 Vol.3, No.4B, 8-19 (2013) Open Journal of Animal Sciences http://dx.doi.org/10.4236/ojas.2013.34A2002 Effect of sublethal doses of the insecticide imidacloprid on adaptive traits of Drosophila melanogaster: Response to treatment over and after 15 consecutive generations Thais de França Patarro1, Antonio José Manzato2, Lílian Madi-Ravazzi1, Hermione E. M. de Campos Bicudo1* 1Departmento de Biologia, Universidade Estadual Paulista-UNESP, Instituto de Biociências, Letras e Ciências Exatas-IBILCE, São José do Rio Preto, Brazil; *Corresponding Author: bicudo@ibilce.unesp.br 2Departmento de Computação, Universidade Estadual Paulista-UNESP, Instituto de Biociências, Letras e Ciências Exatas-IBILCE, São José do Rio Preto, Brazil Received 3 September 2013; revised 8 October 2013; accepted 22 October 2013 Copyright © 2013 Thais de França Patarro et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT A sublethal dose of Imidacloprid, considered actually as the most widely used insecticide against biting and sucking insects, was admin- istered to Dro sophila mel anoga ster fo r dete cting effects on biological traits. The choice of this species as organism-model potentially opens the possibility to explore more deeply the proc- esses involved in those effects because, among other reasons, there is a large accumulation of biological knowledge on this species and be- cause it propitiates multiple approaches in laboratory and nature. The flies were treated along 15 consecutive generations. F1 parents were randomly taken among virgin flies from the stocks, but the parents of the successive gen- erations were the firs t 15 couples e merged in th e previous one. The number of progeny (produc- tivity) and the duration of the emergence period were analyzed in every generation revealing in- secticide toxicity in 12 of the 15 generations. The observation of an increase in the number of progeny over the generations, which occurred in both control and treated experiments (although maintaining higher productivity in the control), suggested an effect of the use of the first 15 emerged couples in successive generations. A comparative analysis of the mortality of the F15 adult flies exposed to imidacloprid by contact, which involved flies from the control, treatment and from the stocks that originated the experi- ments, reinforced this idea, indicating a genetic interplay of the emergence speed with produc- tivity and adult tolerance to the insecticide, a subject that may be better explored in another study. Toxicity was also observed for the traits longevity, viability during development from egg to adult and oviposition rate. Considering the present intensive use of imidacloprid, the harm- ful effects observed in these important biologi- cal characteristics may be considered able to decrease the adaptive value of D. melanogaster populations exposing them at risk of decline. Keywords: Productivity; Longevity; Emergence Time Period; Egg-Adult Viability; Oviposition; Tolerance 1. INTRODUCTION Imidacloprid {1-[(6-chloro-3-pyridinyl)methyl]-N-nitro- 2-imidazolidinimine, which is registered in more than a hundred countries in the world, is considered actually the most widely used insecticide for killing sucking and bit- ing insects [1]. Currently, it is intensively used as seed treatment in citrus, cotton, fruits, grapes, potatoes, rice soybeans, sugarcane, tobacco and vegetables [2]. Several studies have provided strong evidence that, in addition to direct mortality, imidacloprid impacts popu- lations through sublethal effects. The exposure to sub- lethal doses of imidacloprid causes deleterious effects on biological traits of target and non-target organisms. De- crease of the progeny number is one of the most fre- quent effects in different organisms, but the decreases of Copyright © 2013 SciRes. OPEN AC CESS  T. de França Patarro et al. / Open Journal of Animal Sc iences 3 (2013) 8-19 9 survival rate and longevity have also been observed in some cases [3,4]. In honeybees (Apis mellifera), pro- bably the more intensively studied non-target organism, imidacloprid is considered one of the causes (or the main cause) of bee populations decline occurring since 1990 [5-8]. In addition, this insecticide has been assigned to a bee malady termed Colony Collapse Disorder (CCD) [9]. Imidacloprid is a neonicotinoid that, similarly to nico- tine, acts as an agonist at the postsynaptic acetylcholine receptor (nAChR) [10]. Like other neonicotinoids, imi- dacloprid causes persistent activation of the receptors leading to hyperexcitation and death [11]. It is effective on contact and via stomach action [12,8]. The wide use of the neonicotinoids is due to that they were considered to kill insects by paralyzing nerves but to have low toxic- ity for other animals. However, data in literature have shown that they affect organisms other than insects. For example, significant adverse effects of this insecticide have been reported in aquatic invertebrates (freshwater and estuarine/marine), being ascribed, at least partially, to its high solubility in water and moderate persistence [13]. In mammals, high doses as well low doses and long exposures were associated with degenerative changes in several organs and other health problems [14]. We used Drosophila melanogaster as organism-model to study how sublethal doses of imidacloprid affect some biological traits. The importance of using Drosophila is that it metabolizes toxic compounds in a way very simi- lar to humans and its biological characteristics favor many possibilities