 International Journal of Clinical Medicine, 2013, 4, 488-495 Published Online November 2013 (http://www.scirp.org/journal/ijcm) http://dx.doi.org/10.4236/ijcm.2013.411086 Open Access IJCM The Concept Study of Recombinant Human Soluble Thrombomodulin in Patients with Acute Respiratory Distress Syndrome Kenji Tsushima1,3*, Toshiki Yokoyama2, Tomonobu Koizumi2, Keishi Kubo2, Koichiro Tatsumi3 1Department of Pulmonary Medicine, Shinonoi General Hospital, Nagano, Japan; 2First Department of Internal Medicine, Shinshu University School of Medicine, Nagano, Japan; 3Department of Respirology, Graduate School of Medicine, Chiba University, Chiba Japan. Email: *tsushimakenji@yahoo.co.jp Received August 27th, 2013; revised September 28th, 2013; accepted October 15th, 2013 Copyright © 2013 Kenji Tsushima et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Recombinant human soluble thrombomodulin (rhTM) was approved for the treatment of disseminated intravascular coagulation in Japan, and rhTM has anti-inflammatory effects. Disordered coagulation is a part of the acute respiratory distress syndrome (ARDS) pathophysiology and thus we hypothesize that anticoagulant therapy may help. This preliminary study was to observe the safety of rhTM administration and the improvement on biomarker lev- els after the therapy for ARDS-patients. Objectives: Case series of ARDS-patients. Methods: Seventeen ARDS-pa- tients that required ventilatory management were treated with rhTM and clinical and laboratory data were collected in- cluding platelets, thrombin-antithrombin complex (TAT), fibrinogen degradation products, oxygen saturation/the frac- tion of inspired oxygen (SpO2/FIO2), and high-mobility group-1 (HMG-1). The administration of rhTM was started dur- ing 6 days at a bolus dose of 0.06 mg/kg/day immediately after the diagnosis of ARDS. Results: Eleven of the 17 ARDS-patients were alive at 28 days after the beginning of the administration of rhTM. The serial pattern of the SpO2/FIO2 showed remarkable differences between the survivors and nonsurvivors from day 5 to day 7. The TAT in the survivors significantly decreased after treatment, and there were significantly lower levels in the TAT on day 7 in com- parison to that of the nonsurvivors. The serial changes of HMG-1 showed increased levels in the nonsurvivors until day 5 after the administration of rhTM. Conclusions: Additional rhTM administration can safely improve the parameters in survival ARDS-patients, as demonstrated by significant improvements in the SpO2/FIO2, HMG-1 and TAT. Keywords: Acute Respiratory Distress Syndrome; Recombinant Human Soluble Thrombomodulin; Thrombin-Antithrombin Complex; SpO2/FIO2; High-Mobility Group-1 1. Introduction Thrombomodulin (TM) is a transmembrane protein ex- pressed on the endothelial cell surface that plays an im- portant role in the regulation of intravascular coagulation [1]. The novel biological agent, recombinant human soluble thrombomodulin (rhTM), was approved and is being used clinically for the treatment of disseminated intravascular coagulation (DIC) in Japan. The effects of rhTM on DIC were previously examined in a multicenter, randomized clinical trial in Japan [2], and the resolution of DIC was significantly better in the group treated with rhTM than in the group treated with unfractionated heap- rin. The rhTM binds to thrombin to inactivate coagula- tion, and the thrombin-rhTM complex activates protein C to produce activated protein C (APC), which, in the presence of protein S, inactivates factors VIIIa and Va, thereby inhibiting the further thrombin formation. Acute respiratory distress syndrome (ARDS) is also characterized by excessive intra-alveolar fibrin deposi- tion, driven at least partly by inflammation [3]. The im- balance between the activation of coagulation and inhibi- tion of fibrinolysis in patients with ARDS appears to occur both systemically in the lungs and the alveoli [4]. The activation of tissue factor (TF) is a critical event that results in thrombin formation. Thrombin is a key inter- mediate molecule with several biological functions, in- *Corresponding author.  