A. A. A. EL-RADY ET AL.
376
initial TOCundoped TiO 20.05 % pd doped T iO20.1 % pd doped TiO20.3% pd doped TiO2
0
10
20
30
40
50
60
TOC (ppm)
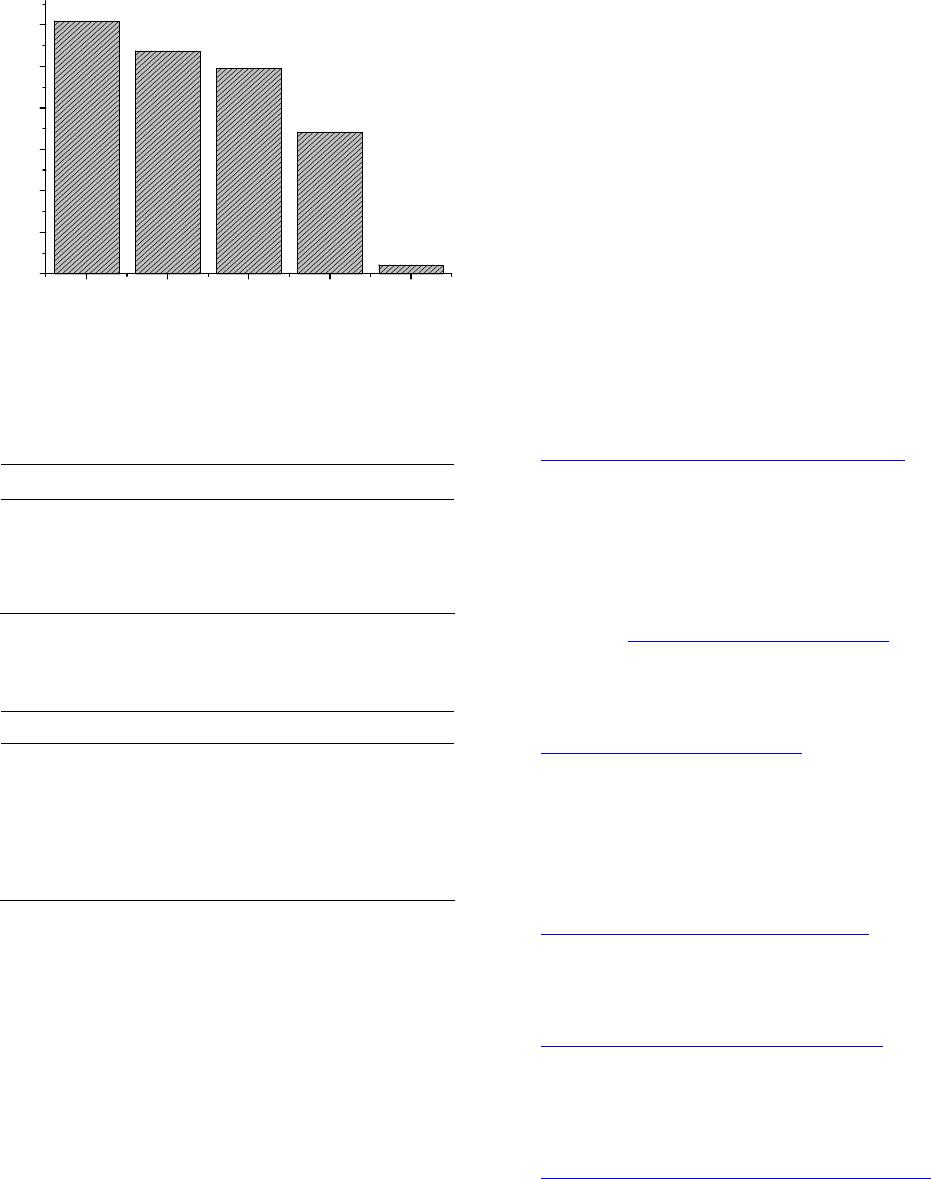
Figure 9. Removal of formic acid by the undoped TiO2,
0.05%, 0.1% and 0.3% Pd/TiO2. Catalyst wt 150 mg under
sun light irradiation.
Table 3. TOC and pH values for formic acid solution
treated by doped and undoped TiO2 nanoparticles prepared
by calcinations at 400˚C under UV irradiation for 6 hrs.
Sample Name pH TOC Degradation Rate (%)
Formic acid Solution 3.06 52.2 -
Undoped TiO2 3.3 23.6 54.8
0.05% Pd Doped TiO2 3.17 35 33
Table 4. TOC results for doped and undoped TiO2 nano-
particles annealed at 400˚C under sun light irradiation for 4
hrs.