for methodological approaches, in laboratory and nature. Specifically D. melanogaster, with a great amount of biological information accumulated in more than a hundred years of studies, may favor the un- derstanding of new observations foreseeing the possibil- ity of a deeper study about the findings. This species has been successfully used as an organism-model for analy- sis of normal and pathological mechanisms involved with essential human biological processes, including metabolism, development and physiology [15,16]. The treatment in 15 successive generations (nine months of duration) was used to study the impact of the insecticide on a set of biological traits that are very im- portant to preserve the continuity of the species. The re- sults in D. melanogaster confirmed the harmful effects of imidacloprid and raised interesting questions to be an- swered in future works. 2. MATERIALS AND METHODS 2.1. Strain Origin and Culture in the Laboratory The strain used was Drosophila melanogaster from São José do Rio Preto, State of São Paulo, Brazil, col- lected approximately two years before the beginning of the study, and kept in the Department of Biology of the IBILCE-UNESP. The original mass crosses for preparing the stocks involved more than 20 couples collected in nature. Stocks and experiments were maintained in ba- nana-agar culture medium, at 20˚C ± 1˚C. 2.2. Experiments Imidacloprid (Confidor 700WG, Bayer, 70% imida- cloprid) was administered orally to the flies, being added to the culture medium at 5 μg/mL concentration (value corrected for the percentage contained in the product). This is a sublethal dose, since data unpublished, obtained by L. M. Ravazzi, one of the authors of this paper, for adult flies from the same D. melanogaster strain, showed LC50 value = 49.27 μg/ml water. The present study involved 15 successive generations. In each of them three replicates were prepared for control and three for treatment. Each generation started with the 15 virgin couples first emerged in the previous genera- tion, except the first generation that started with 15 virgin males and females taken randomly from the stocks. Most flies used in F2 to F15 were from the first and second days of emergence, but in some cases, flies from the third day were used to complete the 15 couples. 2.3. Adaptive T rait s Analy zed Flies treated with imidacloprid and controls were com- paratively analyzed as to their effects on the following traits: 1) productivity (number of progeny); 2) duration of the emergence period (the time elapsed between the emergence of the first and last fly); 3) longevity; 4) ovi- position rate; 5) viability from egg to adult stages and 6) mortality of adult flies. Productivity and duration of the emergence period were evaluated in each of the fifteen generations while the other traits were analyzed in the generation F15, ex- cept longevity that was analyzed in F12. 2.3.1. Productivity and Duration of the Emergence Period The number of progeny (productivity) was computed daily in the control (C) and treated (T) replicates, sepa- rately by sex, from the beginning to the end of the adults’ emergence period, in every generation till the 15th. 2.3.2. Longevity Twenty five virgin, recently emerged males and fe- males taken from F12 generation in T and C groups were transferred to tubes containing culture medium with and without the insecticide, respectively. F12 was chosen for this analysis in order to decrease the amount of simulta- neous work that would be done in F15. Dead flies were Copyright © 2013 SciRes. OPEN A CCESS  T. de F r ança Patarro et al. / Open Journal of Animal Sciences 3 (2013) 8-19 10 computed daily, separately by sex and treatment, till the last one had died. Half-life (the time required for mortal- ity of half of the total number of flies) and mean longev- ity of the flies were computed. 2.3.3. Oviposition Rate Ten virgin, three days aged males and females from each experimental groups C and T, and also from the stock that originated the experiments (S), were separately put to cross in tubes containing culture medium (without insecticide for C and S, and with it for T flies). The cou- ples were left in the tubes for 8 hours and then the fe- males were individually transferred to empty, clean tubes containing a transparent plastic tea spoon full of agar- sugar culture medium (prepared with 0.5 g agar-agar dis- solved in 100 mL of hot water and addition of 2.5 g sugar). Twenty-two hours latter, the spoons were re- moved for counting the eggs (in a stereomicroscope) and a second set of spoons was included in the same vials. The eggs in this second set of spoons were counted again after 22 h, totalizing 44 h observation. 2.3.4. Viability Egg-Adult The eggs obtained in the study of the oviposition rate were used in the analysis of viability egg-adult. After being computed for oviposition rate, the eggs from T, C and S flies were put for development in the respective culture medium and the percentage of adults obtained in relation to the initial number of eggs gave the viability egg-adult. Males and females were computed separately. 