The Concept Study of Recombinant Human Soluble Thrombomodulin in Patients with Acute Respiratory Distress Syndrome 489 cluding the augmentation of vascular permeability [5] and enhancement of inflammation [6]. Thrombin genera- tion leads to fibrin polymerization and deposition, with the resultant formation of hyaline membranes and a pa- thological hallmark of ARDS. The modulation of coagu- lation and fibrinolysis has a complex effect on both he- mostatic and inflammatory pathways. Therefore, antico- agulation therapy is beginning to be recognized as a po- tential new strategy for the treatment of ARDS patients. The aim of the present preliminary study was to evalu- ate whether the additional administration of rhTM has the improvement in biomarker levels with the onset of therapy between survival and nonsurivival patients with ARDS. 2. Methods Our study was approved by the ethics committee of Shi- nonoi General Hospital, and all patients or their family gave written informed consent on admission. We per- formed a prospective study of adult patients with ARDS who were admitted between April 2010 and September 2012. Our clinical study was registered to Shinonoi Gen- eral Hospital, Chikuma Central Hospital and Shinshu University School of Medicine. 3. The Diagnosis of ARDS The criteria for the diagnosis of ARDS, as set by the Ber- lin Definition [7], were used as follows: acute onset of lung injury, diffuse bilateral infiltrates seen upon chest X-ray, a PaO2/FIO2 (PF) ratio for ARDS, pulmonary ar- tery occlusion pressure <19 mmHg, or no clinical evi- dence of congestive heart failure. To rule out congestive heart failure, we performed echocardiographic examina- tions. Enrolled patients were diagnosed as having ARDS on admission. 4. Noninvasive Ventilation and Endotracheal Intubation Enrolled patients were performed chest X-ray on admis- sion. All patients initially received oxygen via a nasal cannula to maintain an oxygen concentration of more than 60 mmHg. If they could not maintain oxygen satu- ration (SpO2) of more than 90% or an oxygen concentra- tion (PaO2) of more than 60 mmHg with 3 - 5 L/ minute of oxygen via the nasal cannula, we recommended non- invasive ventilation (NIV) with an oronasal mask and bi-level positive airway pressure ventilation (BiPAP) with a BiPAP Vision device (Respironics; Murrysville, PA). All patients received NIV with the bilevel mode at a maximal positive pressure of 30 cmH2O. The spontane- ous timing mode was used for all patients, with a back-up rate of 10 breaths/minute. Inspiratory positive airway pressure (IPAP) was commenced at 4 cm H2O, and ad- justed to achieve a respiratory rate of less than 30 breaths/minute. The expiratory positive airway pressure (EPAP) was adjusted to achieve the target level of oxy- genation with minimum carbon dioxide rebreathing. The oronasal mask or full face mask was fitted by a trained pulmonologist. Straps were adjusted to allow one finger to pass easily between the strap and the patient’s face. The fraction of inspired oxygen (FIO2) and IPAP were adjusted to maintain a SpO2 of more than 90% or a PaO2 of more than 60 mmHg. Initial