and 10-U bolus in 1000-mL drip [9]. Before incision, a cock- tail of fentanyl, atropine, and vecuronium is administered intramuscularly to provide for postoperative care [44]. 5. EXIT INDICATIONS FROM KNOWN CONDITIONS Several conditions are likely suitable for the usage of EXIT. In rare cases if diagnosed in utero, EXIT can be performed for amniotic band syndrome (ABS), if the congenital disorder starts to cause entrapment of fetal parts. However, unless to save a limb considered in seri- ous danger of amputation or deformity, EXIT is typically not considered until identifiable vital organs or the um- bilical cord are directly affected. Cervical teratomas (CT) are difficult tumors with high mortality and morbidity. Though most tumors are benign, CT must be dealt with through timely antenatal diagnosis and care must be taken to avoid upper airway obstruction. EXIT is cited through sources to one part of a structured approach to the treatment of CT [28,38,45]. Along with EXIT, fMRI for evaluation of giant fetal mass must be used as fMRI provides the essential infor- mation prior to selection for the EXIT [21,23,24]. En- cephaloceles known to be abnormalities in the pediatric age group can likewise be treated. For the otolaryngolo- gist, encephaloceles will mostly be encountered adjacent to the brain and in the nasopharynx, which might de- velop through mastoid surgery, trauma, or infections [46]. However, it is rare for encephaloceles to occur congeni- tally, but nonetheless, instances are found in the mastoid. It is relatively more common for fronto-ethmoidal en- cephaloceles to be found in about 1 in 10 of all encepha- loceles [47]. The infant presented with cystic mediastinal mass or enlarged echogenic lungs can be treated with bronchoscopic evaluation during EXIT [15,48,49]. The presence of CDH in infants with liver herniation into the chest show prenatal repair with unsuccessful outcomes. In understanding the pathophysiology of CDH and its repair, the normal egress of fetal lung fluid enlarge the lungs and reduces herniated viscera, leading to improved pulmonary function. The development of methods to temporarily occlude the fetal trachea allows growth of the lungs and reverse obstruction, unplugging the trachea at the time of birth. Signs of improvements in the lungs in utero, with reversal of pulmonary hypoplasia, is docu-  S. Pentyala et al. / Open Journal of Obstetrics and Gynecology 3 (2013) 51-60 57 mented after birth and temporary occlusion of the trachea accelerates growth of the lungs and ameliorates the pul- monary hypoplasia associated with CDH [50]. Cases of syringomyelia with coexisting hydrocephalus establish a pathogenic relationship between several con- ditions. It is reported that hydrocephalus can aggravate conditions through the hydraulic pressure effect [51,52]. Myelomeningocele is a congenital occurrence in the backbone and spinal canal and is a type of spina bifida associated with the lack of dietary folate or neural tube defects. Detection of neural tube defects can usually be done during pregnancy by AFP screening or detailed US, among other imaging [53-55]. Intrauterine surgery for myelomeningocele has also been performed and the safety and efficacy is currently being investigated. The incidence of spina bifida can be decreased significantly with dietary folate within three months of pregnancy. Sacrococcygeal teratoma (SCT) is a tumor located at the base of the coccyx. Specifically, SCT is a type of tera- toma neoplasm belonging to a class of nonseminomatous germ cell tumor and is a result of abnormal development of pluripotent cells. SCT are idiopathic in terms of whether the condition is congenital and the pluripotent cell seem unimportant in the body [56,57]. Recent case reports should however be noted for other indications for the use of EXIT (Table 1). 