2.3.5. Mort ality of Adult Flies Exposed to Imidacloprid by Contact This experiment was done in order to detect possible changes in the degree of tolerance of the adults after 15 generations of treatment. Adult flies from C and T groups taken in F15, and S flies were put in contact with the insecticide at 10 mg/mL water concentration) imbibed in pieces of filter paper introduced in the vials. This analy- sis intended also to detect the existence of interference of the method used (selection of the first 15 couples), in the results. Ninety couples, three days old (virgin males and females), from each experimental group, were put in contact with the imidacloprid. Strips of filter paper were impregnated with the aqueous solution of the insecticide, put to air dry and placed into the tubes (9.0 cm × 6.5 cm), covering their interior. Females and males were placed separately in these tubes. The count of dead flies was done at 24 and 48 hours after exposing the flies to the impregnated paper. For making sure that the flies didn’t die due to desiccation or hunger, a piece of cotton im- bibed in aqueous solution of glucose (0.8%) was fixed in the upper part of the tube. 2.4. Statistical Analysis Statistical analysis of the data involved exploratory analysis, Student’s t-test, non-parametric test of Kruskal- Wallis, χ2 for comparison of several proportions, Tukey test for multiple comparisons of proportions two by two (involving the Tukey’s angular transformation). and the Z test with normal approximation. The statistical meth- ods used were based on [17] and [18]. The software was the Minitab Release Package 14. 3. RESULTS 3.1. Characteristics Analyzed in the 15 Generations 3.1.1. Productivity The descriptive statistics (Table 1) shows, for each generation, the data on the mean number of progeny and standard error, separately for males and females, in C and T experiments. The total number of progeny produced in both experiments was 16,620 flies. The smallest productivity was obtained in the first generation. The mean values for C and T replicates of this generation were 98.3 and 84.7, respectively, while the mean for F2 to F15 was 219.21 for C and 163.46 for T. The mean of the means of the progeny in the 15 gen- erations was: for males, C = 104.23 and T = 79.93; for females, C = 106.44 and T= 78.23; and for males plus females, C= 211.15 and T = 158.21. Student’s t-test for comparison between C and T ex- periments relative to the mean productivity of males, females and their sum, in each generation (Table 2), showed that the differences between C and T were sig- nificant for males in the generations F4 (t = 3.37, P = 0.003), F9 (t = 3.05, P = 0.039 and F12 T = 17.17, P = 0.00+ [P value close to zero]). For females, the significant differences were detected in F6 (t = 3.14, P = 0.003) and F12 (t = 5.23, P = 0.001), and for the mean total progeny (males plus females), in F1 (t = 3.04, P = 0.039, F4 (t = 2.75, P = 0.048), F6 (t = 2.80, P = 0.049), F9 (t = 2.82, P = 0.047) and F12 (t = 9.31, P = 0.000). In every case of significant difference, values in C were greater than in T. However, in the light of the numbers, male progeny was greater in C than in T in 12 of the 15 generations, female progeny, in 10, and males plus females in 12. Boxplot of data (Figure 1) showed the wide variation of the progeny number among replicates, mainly for C group. To better visualize the difference between C and T over generations the results were submitted to the statistical method of smoothing 4253H (Figure 2). With this method, significant differences on progeny number were found for males in F3 to F12, for females in F5 and F9, and for males plus females, in F6 to F11. Ex- cept in F11, the productivity was lower in T experi- Copyright © 2013 SciRes. OPEN AC CESS 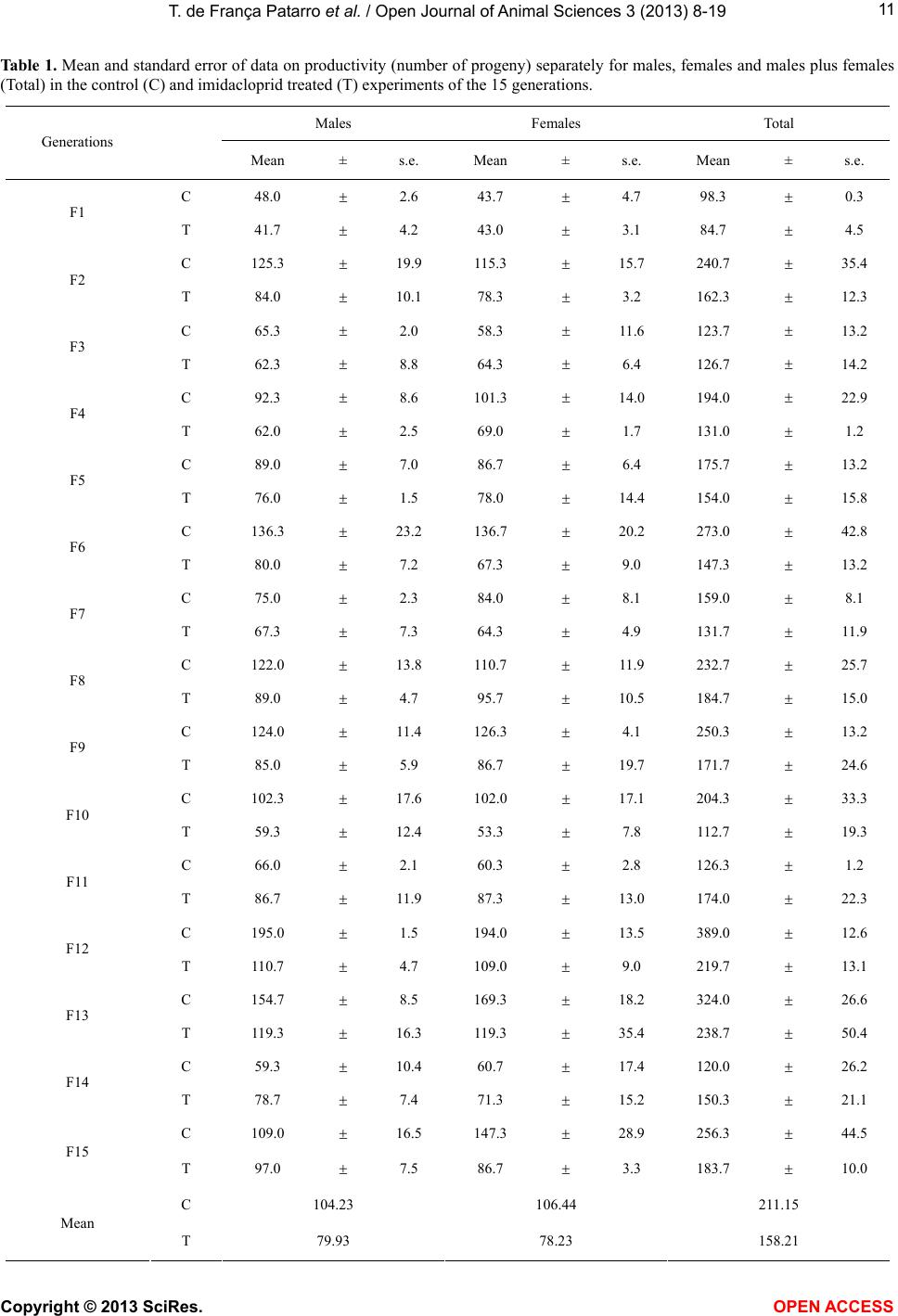 T. de F r ança Patarro et al. / Open Journal of Animal Sciences 3 (2013) 8-19 Copyright © 2013 SciRes. OPEN A CCESS 11 Tabl e 1. Mean and standard error of data on productivity (number of progeny) separately for males, females and males plus females (Total) in the control (C) and imidacloprid treated (T) experiments of the 15 generations. Males Females Total Generations Mean ± s.e. Mean ± s.e. Mean ± s.e. C 48.0 2.6 43.7 4.7 98.3 0.3 F1 T 41.7 4.2 43.0 3.1 84.7 4.5 C 125.3 19.9 115.3 15.7 240.7 35.4 F2 T 84.0 10.1 78.3 3.2 162.3 12.3 C 65.3 2.0 58.3 11.6 123.7 13.2 F3 T 62.3 8.8 64.3 6.4 126.7 14.2 C 92.3 8.6 101.3 14.0 194.0 22.9 F4 T 62.0 2.5 69.0 1.7 131.0 1.2 C 89.0 7.0 86.7 6.4 175.7 13.2 F5 T 76.0 1.5 78.0 14.4 154.0 15.8 C 136.3 23.2 136.7 20.2 273.0 42.8 F6 T 80.0 7.2 67.3 9.0 147.3 13.2 C 75.0 2.3 84.0 8.1 159.0 8.1 F7 T 67.3 7.3 64.3 4.9 131.7 11.9 C 122.0 13.8 110.7 11.9 232.7 25.7 F8 T 89.0 4.7 95.7 10.5 184.7 15.0 C 124.0 11.4 126.3 4.1 250.3 13.2 F9 T 85.0 5.9 86.7 19.7 171.7 24.6 C 102.3 17.6 102.0 17.1 204.3 33.3 F10 T 59.3 12.4 53.3 7.8 112.7 19.3 C 66.0 2.1 60.3 2.8 126.3 1.2 F11 T 86.7 11.9 87.3 13.0 174.0 22.3 C 195.0 1.5 194.0 13.5 389.0 12.6 F12 T 110.7 4.7 109.0 9.0 219.7 13.1 C 154.7 8.5 169.3 18.2 324.0 26.6 F13 T 119.3 16.3 119.3 35.4 238.7 50.4 C 59.3 10.4 60.7 17.4 120.0 26.2 F14 T 78.7 7.4 71.3 15.2 150.3 21.1 C 109.0 16.5 147.3 28.9 256.3 44.5 F15 T 97.0 7.5 86.7 3.3 183.7 10.0 C 104.23 106.44 211.15 Mean T 79.93 78.23 158.21 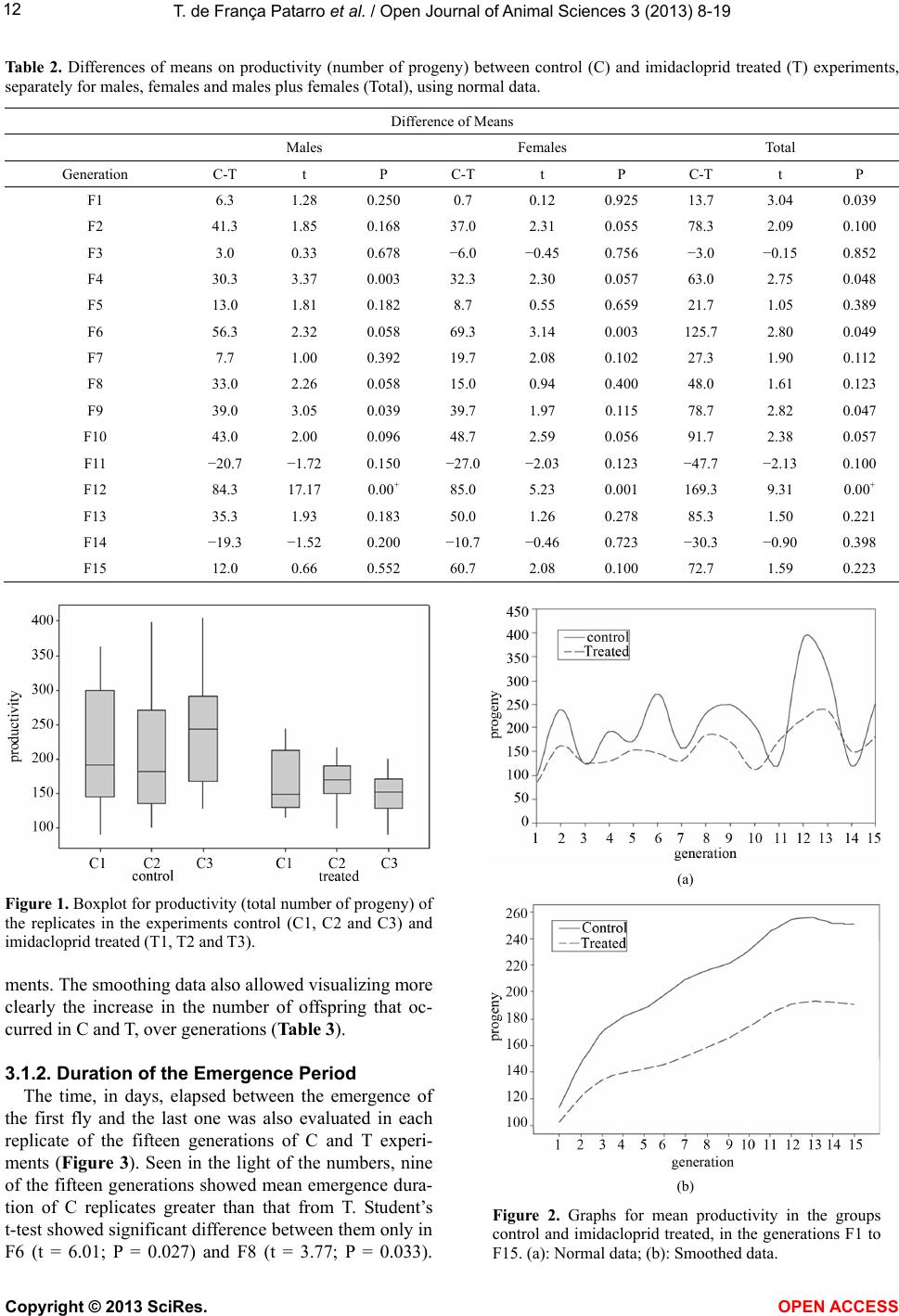 T. de F r ança Patarro et al. / Open Journal of Animal Sciences 3 (2013) 8-19 12 Ta ble 2. Differences of means on productivity (number of progeny) between control (C) and imidacloprid treated (T) experiments, separately for males, females and males plus females (Total), using normal data. Difference of Means Males Females Total Generation C-T t P C-T t P C-T t P F1 6.3 1.28 0.250 0.7 0.12 0.925 13.7 3.04 0.039 F2 41.3 1.85 0.168 37.0 2.31 0.055 78.3 2.09 0.100 F3 3.0 0.33 0.678 −6.0 −0.45 0.756 −3.0 −0.15 0.852 F4 30.3 3.37 0.003 32.3 2.30 0.057 63.0 2.75 0.048 F5 13.0 1.81 0.182 8.7 0.55 0.659 21.7 1.05 0.389 F6 56.3 2.32 0.058 69.3 3.14 0.003 125.7 2.80 0.049 F7 7.7 1.00 0.392 19.7 2.08 0.102 27.3 1.90 0.112 F8 33.0 2.26 0.058 15.0 0.94 0.400 48.0 1.61 0.123 F9 39.0 3.05 0.039 39.7 1.97 0.115 78.7 2.82 0.047 F10 43.0 2.00 0.096 48.7 2.59 0.056 91.7 2.38 0.057 F11 −20.7 −1.72 0.150 −27.0 −2.03 0.123 −47.7 −2.13 0.100 F12 84.3 17.17 0.00+ 85.0 5.23 0.001 169.3 9.31 0.00+ F13 35.3 1.93 0.183 50.0 1.26 0.278 85.3 1.50 0.221 F14 −19.3 −1.52 0.200 −10.7 −0.46 0.723 −30.3 −0.90 0.398 F15 12.0 0.66 0.552 60.7 2.08 0.100 72.7 1.59 0.223 Figure 1. Boxplot for productivity (total number of progeny) of the replicates in the experiments control (C1, C2 and C3) and imidacloprid treated (T1, T2 and T3). ments. The smoothing data also allowed visualizing more clearly the increase in the number of offspring that oc- curred in C and T, over generations (Table 3). 