settings for NIV were chosen empirically by the attending pulmonologists. Patients were initiated on IPAP of 4 cmH2O and a FIO2 of 100%, after which the NIV was titrated by the pulmonologist to maintain pa- tient comfort and to keep the respiration rate at <35 breaths/minute, pH >7.3, and oxygen saturation >90%. If the patients could not maintain an oxygen saturation of more than 90% or an arterial oxygen concentration of more than 60 mmHg with the BiPAP Vision at 2 hours and 24 hours after initiation of NIV, we recommended endotracheal intubation. Endotracheal intubation was performed for patients with decreased alertness or major agitation requiring sedation, clinical signs of exhaustion (active contraction of the accessory muscles with para- doxical abdominal or thoracic motion), hemodynamic instability, cardiac arrest, refractory hypoxemia, or a PaO2/FIO2 (PF) ratio of less than 100 after treatment with NIV. When an improvement of the respiration rate and a decrease of the oxygen requirement became evident, the patients were gradually weaned off ventilation during the daytime and then during sleeping hours. Patient or family refusal of invasive mechanical ventilation was an exclu- sionary criterion for endotracheal intubation. These pa- tients were excluded from this study. 5. Treatment Standard microbiological investigations (e.g., blood and sputum cultures) were performed before the start of treatment to exclude pulmonary infection. Bronchoal- veolar lavage (BAL) was performed at admission, except in patients with refractory hypoxaemia despite sufficient mechanical ventilation, those with hemodynamic insta- bility, and those who rejected BAL. BAL fluid was as- sessed to exclude infectious disease. The administration of rhTM (recomodulin®) was started during 6 days at a bolus dose of 0.06 mg/kg/day. Microbiological investiga- tions (e.g., blood and sputum cultures) were performed before the start of antibiotic therapy. The exclusion of this study was done on suspicion of infection at admis- sion when the microbiological cultures were assessed as positive. Broad-spectrum antibiotics were then adminis- tered until the offending pathogen was identified for all Open Access IJCM  The Concept Study of Recombinant Human Soluble Thrombomodulin in Patients with Acute Respiratory Distress Syndrome 490 ARDS patients. 6. Data Collection rhTM administration can use for 6 days, and is effective for acute phase of ARDS patients. Therefore, patients were followed until 28 days after entry into the study. Patients were defined as survivors and nonsurvivors if they were alive or dead 28 days after beginning rhTM treatment, respectively. The collected data were age, sex, PF ratio, SpO2/FIO2, Sequential Organ Failure Assess- ment (SOFA) score and DIC score on admission. We evaluated the 28-day mortality after admission, and the physiological and biochemical variables. White blood cell counts (WBC, normal range: 4000-9000/μL), platelet counts (normal range: 14 - 30 × 104/μL), the serum lac- tate dehydrogenase level (LDH, normal range: 120 - 235 IU/L), fibrinogen degradation products (FDP, normal range: less than 5 μg/mL), and C-reactive protein (CRP, normal range: less than 0.3 mg/dl) were recorded on days 0 (before), 1, 2, 3, 5, and 7 after admission. The Krebs von den Lungen-6 (KL-6, normal range: less than 500 U/mL) and thrombin-antithrombin complex (TAT, nor- mal range: less than 4 ng/ml) values were recorded on days 0 (before), and 7 after admission. Cytokines (inter- leukin-6 (IL-6, normal range: less than 4.0 pg/mL), and high-mobility group-1 (HMG-1) protein were measured on days 0 (before), 