6. FUTURE AND IMPROVEMENTS OF EXIT PROCEDURE There are several rules for the future success and expan- sion of the EXIT procedure. In the short-term, the most important rule is accountability. The EXIT procedure has been concluded as an optimal strategy for delivery in multiple cases. In order for the EXIT procedure to be- come permanently established, patients must be random- ized in clinical trials when applicable to demonstrate the benefits. In the long-term, it is predicted that the EXIT procedure, as now practiced, will be entirely eliminated. The uterine incisions with attendant risk and morbidity will likely be deduced or entirely minimized for less in- vasive procedures using superior imaging, instruments, and technological innovations and advances. In the same area, the developed method will likely be required to correct the specific defects with discrete interventions [58]. The randomized trails would need to be validated through outcome-based research [59,60]. Due to the rar- ity of anomalies or high mortality, it had been impracti- cal or unethical to perform clinical trials, but it has be- come imperative that EXIT can be subjected to the scru- tiny of randomized trails as found in fetoscopic laser separation cases and fetoscopic tracheal occlusion for diaphragmatic hernia. In addition, though present time fetoscopy is considered minimally invasive, there is con- sideration for treatment associated morbidity with mini- mal procedures [61,62]. Though procedures are currently performed fetoscopically, progress has been slow [63]. Ultimately, fetal imaging is the realization of immediate ultra-high resolution imaging in all aspects [58]. The pro- cession of US and fMRI technologies seems most prom- ising and the most modern is the high Tesla fMRI tech- nologies, which achieves 25-µm resolution and provides internal and external anatomy [64]. Additionally, it has been suggested that Near-Infrared Spectroscopy (NIRS) can be used in the monitoring of fetal health during the EXIT procedure considering the advantageous ability of measuring hemoglobin oxygen saturation and umbilical venous oxygenation [44]. 7. SUMMARY While EXIT procedure is being used, advances have been made in both the neonatal and uteroplacental as- pects surrounding CS. The methodology of EXIT from preoperative to postoperative care has improved drasti- cally with the additional influx of information from re- cent research. It is now definitely known that the benefits of the procedure are formulated through accuracies in imagining diagnostics and accommodations to the needs of the mother through the multidisciplinary team of spe- cialists, surgeons, and other personnel. With several con- ditions mentioned about the EXIT procedure, steps to avoid complications are known, and imperfections in the art are noted. The direction of the EXIT procedure will allow attendant risk and morbidity to be deduced, and methods will correct defects. As further information be- comes accessible, the psychosocial concerns of women about CS and EXIT procedure will likewise be addressed, assuming CS can occur with the link of anesthesia in terms of proper EXIT procedure. REFERENCES [1] Meddings, F., Phipps, F.M., Haith-Cooper, M. and Haigh, J. (2007) Vaginal birth after caesarean section (VBAC): Exploring women’s perceptions. Journal of Clinical Nurs- ing, 16, 