3.1.2. Duration of the Emergence Period The time, in days, elapsed between the emergence of the first fly and the last one was also evaluated in each replicate of the fifteen generations of C and T experi- ments (Figure 3). Seen in the light of the numbers, nine of the fifteen generations showed mean emergence dura- tion of C replicates greater than that from T. Student’s t-test showed significant difference between them only in F6 (t = 6.01; P = 0.027) and F8 (t = 3.77; P = 0.033). (a) (b) Figure 2. Graphs for mean productivity in the groups control and imidacloprid treated, in the generations F1 to F15. (a): Normal data; (b): Smoothed data. Copyright © 2013 SciRes. OPEN AC CESS  T. de F r ança Patarro et al. / Open Journal of Animal Sciences 3 (2013) 8-19 13 Ta ble 3. Differences of means on productivity (number of progeny) between control (C) and imidacloprid treated (T) experiments, separately for males, females and males plus females (Total), using smoothed data. Males Females Total Generation C-T t P C-T t P C-T t P F1 7.4 1.2 0.18 −3.6 −1.07 0.191 11.5 1.12 0.191 F2 13.8 2.3 0.05 10.9 1.55 0.13 26.2 1.84 0.07 F3 17.8 2.5 0.04 20.1 2.00 0.055 36.6 2.04 0.085 F4 19.5 2.4 0.02 24.0 2.26 0.052 42.2 2.16 0.058 F5 21.6 2.4 0.02 25.9 2.49 0.03 45.7 2.26 0.056 F6 26.2 2.6 0.03 28.0 2.73 0.028 51.9 2.53 0.041 F7 30.3 2.8 0.03 29.0 3.2 0.024 57.4 2.92 0.027 F8 30.7 2.9 0.03 28.9 3.54 0.012 58 3.07 0.025 F9 28.6 2.7 0.03 28.2 3.00 0.026 56.2 2.96 0.031 F10 26.6 2.7 0.03 29.6 2.35 0.051 57.4 2.74 0.032 F11 25.7 2.7 0.03 34.1 2.12 0.061 61.3 2.44 0.034 F12 24.6 2.6 0.03 38.2 2.18 0.06 63.5 2.31 0.057 F13 21.1 2.2 0.06 41.0 2.36 0.062 62.4 2.16 0.059 F14 15.6 1.3 0.11 42.6 2.46 0.046 58.9 1.79 0.085 F15 12.6 0.7 0.18 43.0 2.28 0.055 60.1 1.52 0.089 Figure 3. Means of emergence time duration of progeny (in days) from the control and imidacloprid treated experiments, in each of the 15 generations. However, among the other seven non-significant genera- tions with mean duration of emergence period greater in C, the difference from T varied from two to six days. The mean of the replicate means for all generations was 13.66 days for C and 11.54 days for T (Tables 4 and 5). Due to the similarity of profiles in the graphs of num- ber of progeny and emergence time duration, the coeffi- cient of correlation was calculated for each replicate us- ing Pearson’s coefficient of linear correlation and sig- nificance test for P = 0.00+. Except in the replicate num- ber 3 of T, the two characteristics showed high correla- tion: control P = 0.95; treated P = 0.85 (Figure 4). Ta bl e 4 . Duration (in days) of the emergence period computed from the first to the last fly emerged in the replicates from ex- periments control (C1, C2, C3) and imidacloprid treated (T1, T2, T3), in the 15 generations. Emergence Time (days) GenerationC1C2C3 Mean T1 T2 T3Mean F1 7787.33 5 7 8 6.67 F2 16 91613.67 16 15 1615.67 F3 8798.00 6 11 119.33 F4 1081210.00 8 8 9 8.33 F5 1181411.00 8 13 7 9.33 F6 16131615.00 9 9 9 9.00 F7 1411911.33 12 9 9 10.00 F8 17131715.67 11 10 8 9.67 F9 17131214.00 15 10 9 11.33 F10 19101514.67 10 9 8 9.00 F11 1112 810.33 10 12 1311.67 F12 282524 25.67 21 19 1217.33 F13 261923 22.67 31 22 1522.67 F14 6 11119.33 13 10 1011.00 F15 25101215.67 13 14 8 11.67 Copyright © 2013 SciRes. OPEN A CCESS  T. de F r ança Patarro et al. / Open Journal of Animal Sciences 3 (2013) 8-19 14 Table 5. Student’s t-test for comparison on duration of emer- gence time of progeny from control and imidacloprid treated experiments, in the 15 generations. Generation t P F1 0.71 0.549 F2 0.85 0.485 F3 0.76 0.529 F4 1.39 0.299 F5 0.66 0.557 F6 6.01 0.027* F7 0.76 0.505 F8 3.77 0.033* F9 1.11 0.347 F10 2.13 0.167 F11 0.89 0.437 F12 2.80 0.108 F13 0.00 0.999 F14 0.86 0.453 F15 0.79 0.511 *= significant values. (a) (b) Figure 4. Dispersion graphs between total productivity and mean emergence time for control (a) and imidaclo- prid treated (b) experiments (Pearson’s linear correlation r = 0.95 and r = 0.85, respectively). 3.2. Characteristics Analyzed in a Single Generation 3.2.1. Longevity The time elapsed between the emergence and the death of the adult flies was studied for virgin males and fe- males from F12, in C and T groups. Longevity graphs (Figures 5 and 6), showed that, in C experiments, half life was approximately 58 days for females and 57 days for males while, in T, they were about 50 and 48 days, respectively Mean longevity values for C were 54.32 days for females and 56.84 days for males while for T, they were 50.52 and 48.92, respectively. Thus, in the T experiments, the mean longevity was reduced in 7.92 days for males and 3.80 days for females. 