Sample Name pH TOC Degradation Rate (%)
Formic Acid Solution 2.98 61 -
Undoped TiO2 2.99 53.8 11.8
0.05% Pd Doped TiO2 3.08 49.6 18.69
0.1% Pd Doped TiO2 3.02 34.2 43.93
0.3% Pd Doped TiO2 3.58 2.19 96.4
53.8 mg/L for 0.05%, 0.1%, 0.3% Pd doped TiO2 and
undoped TiO2, respectively under sun light irradiation
within the same time (see Table 4). The pH of the
solution also was changed from 2.98 to 3.08, 3.02, 3.58
and 2.98 for 0.05%, 0.1%, and 0.3% Pd doped TiO2 and
undoped TiO2 photocatalysts, respectively, within the
same time.
4. Conclusion
The pure and palladium doped TiO2 (Pd/TiO2) nano-
particles were prepared by the sol gel method. Samples
prepared by calcinations at 380˚C contain anatase phase
only. A mixture of anatase and rutile was obtained at
higher calcination temperatures. Doping TiO2 with
palladium in the concentration range of 0.05 to 0.3 has
no significant effect on the particle sizes and did not
result in the formation of a new crystalline phase. It was
confirmed that the incorporation of Pd in TiO2 matrix
shifts the onset wave length of absorption to higher
values (red shift). Under UV irradiation, the pure TiO2
exhibited higher efficiency than the palladium doped
TiO2 for formic acid removal from water. However,
when sun light was used as the radiation source, the
palladium doped photocatalyst exhibited higher effi-
ciency than the pure TiO2 and the photocatalytic ef-
ficiency increases with increasing palladium content up
to a concentration of 0.3% (0.3% Pd/TiO2).
REFERENCES
[1] D. A. Tryk, A. Fujishima and K. Honda, “Recent Topics
in Photoelectrochemistry: Achievements and Future Pros-
pects,” Electrochimica Acta, Vol. 45, No. 15-16, 2000, pp.
2363-2376.
http://dx.doi.org/10.1016/S0013-4686(00)00337-6
[2] M. Kunst, T. Moehl, F. Wunsch and H. Tributsch, “Opto-
electronic Properties of SnO2/TiO2 Junctions,” Super Lat-
tice Microst., Vol. 39, No. 1-4, 2006, pp. 376-380.
[3] S. M. Karvinen, “The Effects of Trace Element Doping
on the Optical Properties and Photocatalytic Activity of
Nanostructured Titanium Dioxide,” Industrial & Engi-
neering Chemistry Research, Vol. 42, No. 5, 2003, pp.
1035-1043. http://dx.doi.org/10.1021/ie020358z
[4] J. Chen, S. L. Li, Z. L. Tao, Y. T. Shen and C. X. Cui,
“Titanium Disulfide Nanotubes as Hydrogen-Storage
Materials,” Journal of the American Chemical Society,
Vol. 125, No. 18, 2003, pp. 5284-5285.
http://dx.doi.org/10.1021/ja034601c
[5] A. Fujishima, X. Zhang and D. A. Tryk, “TiO2 Photo-
catalysis and Related Surface Phenomena,” Surface Sci-
ence Reports, Vol. 63, No. 12, 2008, pp. 515-582.
[6] L. Ge and M. X. Xu, “Influences of the Pd Doping on the
Visible Light Photocatalytic Activities of InVO4-TiO2
Thin Films,” Materials Science and Engineering: B, Vol.
131, No. 1-3, 2006, pp. 222-229.
http://dx.doi.org/10.1016/j.mseb.2006.04.021
[7] B. P. Xie, Y. Xiong, R. M. Chen, J. Chen and P. X. Cai,
“Catalytic Activities of Pd-TiO2 Film towards the Oxida-
tion of Formic Acid,” Catalysis Communications, Vol. 6,
No. 11, 2005, pp. 699-704.
http://dx.doi.org/10.1016/j.catcom.2005.06.003
[8] X. Zhang, F. Zhang and K. Y. Chan, “The Synthesis of
Pt-Modified Titanium Dioxide Thin Films by Microe-
mulsion Templating, Their Characterization and Visible-
Light Photocatalytic Properties,” Materials Chemistry
and Physics, Vol. 97, No. 2-3, 2006, pp. 384-389.
http://dx.doi.org/10.1016/j.matchemphys.2005.08.060
[9] H. Li, B. L. Zhu, Y. F. Feng, S. R. Wang, S. M. Zhang
and W. P. Huang, “Synthesis, Characterization of TiO2
Nanotubes-Supported MS (TiO2NTs@MS, M = Cd, Zn)
and Their Photocatalytic Activity,” Journal of Solid State
Chemistry, Vol. 180, No. 7, 2007, pp. 2136-2142.
Open Access ANP