160-167. http://dx.doi.org/10.1111/j.1365-2702.2005.01496.x [2] Srinivas, S.K., Stamilio, D.M., Sammel, M.D., Stevens, E.J., Peipert, J.F., Odibo, A.O., et al. (2007) Vaginal birth after caesarean delivery: Does maternal age affect safety and success? Paediatric and Perinatal Epidemiology, 21, 114-120. http://dx.doi.org/10.1111/j.1365-3016.2007.00794.x [3] Cahill, A.G., Stamilio, D.M., Odibo, A.O., Peipert, J.F., Ratcliffe, S.J., Stevens, E.J., et al. (2006) Is vaginal birth after cesarean (VBAC) or elective repeat cesarean safer in women with a prior vaginal delivery? American Jour- nal of Obstetrics & Gynecology, 195, 1143-1147. http://dx.doi.org/10.1016/j.ajog.2006.06.045 [4] Crawford, P. and Kaufmann, L. (2006) How safe is vagi- Copyright © 2013 SciRes. OPEN ACCESS  S. Pentyala et al. / Open Journal of Obstetrics and Gynecology 3 (2013) 51-60 58 nal birth after cesarean section for the mother and fetus? Journal of Family Practice, 55, 149. [5] Guise, J.M., McDonagh, M.S., Hashima, J., Kraemer, D.F., Eden, K.B., Berlin, M., et al. (2003) Vaginal birth after cesarean (VBAC). Evid Rep Technol Assess (Summ), 71, 1-8. [6] Martel, M.J. and MacKinnon, C.J. (2005) Guidelines for vaginal birth after previous Caesarean birth. Journal of Obstetrics and Gynaecology Canada, 27, 164-188. [7] Rozenberg, P. (2005) The counselling of patient with prior C-section. Gynecologie, Obstetrique & Fertilite, 33, 1003. http://dx.doi.org/10.1016/j.gyobfe.2005.10.001 [8] Cook, T.M. (2000) Combined spinal-epidural techniques. Anaesthesia, 55, 42-64. http://dx.doi.org/10.1046/j.1365-2044.2000.01157.x [9] Marwan, A. and Crombleholme, T.M. (2006) The EXIT procedure: Principles, pitfalls, and progress. Seminars in Pediatric Surgery, 15, 107-115. http://dx.doi.org/10.1053/j.sempedsurg.2006.02.008 [10] Garcia, P.J., Olutoye, O.O., Ivey, R.T. and Olutoye, O.A. (2011) Case scenario: Anesthesia for maternal-fetal sur- gery: The Ex Utero Intrapartum Therapy (EXIT) proce- dure. Anesthesiology, 114, 1446-1452. http://dx.doi.org/10.1097/ALN.0b013e31821b173e [11] Mota, R., Ramalho, C., Monteiro, J., Correia-Pinto, J., Rodrigues, M., Guimaraes, H., et al. (2007) Evolving in- dications for the EXIT procedure: The usefulness of com- bining ultrasound and fetal MRI. Fetal Diagnosis and Therapy, 22, 107-111. http://dx.doi.org/10.1159/000097106 [12] Bouchard, S., Johnson, M.P., Flake, A.W., Howell, L.J., Myers, L.B., Adzick, N.S., et al. (2002) The EXIT pro- cedure: Experience and outcome in 31 cases. Journal of Pediatric Surgery, 37, 418-426. http://dx.doi.org/10.1053/jpsu.2002.30839 [13] Hirose, S. and Harrison, M.R. (2003) The ex utero in- trapartum treatment (EXIT) procedure. Seminars in Neo- natology, 8, 207-214. http://dx.doi.org/10.1016/S1084-2756(03)00029-0 [14] Nio, M. (2004) Congenital diaphragmatic hernia. Kyobu Geka, 57, 800-806. [15] Kunisaki, S.M., Fauza, D.O., Barnewolt, C.E., Estroff, J.A., Myers, L.B., Bulich, L.A., et al. (2007) Ex utero in- trapartum treatment with placement on extracorporeal membrane oxygenation for fetal thoracic masses. Journal of Pediatric Surgery, 42, 420-425. http://dx.doi.org/10.1016/j.jpedsurg.2006.10.035 [16] Partridge, E.A. and Flake, A.W. (2012) Maternal-fetal surgery for structural malformations. Best Practice & Research Clinical Obstetrics & Gynaecology, 26, 669- 682. http://dx.doi.org/10.1016/j.bpobgyn.2012.03.003 [17] Gershanik, J.J., Lacassie, Y., Sargent, W., Thelin, O., Florez, L. and Dildy III, G.A. (2003) EXIT procedure: A case report. Journal of the Louisiana State Medical Soci- ety, 155, 46-50. [18] Midrio, P., Grismondi, G., Meneghini, L., Suma, V., Pit- ton, M.A., Salvadori, S., et