3.2.2. Oviposition Rate Egg numbers were counted at the first and second 22h periods after flies from groups C, T and from S (stock) were put in contact with the specific culture medium (Figure 7). Due to the variability of the standard error, (a) (b) Figure 5. Graphs for comparison of longevity between males and females from F12 generation, in the control (a) and imidacloprid treated (b) groups. Horizontal line at 0.5 proportion indicates half-life value. Copyright © 2013 SciRes. OPEN AC CESS 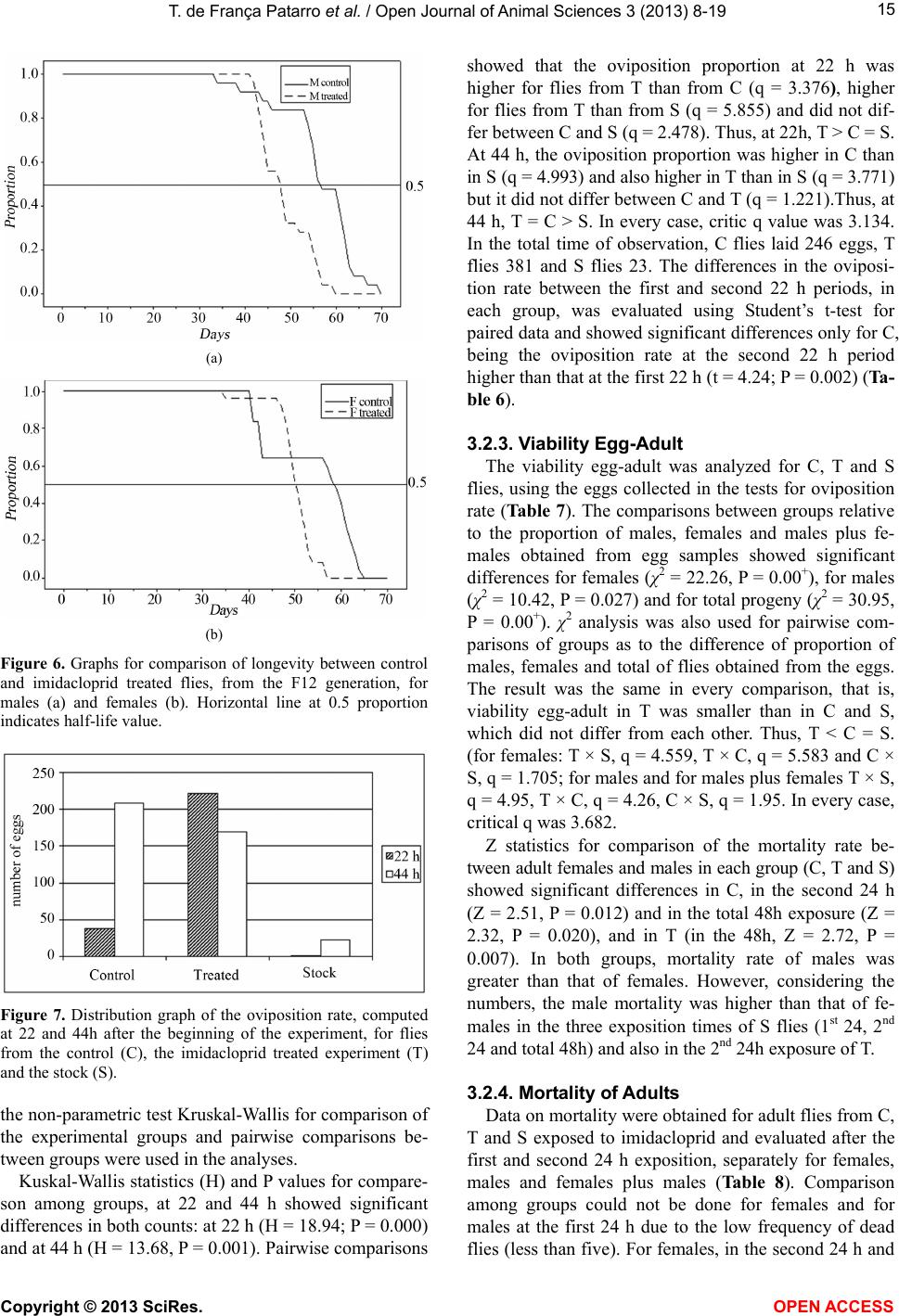 T. de F r ança Patarro et al. / Open Journal of Animal Sciences 3 (2013) 8-19 15 (a) (b) Figure 6. Graphs for comparison of longevity between control and imidacloprid treated flies, from the F12 generation, for males (a) and females (b). Horizontal line at 0.5 proportion indicates half-life value. Figure 7. Distribution graph of the oviposition rate, computed at 22 and 44h after the beginning of the experiment, for flies from the control (C), the imidacloprid treated experiment (T) and the stock (S). the non-parametric test Kruskal-Wallis for comparison of the experimental groups and pairwise comparisons be- tween groups were used in the analyses. Kuskal-Wallis statistics (H) and P values for compare- son among groups, at 22 and 44 h showed significant differences in both counts: at 22 h (H = 18.94; P = 0.000) and at 44 h (H = 13.68, P = 0.001). Pairwise comparisons showed that the oviposition proportion at 22 h was higher for flies from T than from C (q = 3.376), higher for flies from T than from S (q = 5.855) and did not dif- fer between C and S (q = 2.478). Thus, at 22h, T > C = S. At 44 h, the oviposition proportion was higher in C than in S (q = 4.993) and also higher in T than in S (q = 3.771) but it did not differ between C and T (q = 1.221).Thus, at 44 h, T = C > S. In every case, critic q value was 3.134. In the total time of observation, C flies laid 246 eggs, T flies 381 and S flies 23. The differences in the oviposi- tion rate between the first and second 22 h periods, in each group, was evaluated using Student’s t-test for paired data and showed significant differences only for C, being the oviposition rate at the second 22 h period higher than that at the first 22 h (t = 4.24; P = 0.002) (Ta- ble 6). 3.2.3. Viability Egg-Adult The viability egg-adult was analyzed for C, T and S flies, using the eggs collected in the tests for oviposition rate (Tabl e 7). The comparisons between groups relative to the proportion of males, females and males plus fe- males obtained from egg samples showed significant differences for females (χ2 = 22.26, P = 0.00+), for males (χ2 = 10.42, P = 0.027) and for total progeny (χ2 = 30.95, P = 0.00+). χ2 analysis was also used for pairwise com- parisons of groups as to the difference of proportion of males, females and total of flies obtained from the eggs. The result was the same in every comparison, that is, viability egg-adult in T was smaller than in C and S, which did not differ from each other. Thus, T < C = S. (for females: T × S, q = 4.559, T × C, q = 5.583 and C × S, q = 1.705; for males and for males plus females T × S, q = 4.95, T × C, q = 4.26, C × S, q = 1.95. In every case, critical q was 3.682. Z statistics for comparison of the mortality rate be- tween adult females and males in each group (C, T and S) showed significant differences in C, in the second 24 h (Z = 2.51, P = 0.012) and in the total 48h exposure (Z = 2.32, P = 0.020), and in T (in the 48h, Z = 2.72, P = 0.007). In both groups, mortality rate of males was greater than that of females. However, considering the numbers, the male mortality was higher than that of fe- males in the three exposition times of S flies (1st 24, 2nd 24 and total 48h) and also in the 2nd 24h exposure of T. 3.2.4. Mort ality of Adults Data on mortality were obtained for adult flies from C, T and S exposed to imidacloprid and evaluated after the first and second 24 h exposition, separately for females, males and females plus males (Table 8). Comparison among groups could not be done for females and for males at the first 24 h due to the low frequency of dead lies (less than five). For females, in the second 24 h and f Copyright © 2013 SciRes. OPEN A CCESS 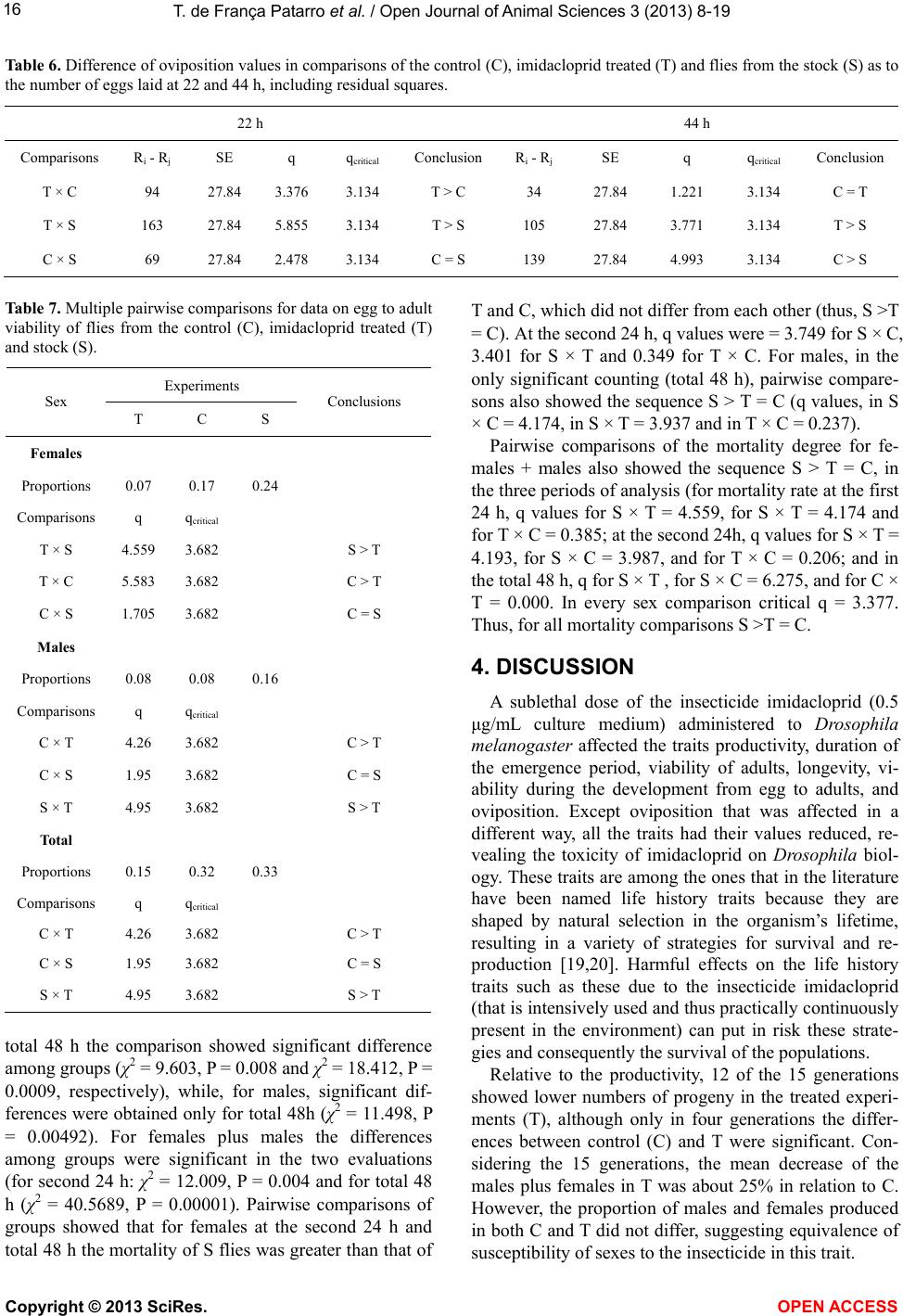 T. de F r ança Patarro et al. / Open Journal of Animal Sciences 3 (2013) 8-19 Copyright © 2013 SciRes. OPEN A CCESS 16 Table 6. Difference of oviposition values in comparisons of the control (C), imidacloprid treated (T) and flies from the stock (S) as to the number of eggs laid at 22 and 44 h, including residual squares. 22 h 44 h Comparisons Ri - Rj SE q qcritical ConclusionRi - Rj SE q qcritical Conclusion T × C 94 27.84 3.376 3.134 T > C 34 27.84 1.221 3.134 C = T T × S 163 27.84 5.855 3.134 T > S 105 27.84 3.771 3.134 T > S C × S 69 27.84 2.478 3.134 C = S 139 27.84 4.993 3.134 C > S Table 7. Multiple pairwise comparisons for data on egg to adult viability of flies from the control (C), imidacloprid treated (T) and stock (S). Experiments Sex T C S Conclusions Females Proportions 0.07 0.17 0.24 Comparisons q qcritical T × S 4.559 3.682 S > T T × C 5.583 3.682 C > T C × S 1.705 3.682 C = S Males Proportions 0.08 0.08 0.16 Comparisons q qcritical C × T 4.26 3.682 C > T C × S 1.95 3.682 C = S S × T 4.95 3.682 S > T Total Proportions 0.15 0.32 0.33 Comparisons q qcritical C × T 4.26 3.682 C > T C × S 1.95 3.682 C = S S × T 4.95 3.682 S > T total 48 h the comparison showed significant difference among groups (χ2 = 9.603, P = 0.008 and χ2 = 18.412, P = 0.0009, respectively), while, for males, significant dif- ferences were obtained only for total 48h (χ2 = 11.498, P = 0.00492). For females plus males the differences among groups were significant in the two evaluations (for second 24 h: χ2 = 12.009, P = 0.004 and for total 48 h (χ2 = 40.5689, P = 0.00001). Pairwise comparisons of groups showed that for females at the second 24 h and total 48 h the mortality of S flies was greater than that of T and C, which did not differ from each other (thus, S >T = C). At the second 24 h, q values were = 3.749 for S × C, 3.401 for S × T and 0.349 for T × C. For males, in the only significant counting (total 48 h), pairwise compare- sons also showed the sequence S > T = C (q values, in S × C = 4.174, in S × T = 3.937 and in T × C = 0.237). Pairwise comparisons of the mortality degree for fe- males + males also showed the sequence S > T = C, in the three periods of analysis (for mortality rate at the first 24 h, q values for S × T = 4.559, for S × T = 4.174 and for T × C = 0.385; at the second 24h, q values for S × T = 4.193, for S × C = 3.987, and for T × C = 0.206; and in the total 48 h, q for S × T , for S × C = 6.275, and for C × T = 0.000. In every sex comparison critical q = 3.377. Thus, for all mortality comparisons S >T = C. 4. DISCUSSION A sublethal dose of the insecticide imidacloprid (0.5 μg/mL culture medium) administered to Drosophila melanogaster affected the traits productivity, duration of the emergence period, viability of adults, longevity, vi- ability during the development from egg to adults, and oviposition. Except oviposition that was affected in a different way, all the traits had their values reduced, re- vealing the toxicity of imidacloprid on Drosophila biol- ogy. These traits are among the ones that in the literature have been named life history traits because they are shaped by natural selection in the organism’s lifetime, resulting in a variety of strategies for survival and re- production [19,20]. Harmful effects on the life history traits such as these due to the insecticide imidacloprid (that is intensively used and thus practically continuously present in the environment) can put in risk these strate- gies and consequently the survival of the populations. Relative to the productivity, 12 of the 15 generations showed lower numbers of progeny in the treated experi- ments (T), although only in four generations the differ- ences between control (C) and T were significant. Con- sidering the 15 generations, the mean decrease of the males plus females in T was about 25% in relation to C. However, the proportion of males and females produced in both C and T did not differ, suggesting equivalence of susceptibility of sexes to the insecticide in this trait.  T. de F r ança Patarro et al. / Open Journal of Animal Sciences 3 (2013) 8-19 17 Table 8. Mortality (number and percentage) of females and males during 48 h to 10 μg/ml imidacloprid, computed in the first and the second 24 h and in the total 48 h. Ninety males and ninety females were used for tests in control (C) and imidacloprid treated (T) groups, taken in the F15 generation, and also from the stocks which originated the experiments (S). Females Males Group 1st 24h 2nd 24h Total 1st 24h 2nd 24h Total C 3 (3.4%) 8 (8.9%) 11 (12.2%) 3 (3.4%) 20 (22.3%) 23 (25.56%) T 1 (1.2%) 9 (10.0%) 10 (11.1%) 6 (6.7%) 18 (20.0%) 24 (26.7%) S 9(10.0%) 21 (23.4%) 30 (33.3%) 13 (14.4%) 29 (32.2%) 42 (46.7%) The duration of the emergence period was also ana- lyzed in every generation. In the light of the numbers, the mean emergence period of C flies was greater than that of T, in nine of the 15 generations, although significant results were observed only in two of them. Taking into account all the generations, we observed for T a mean emergence period 2.13 days (15.5%) shorter than that for C, a difference that may not be neglected considering the mean value of 13.6 days for the duration of emergence in the control experiments. The correlation between the number of progeny and the emergence time duration also opened a new problem to be studied: is the decrease of emergence days due to a greater mortality of the larvae with longer developmental time and consequently with longer exposure to the insecticide? The longevity (or lifespan) of D. melanogaster, in laboratory, is known since long ago as being about eight weeks. The influence of the genetic background and the environmental variation in their expression have also been demonstrated in laboratory tests as well by the sig- nificant variation in longevity observed within and among natural populations [21]. Our measures of longevity in C experiments showed values included in the known limits, while the treated flies showed mean longevity decrease of about four and eight days for females and males, re- spectively, showing that, for longevity, imidacloprid was more toxic for males than for females. The viability egg-adult of the imidacloprid treated flies differed significantly between T and C experiments, with T producing lower percentage of adults than C and S, which did not differ from each other. In the study of this and also the traits oviposition and tolerance, comparisons of C and T flies with flies from the stock (that had been normally maintained in the laboratory during the time of the study) were performed in order to detect possible effects of using the 15 first couples for producing the consecutive generations. Thus, for viability egg to adult this effect apparently does not occur. The lower viability of the treated flies during development may be an im- portant factor to explain the lower productivity