al. (2001) The EX-utero In- trapartum Technique (EXIT) procedure in Italy. Minerva Ginecologica, 53, 209-214. [19] Stevens, G.H., Schoot, B.C., Smets, M.J., Kremer, B., Manni, J.J., Gavilanes, A.W., et al. (2002) The ex utero intrapartum treatment (EXIT) procedure in fetal neck masses: A case report and review of the literature. Euro- pean Journal of Obstetrics & Gynecology and Reproduc- tive Biology, 100, 246-250. http://dx.doi.org/10.1016/S0301-2115(01)00467-5 [20] Cass, D.L., Olutoye, O.O., Cassady, C.I., Zamora, I.J., Ivey, R.T., Ayres, N.A., et al. (2013) EXIT-to-resection for fetuses with large lung masses and persistent medi- astinal compression near birth. Journal of Pediatric Sur- gery, 48, 138-144. http://dx.doi.org/10.1016/j.jpedsurg.2012.10.067 [21] Ogamo, M., Sugiyama, T., Maeda, Y., Kusaka, H., Utsu- nomiya, H., Tsubouchi, M., et al. (2005) The ex utero in- trapartum treatment (EXIT) procedure in giant fetal neck masses. Fetal Diagnosis and Therapy, 20, 214-218. http://dx.doi.org/10.1159/000083908 [22] Wagner, W. and Harrison, M.R. (2002) Fetal operations in the head and neck area: Current state. Head & Neck, 24, 482-490. http://dx.doi.org/10.1002/hed.10083 [23] De Wilde, J., Rivers, A. and Price, D. (2005) A review of the current use of magnetic resonance imaging in preg- nancy and safety implications for the fetus. Progress in Biophysics and Molecular Biology, 87, 335-353. http://dx.doi.org/10.1016/j.pbiomolbio.2004.08.010 [24] Leva, E., Pansini, L., Fava, G., Maestri, L., Pansini, A. and Selvaggio, G. (2005) The role of the surgeon in the case of a giant neck mass in the EXIT procedure. Journal of Pediatric Surgery, 40, 748-750. http://dx.doi.org/10.1016/j.jpedsurg.2005.01.035 [25] Kill, C., Gebhardt, B., Schmidt, S., Werner, J.A., Maier, R.F. and Wulf, H. (2005) Anesthesiological management of the EXIT procedure. Case report and literature review. Anaesthesist, 54, 1105-1110. http://dx.doi.org/10.1007/s00101-005-0898-y [26] Glynn, F., Sheahan, P., Hughes, J. and Russell, J. (2006) Successful ex utero intrapartum treatment (EXIT) proce- dure for congenital high airway obstruction syndrome (CHAOS) owing to a large oropharyngeal teratoma. Irish Medical Journal, 99, 242-243. [27] Kanamori, Y., Kitano, Y., Hashizume, K., Sugiyama, M., Tomonaga, T., Takayasu, H., et al. (2004) A case of la- ryngeal atresia (congenital high airway obstruction syn- drome) with chromosome 5p deletion syndrome rescued by ex utero intrapartum treatment. Journal of Pediatric Surgery, 39, E25-E28. http://dx.doi.org/10.1016/j.jpedsurg.2003.09.041 [28] Myers, L.B., Bulich, L.A., Mizrahi, A., Barnewolt, C., Estroff, J., Benson, C., et al. (2003) Ultrasonographic guidance for location of the trachea during the EXIT procedure for cervical teratoma. Journal of Pediatric Sur- gery, 38, E12. http://dx.doi.org/10.1053/jpsu.2003.50150 [29] Shih, J.C., Hsu, W.C., Chou, H.C., Peng, S.S., Chen, L.K., Chang, Y.L., et al. (2005) Prenatal three-dimensional ul- trasound and magnetic resonance imaging evaluation of a fetal oral tumor in preparation for the ex-utero intrapar- tum treatment (EXIT) procedure. Ultrasound in Obstet- Copyright © 2013 SciRes. OPEN ACCESS  S. Pentyala et al. / Open Journal of Obstetrics and Gynecology 3 (2013) 51-60 59 rics & Gynecology, 25, 76-79. http://dx.doi.org/10.1002/uog.1791 [30] Dahlgren, G., Tornberg, D.C., Pregner, K. and Irestedt, L. (2004) Four cases of the ex utero intrapartum treatment (EXIT) procedure: Anesthetic implications. International Journal of Obstetric Anesthesia, 13, 178-182. http://dx.doi.org/10.1016/j.ijoa.2004.01.007 [31] Recasens Urbez, J., Boada Pie, S., Solsona Della, E., Saludes Serra, J., Bueno Izquierdo, J.M. and Rull Bar- tomeu, M. (2000) Elective cesarean section with epidural anesthesia in a pregnant woman with obstructive hyper- trophic myocardiopathy. Revista Española de Anestesi- ología y Reanimació n, 47, 320-322. [32] Myers, L.B., Cohen, D., Galinkin, J., Gaiser, R. and Kurth, C.D. (2002) Anaesthesia for fetal surgery. Pediat- ric Anesthesia, 12, 569-578. http://dx.doi.org/10.1046/j.1460-9592.2002.00840.x [33] Kuczkowski, K.M. (2006) Images in Anesthesia: Ex utero intrapartum treatment (exit procedure): Fetal airway man- agement. Canadian Journal of Anesthesia, 53, 1117. http://dx.doi.org/10.1007/BF03022880 [34] Lee, S.J., Ralston, H.J., Drey, E.A., Partridge, J.C. and Rosen, M.A. (2005) Fetal pain: A systematic multidisci- plinary review of the evidence. JAMA, 294, 947-954. http://dx.doi.org/10.1001/jama.294.8.947 [35] Klein, L.V. and Wilson, D.V. (1989) An unusual cause of increasing airway pressure during anesthesia. Veterinary Surgery, 18, 239-241. http://dx.doi.org/10.1111/j.1532-950X.1989.tb01078.x [36] Zadra, N., Giusti, F. and Midrio, P. (2004) Ex utero intra- partum surgery (EXIT): Indications and anaesthetic man- agement. Best Practice & Research Clinical Anaesthesi- ology, 18, 259-271. http://dx.doi.org/10.1016/j.bpa.2003.11.001 [37] Farrell, J. and Howell, L.J. (2012) An overview of surgi- cal techniques, research trials, and future directions of fe- tal therapy. Journal of Obstetric, Gynecologic, & Neona- tal Nursing, 41, 419-425. http://dx.doi.org/10.1111/j.1552-6909.2012.01356.x [38] Hullett, B.J., Shine, N.P. and Chambers, N.A. (2006) Air- way management of three cases of congenital cervical te- ratoma. Pediatric Anesthesia, 16, 794-798. http://dx.doi.org/10.1111/j.1460-9592.2006.01859.x [39] Froese, A.B. and Kinsella, J.P. (2005) High-frequency oscillatory ventilation: Lessons from the neonatal/pedia- tric experience. Critical Care Medicine, 33, S115-S121. http://dx.doi.org/10.1097/01.CCM.0000155923.97849.6D [40] Konduri, G.G. and Kim, U.O. (2009) Advances in the di- agnosis and management of persistent pulmonary hyper- tension of the newborn. Pediatric Clinics of North Amer- ica, 56, 579-600. http://dx.doi.org/10.1016/j.pcl.2009.04.004 [41] Liechty, K.W. (2010) Ex-utero intrapartum therapy. Se- minars in Fetal & Neonatal Medicine, 15, 34-39. http://dx.doi.org/10.1016/j.siny.2009.05.007 [42] Bayrakci, B., Josephson, C. and Fackler, J. (2007) Oxy- genation index for extracorporeal membrane oxygenation: is there predictive significance? Journal of Artificial Or- gans, 10, 6-9. http://dx.doi.org/10.1007/s10047-006-0359-7 [43] Schwarz, U. and Galinkin, J.L. (2003) Anesthesia for fe- tal surgery. Seminars in Pediatric Surgery, 12, 196-201. http://dx.doi.org/10.1016/S1055-8586(03)00025-8 [44] Elliott, R., Vallera, C., Heitmiller, E.S., Isaac, G., Lee, M., Crino, J., Boss, E.F. and Ishman, S.L. (2013) Ex utero in- trapartum treatment procedure for management of conge- nital high airway obstruction syndrome in a vertex/breech twin gestation. International Journal of Pediatric Otorhi- nolaryngology, 77, 439-442. http://dx.doi.org/10.1016/j.ijporl.2012.11.023 [45] De Backer, A., Madern, G.C., van de Ven, C.P., Tibboel, D. and Hazebroek, F.W. (2004) Strategy for management of newborns with cervical teratoma. Journal of Perinatal Medicine, 32, 500-508. http://dx.doi.org/10.1515/JPM.2004.122 [46] Gyure, K.A., Thompson, L.D. and Morrison, A.L. (2000) A clinicopathological study of 15 patients with neuroglial heterotopias and encephaloceles of