observed under the imidacloprid effect. The harmful effect on the viability egg-adult of Drosophila has also been described in experiments with other insecticides [22]. Oviposition was affected by imidacloprid in a different way. It provoked a decrease of the pre-oviposition time. At 22 h, T flies had laid 55% of the total number of eggs counted in the complete period of analysis (44 h), a per- centage significantly higher than the 15% laid by C flies in the same period. Since in S flies the egg-laying time did not differ significantly from C, the anticipation of oviposition in that period apparently was not influenced by the method of preparing the consecutive generations. However, at 44 h, while S remained in a slow pace, T and C oviposition rates equaled due to the significant in- crease of the egg number in C. Recently, [4] also re- ported a shortening of the pre-oviposition period in the mirid bug Apoligus lucorum treated with imidacloprid, but parallel to this shortening they observed an increase of the embryogenesis time that doesn’t seem to occur in our study in Drosophila because we detected decrease in the duration of the emergence period of the treated flies. The meaning and the mechanism of these changes in oviposition remain to be understood. The development of resistance to imidacloprid has been reported in many insect species, including field populations and laboratory selected strains (in honeybee, [9]; in the whitefly Bemisia tabaci [23,24]; in the brown planthoper Lugaparvata lugens [25] and in the potato beetle Leptinitarsa decemlineata [26]. In the present study, although we had not selected the flies for resis- tance with the usual method (selecting the more resistant flies in each generation to prepare the next), during 15 successive generations the parental adults and the larvae of the T experiments were fed exclusively with the cul- ture medium containing imidacloprid. We expected that, if some tolerance had developed, the number of progeny would increase in T experiments over the generations. The smoothing technique applied to the productivity data revealed more clearly, in graph, a tendency for this in- crease. However, the increase occurred in C and T ex- periments, which allowed hypothesizing that the use of the first 15 emerged couples to prepare the consecutive generations was revealing an interaction between emer- gence speed and productivity. In situations like this, the characteristics that seem to be interdependent must be necessarily considered genetically correlated [27]. Copyright © 2013 SciRes. OPEN A CCESS  T. de F r ança Patarro et al. / Open Journal of Animal Sciences 3 (2013) 8-19 18 A similar idea resulted from the study of mortality of adult flies exposed to the imidacloprid, in which we compared flies from C and T experiments, and also flies from the stock (S). The mortality of flies from S was higher than that of C and T, which, in turn, did not differ from each other. These results indicated that the toler- ance in C and T, as also observed in the productivity in- crease is, in some way, related with the selection for emergence speed. Previous studies on emergence time in Drosophila suggested that this trait can be correlated with fecundity [28] and with stress resistance [29] rein- forcing the present hypothesis. In summary, the present work showed harmful effects of the insecticide imidacloprid on Drosophila, affecting a set of important components of organisms’ fitness. Be- yond this focus on the biological danger due to the ex- tensive presence of this neonicotinoid in the environment, the analysis of different biological traits allowed propos- ing a relationship of cause and effect among some of them (for example, the viability decrease during devel- opment from egg to adult and the productivity decrease, in the treated experiments). There are also other ques- tions such as the possible shortening of the emergence period due to a preferential mortality of the larvae de- layed in development and consequently exposed longer to the insecticide; another question is what the conse- quences of the pre-oviposition time decrease are—Are these eggs viable? Do they develop earlier? Furthermore, the results suggested the interplay of the trait developmental speed of flies with other characteris- tics such as progeny number and development of toler- ance. This aspect also deserves further studies, for Dro- sophila melanogaster is a very propitious model. The clarifying of the mentioned questions will be im- portant, considering, on one side, the need to increase the knowledge on the effects of imidacloprid considered currently the insecticide of prevalent use in the world, and, on the other, the still poor knowledge of the mecha- nisms underlying the adaptive traits. 5. ACKNOWLEDGEMENTS Research supported by FAPESP (Scientific initiation fellowship, Thais de França Patarro). REFERENCES [1] Yamamoto, I. and Casida, J.E. (1999) Nicotinoid insecti- cides and the nicotinic acetylcholine receptor. Springer Verlag, Berlin. [2] Bayer Crop Science (2013) Imidacloprid. http://www.bayergroupindia.com/imidacloprid.html [3] Bao, H., Liu, S., Gu, J., Wang, X., Liang X. and Liu, Z. (2009) Sublethal effects of four insecticides on the re- production and wing formation of brown planthopper, Nilaparvata lugens. Pest Management Science, 65, 170- 174. http://dx.doi.org/10.1002/ps.1664 [4] Tan, Y., Biondi, A., Desneux, N. and Gao, X. (2012) As- sessment of physiological sublethal effects of imidaclo- prid on the mirid bug Apolygus lucorum (Meyer-Dür). Ecotoxicology, 21, 1989-1997. http://dx.doi.org/10.1007/s10646-012-0933-0 [5] Blacquière, T., Smagghe, G., Van Gestel, C.A.M. and Mommaerts, V. (2012) Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology, 21, 973-992. http://dx.doi.org/10.1007/s10646-012-0863-x [6] Bortolotti, L., Montanari, R., Marcelino, J., Medrzycki, P., Maini, S. and Porrini, C., (2003) Effects of sub-lethal imidacloprid doses on the homing rate and foraging ac- tivity of honey bees. Bulletin of Insectology, 56, 63-67. [7] Henry, M., Béguin, M., Requier, F., Rollin, O., Odoux, J., Aupinel, P., Aptel, J., Tchamitchian, S. and Decourtye, A. (2012) A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science, 336, 348-350. http://dx.doi.org/10.1126/science.1215039 [8] Tapparo, A., Marton, D., Giorio, C., Zanella, A., Solda, L., Marzaro, M., Vivan, L. and Girolami,V. (2012) As- sessment of the environmental exposure of honeybees to particulate matter containing neonicotinoid insecticides coming from corn coated seeds. Environmental Science & Technology, 46, 2592-2599. http://dx.doi.org/10.1021/es2035152 [9] Johnson, R.M., Ellis, M.D., Mullin, C.A. and Frazier, M., 2010. Pesticides and honey bee toxicity—USA. Apidolo- gie, 41, 312-331. http://dx.doi.org/10.1051/apido/2010018 [10] Tomizawa, M. and Casida, J.E. (2003) Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annual Review of Ento- mology, 48, 339-364. http://dx.doi.org/10.1146/annurev.ento.48.091801.112731 [11] Jeschke, P. and Nauen, R. (2008) Neonicotinoids—From zero to hero in insecticide chemistry. Pest Management Science, 64, 1084-1098. http://dx.doi.org/10.1002/ps.1631 [12] Magalhães, L.C., Hunt, T.E. and Siegfried, B.D. (2008) Development of methods to evaluate susceptibility of soybean aphid to imidacloprid and thiamethoxam at lethal and sublethal concentrations. Entomologia Experimen- talis et Applicata, 128, 330-336. http://dx.doi.org/10.1111/j.1570-7458.2008.00718.x [13] Sarkar, M.A., Biswas, P.K., Roy, S., Kole, R.K. and Chowdhury, A. (1999) Effect of pH and type of formula- tion on the persistence of lmidacloprid in water. Bulletin of Environmental Contamination and Toxicology, 63, 604-609. http://dx.doi.org/10.1007/s001289901023 [14] Anatra-Cordone, M. and Durkin, P. (2005) Human Health and Ecological Risk Assessment. Final Report, USDA/ Forest Service/ Forest Health Protection, New York. [15] Niwa, R. and Niwa, Y. S. (2011) The fruit fly Drosophila melanogaster as a model system to study cholesterol me- tabolism and homeostasis. Cholesterol, 2011, 1-6. http://dx.doi.org/10.1155/2011/176802 Copyright © 2013 SciRes. OPEN AC CESS  T. de F r ança Patarro et al. / Open Journal of Animal Sciences 3 (2013) 8-19 Copyright © 2013 SciRes. OPEN A CCESS 19 [16] Wolf, M.J. and Rockman, H.A. (2008) Drosophila melanogaster as a model system for the genetics of post- natal cardiac function. Drug Discovery Today, 5, 117- 123. [17] Zar, J.H. (1999) Biostatistical Analysis. Prentice Hall, New Jersey. [18] Moore, D.A. (2004) Estatística Básica e Sua Prática. Livros Técnicos e Científicos S.A., Brasil. [19] Stearns, S.C. (1977) The evolution of life history traits: a critique of the theory and a review of the data. Annual Review of Ecology, Evolution, and Systematics, 8, 145- 171. http://dx.doi.org/10.1146/annurev.es.08.110177.001045 [20] Fabian, D. and Flatt, T. (2012) Life History Evolution. Nature Education Knowledge Project. 3, 24. http://www.nature.com/scitable/knowledge/library/life-his tory-evolution-68245673 [21] Paaby, A.B. and Schmidt, P.S. (2009) Dissecting the ge- netics of longevity in Drosophila melanogaster. Fly, 3, 29-38. http://dx.doi.org/10.4161/fly.3.1.7771 [22] Karataş, A., Bahçeci, Z. and Başpinar, E. (2011) The ef- fect of diazinon on egg fertility and development in Dro- sophila melanogaster. Turkish Journal of Biology, 35, 95-101. [23] Sethi, A., Bons, M.S. and Dilawari, V.K. (2008) Realized heritability and genetic analysis of insecticide resistance in whitefly, Bemisia tabaci (Genn.). Journal of Entomol- ogy, 5, 1-9. http://dx.doi.org/10.3923/je.2008.1.9 [24] Liu, Z. and Han, Z. (2006) Fitness costs of laboratory- selected imidacloprid resistance in the brown planthopper, Nilaparvata lugens Stål. Pest Management Science, 62, 279-282. http://dx.doi.org/10.1002/ps.1169 [25] Wen, Y., Liu, Z., Bao, H. and Han, Z. (2009) Imidacloprid resistance and its mechanisms in field populations of brown planthopper, Nilaparvata lugens Stål in China. Pesticide Biochemistry and Physiology, 94, 36-42. http://dx.doi.org/10.1016/j.pestbp.2009.02.009 [26] Alyokhin, A., Dively, G., Patterson, M., Castaldo, C., Rogers, D., Mahoney, M. and Wollam J. (2006) Resis- tance and cross-resistance to imidacloprid and thiameth- oxam in the Colorado potato beetle Leptinotarsa Decem- lineata. Pest Management Science, 63, 32-41. http://dx.doi.org/10.1002/ps.1305 [27] Fuller, R.C., Baer, C.F. and Travis, J. (2005) How and When Selection Experiments Might Actually Be Useful. Integrative and Comparative Biology, 45, 391-404. http://dx.doi.org/10.1093/icb/45.3.391 [28] Nunney, L. (1996) The response to selection for fast lar- val development in Drosophila melanogaster and its ef- fect on adult weight: An example of a fitness trade-off. Evolution, 50, 1193-1204. http://dx.doi.org/10.2307/2410660 [29] Sørensen, J.G. and Loeschcke, V. (2004) Effects of rela- tive emergence time on heat stress resistance traits, lon- gevity and hsp70 expression level in Drosophila melano- gaster. Journal of Thermal Biology, 29, 195-203. http://dx.doi.org/10.1016/j.jtherbio.2004.02.004
|