the middle ear and mastoid region. The Laryngoscope, 110, 1731-1735. http://dx.doi.org/10.1097/00005537-200010000-00032 [47] Ba, M.C., Kabre, A., Badiane, S.B., Ndoye, N., Ly Ba, A., Gueye, E.M. and Sakho, Y. (2003) Fronto-ethmoidal en- cephaloceles in Dakar. Report of 9 cases. Dakar Medical, 48, 131-133. [48] Adzick, N.S. (2009) Management of fetal lung lesions. Clinics in Perinatology, 36, 363-376. http://dx.doi.org/10.1016/j.clp.2009.03.001 [49] Hedrick, H.L., Flake, A.W., Crombleholme, T.M., How- ell, L.J., Johnson, M.P., Wilson, R.D. and Adzick, N.S. (2005) The ex utero intrapartum therapy procedure for high-risk fetal lung lesions. Journal of Pediatric Surgery, 40, 1038-1043. http://dx.doi.org/10.1016/j.jpedsurg.2005.03.024 [50] Logan, J.W., Rice, H.E., Goldberg, R.N. and Cotton, C.M. (2007) Congenital diaphragmatic hernia: A systematic re- view and summary of best-evidence practice strategies. Journal of Perinatology, 27, 535-549. http://dx.doi.org/10.1038/sj.jp.7211794 [51] Orr, L.S., Osher, R.H. and Savino, P.J. (1978) The syn- drome of facial nevi, anomalous cerebral venous return, and hydrocephalus. Annals of Neurology, 3, 316-318. http://dx.doi.org/10.1002/ana.410030407 [52] Williams, H. (2008) A unifying hypothesis for hydroce- phalus, Chiari malformation, syringomyelia, anencephaly and spina bifida. Cerebrospinal Fluid Research, 5, 7. http://dx.doi.org/10.1186/1743-8454-5-7 [53] Ghi, T., Pilu, G., Falco, P., Segata, M., Carletti, A., Coc- chi, G., Santini, D., Bonasoni, P., Tani, G. and Rizzo, N. (2006) Prenatal diagnosis of open and closed spina bifida. Ultrasound in Obstetrics & Gynecology, 28, 899-903. http://dx.doi.org/10.1002/uog.3865 [54] Golalipour, M.J., Mansourian, A.R. and Keshtkar, A. (2006) Serum zinc levels in newborns with neural tube defects. Indian Pediatrics, 43, 809-812. [55] Kalkan, E., Karabagli, P., Karabagli, H. and Baysefer, A. (2006) Congenital cranial and spinal dermal sinuses: A Copyright © 2013 SciRes. OPEN ACCESS  S. Pentyala et al. / Open Journal of Obstetrics and Gynecology 3 (2013) 51-60 Copyright © 2013 SciRes. 60 OPEN ACCESS report of 3 cases. Advances in Therapy, 23, 543-548. http://dx.doi.org/10.1007/BF02850043 [56] Batukan, C., Ozgun, M.T., Basbug, M., Caglayan, O., Dun- dar, M. and Murat, N. (2007) Sacrococcygeal teratoma in a fetus with prenatally diagnosed partial trisomy 10q (10q24.3-->qter) and partial monosomy 17p (p13.3-->pter). Prenatal Diagnosis, 27, 365-368. http://dx.doi.org/10.1002/pd.1653 [57] Bulic-Jakus, F., Ulamec, M., Vlahovic, M., Sincic, N., Ka- tusic, A., Juric-Lekc, G., Serman, L., Kruslin, B. and Be- licza, M. (2006) Of mice and men: Teratomas and terato- carcinomas. Collegium Antropologicum, 30, 921-924. [58] Turchetti, G., Labella, B., Valdastri, P., Menciassi, A. and Dario, P. (2007) The importance of giving an alternative: The case of fetal surgery. International Journal of Heal th- care Technology and Management, 8, 250-267. http://dx.doi.org/10.1504/IJHTM.2007.013163 [59] Chervenak, F.A. and McCullough, L.B. (2009) Ethics of fetal surgery. Clinics in Perinatology, 36, 237-246. http://dx.doi.org/10.1016/j.clp.2009.03.002 [60] Flake, A.W. (2001) In utero transplantation of haemopoi- etic stem cells. Best Practice & Research Clinical Hae- matology, 14, 671-683. http://dx.doi.org/10.1053/beha.2001.0166 [61] Deprest, J., Jani, J., Lewi, L., Ochsenbein-Kolble, N., Can- nie, M., Done, E., et al. (2006) Fetoscopic surgery: En- couraged by clinical experience and boosted by instrument innovation. Seminars in Fetal & Neonatal Medicine, 11, 398-412. http://dx.doi.org/10.1016/j.siny.2006.09.003 [62] Kohl, T., Hering, R., Van de Vondel, P., Tchatcheva, K., Berg, C., Bartmann, P., Heep, A., Franz, A., Müller, A. and Gembruch, U. (2006) Analysis of the stepwise clinical introduction of experimental percutaneous fetoscopic sur- gical techniques for upcoming minimally invasive fetal cardiac interventions. Surgical Endoscopy and Other In- terventional Techniques, 20, 1134-1143. http://dx.doi.org/10.1007/s00464-005-0662-z [63] Basude, S. and Overton, T.G. (2012) Advances in fetal therapy. Obstetrics, Gynaecology & Reproductive Medi- cine, 22, 223-228. http://dx.doi.org/10.1016/j.ogrm.2012.05.005 [64] Neuman, J., Calvo-Garcia, M.A., Kline-Fath, B.M., Bitters, C., Merrow, A.C., Guimaraes, C.V. and Lim, F.Y. (2012) Prenatal imaging of amniotic band sequence: Utility and role of fetal MRI as an adjunct to prenatal US. Pediatric Radiology, 42, 544-551. http://dx.doi.org/10.1007/s00247-011-2296-8 [65] Helfer, D.C., Clivatti, J., Yamashita, A.M. and Moron, A.F. (2012) Anesthesia for ex utero intrapartum treatment (EXIT procedure) in fetus with prenatal diagnosis of oral and cervical malformations: Case reports. Brazilian Jour- nal of Anesthesiology, 62, 411-423. http://dx.doi.org/10.1016/S0034-7094(12)70141-1 [66] Kudo, T., Kimura, F., Hashimoto, H., Wada, M. and Hi- rota, K. (2012) Anesthetic management for EXIT (ex-utero intrapartum treatment) of a twin gestation: One normal and one with a large epignathus. Masui, 61, 307-310. [67] Choleva, A.J. (2011) Anesthetic management of a patient undergoing an ex utero intrapartum treatment (EXIT) procedure: A case report. Journal of the American Asso- ciation of Nurse Anesthetists, 79, 497-503. [68] Ioscovich, A., Shen, O., Sichel, J.Y., Lajos, Y., Orkin, D., Bromiker, R. and Briskin, A. (2011) Remifentanil-nitro- glycerin combination as an anesthetic support for ex utero intrapartum treatment (EXIT) procedure. Journal of Clini- cal Anesthesia, 23, 142-144. http://dx.doi.org/10.1016/j.jclinane.2009.12.014 [69] Garcia, P.J., Olutoye, O.O., Ivey, R.T. and Olutoye, O.A. (2011) Case scenario: Anesthesia for maternal-fetal sur- gery: The ex utero intrapartum therapy (EXIT) procedure. Anesthesiology, 114, 1446-1452. http://dx.doi.org/10.1097/ALN.0b013e31821b173e [70] Fink, R.J., Allen, T.K. and Habib, A.S. (2011) Remifen- tanil for fetal immobilization and analgesia during the ex utero intrapartum treatment procedure under combined spinal-epidural anaesthesia. British Journal of Anaesthe- sia, 106, 851-855. http://dx.doi.org/10.1093/bja/aer097 [71] Lee, H., Ryu, J.W., Kim, D.Y. and Lee, G.Y. (2010) An- esthetic management of the ex utero intrapartum treatment (EXIT) procedure—A case report. Korean Journal of An- esthesiology, 59, S154-S157. http://dx.doi.org/10.4097/kjae.2010.59.S.S154 [72] Butwick, A., Aleshi, P. and Yamout, I. (2009) Obstetric he- morrhage during an EXIT procedure for severe fetal air- way obstruction. Canadian Jo urnal of Anesthesia, 56, 437- 442. http://dx.doi.org/10.1007/s12630-009-9092-z [73] Bilgin, F., Cekmen, N., Ugur, Y., Kurt, E., Güngör, S. and Atabek, C. (2009) Congenital cervical teratoma: Anaes- thetic management (The EXIT Procedure). Indian Journal of Anaesthesia, 53, 678-682. [74] Cha, H.-H., Seo, E.S., Choi, S.-J., Oh, S., Roh, C. and Seo, J. (2009) The ex utero intrapartum treatment (EXIT) procedure for a fetal neck mass: A case report. Journal of Women’s Medicine, 2, 39-43. [75] Chang, L.C. and Kuczkowski, K.M. (2008) The ex utero intrapartum treatment procedure: Anesthetic considerations. Archives of Gynecology and Obstetrics, 277, 83-85. http://dx.doi.org/10.1007/s00404-007-0402-9
|