1, 3, and 7 after starting the treatment. The presence of serious adverse events was defined as follows: fatal bleeding, nonfatal serious bleeding (de- fined as intracranial hemorrhage confirmed by brain im- aging, gastrointestinal or respiratory tract bleeding un- controllable by conservative treatments, and bleeding at a critical location, such as retinal hemorrhage, major he- marthrosis or spinal hemorrhage) or any life-threatening bleeding that led to discontinuation of the administered study drug. The SpO2/FIO2 ratio was calculated at 1 hour and on days 1, 2, 3, 5, and 7 after initiation of NIV on behalf of the PF ratio. 7. Outcomes The primary outcome was whether rhTM administration was safe treatment without adverse events for ARDS patients. The secondary outcome is whether the addi- tional administration of rhTM has a beneficial effect in biomarker levels with the onset of therapy for patients with ARDS. 8. Statistical Analysis The data are expressed as the group means plus or minus the standard error. Continuous variables were compared between groups by using Student’s t-tests. Categorical variables were analyzed by using Fisher’s exact test. A univariate analysis of the time to mortality was compared by using a log-rank test. The comparisons of the WBC and platelet counts, CRP, FDP, LDH, SpO2/FIO2, IL-6, and HMG-1 between groups over time were analyzed by repeated measure analysis of variance (ANOVA) ad- justed for the baseline values as a covariate. A p value < 0.05 was considered to be statistically significant. 9. Results 9.1. Baseline Characteristics Seventeen patients with ARDS were enrolled. The etiol- ogy of ARDS patients was 12 direct ARDS including 3 aspiration pneumonias and 5 indirect ARDS patients with sepsis. The bacteria or fungus in the BAL were not de- tected. Their mean age was 75.5 ± 19.9 years. Enrolled ARDS patients were included in 1 mild ARDS, 12 mod- erate ARDS and 4 severe ARDS patients. Table 1 show- ed that thrombosis showing elevated plasma TAT and FDP levels in the pulmonary vasculature due to lung in- flammation was present in patients with ARDS. 9.2. The Effects of Treatment on Mortality The 28-day mortality rate was 35.3%. The cause of death among the patients who died was the deterioration of ARDS. Enrolled patients were classified into survivors and nonsurvivors at 28 day after administration of rhTM. Table 1 showed that endotracheal intubation was re- quired for13 with ARDS, and NIV was performed for 4 patients with ARDS. Nonsurvivors showed significantly increased levels of TAT on admission compared to that of survivors (P = 0.0081). Five of the 6 nonsurvivors showed high levels of serum KL-6 on admission, without fibrosis and traction bronchiectasis on chest HRCT. ARDS patients were classified into high KL-6 (abnormal levels, more than 500 U/mL) and normal KL-6 sub- groups according to their serum KL-6 levels on admis- sion. There was a significant difference in the mortality rate between normal KL-6 and high KL-6 groups (P= 0.005) (Figure 1). 9.3. The Effects of Treatment on the Clinical Data in the Patients with ARDS In the serial changes in survivors, there was a significant difference in the WBC counts (Figure 2(a)) on days 5 and 7 as compared to that in nonsurvivors (P = 0.024 and 0.0076, respectively). There were no significant differ- ences in the serial changes in the CRP (Figure 2(b)) and LDH (Figure 2(c)) from before treatment until day 7 be- tween the two groups. The SpO2/FIO2 (Figure 2(d)) rap- idly increased on 1hour in survivors, and increased from Open Access IJCM 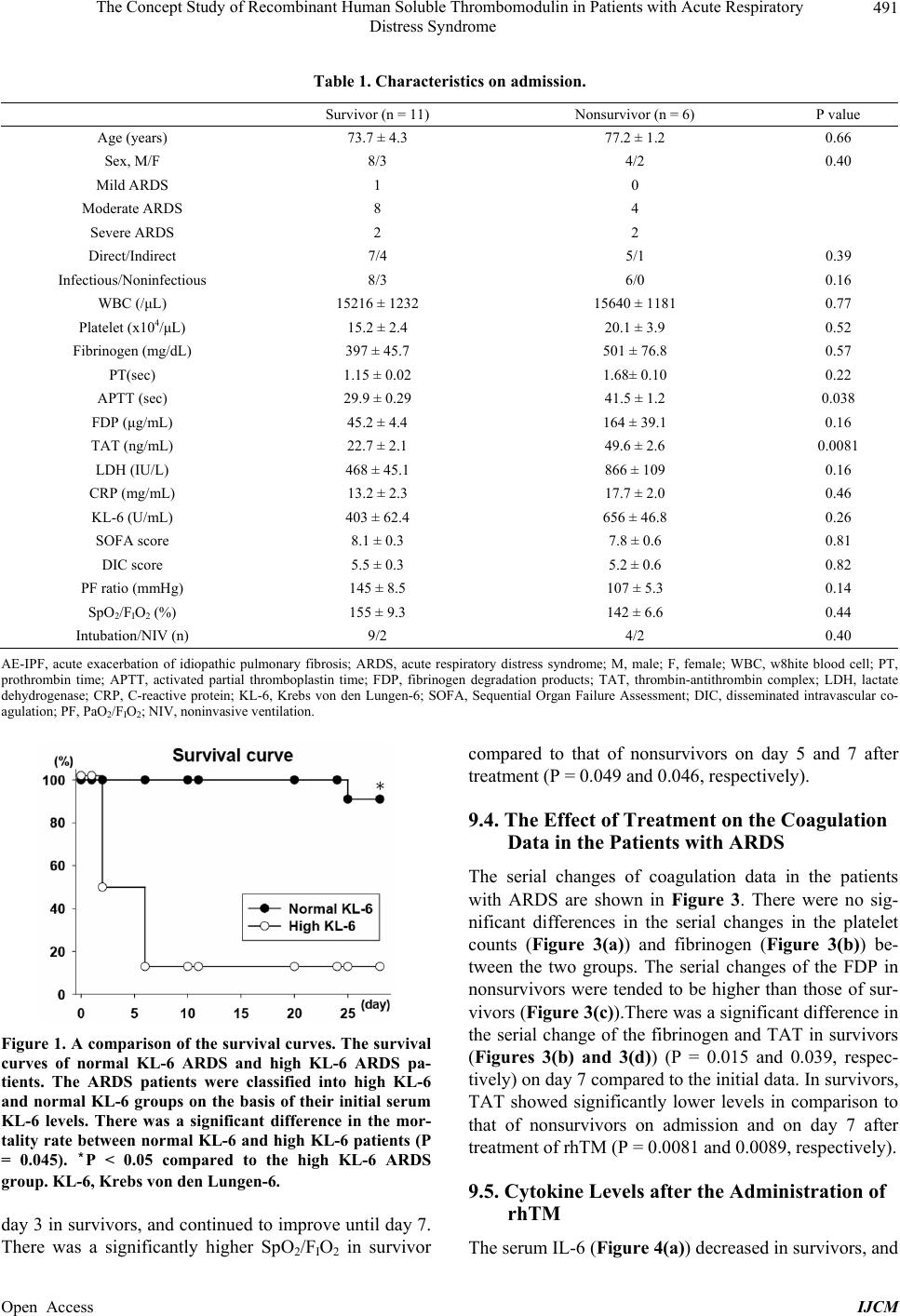 The Concept Study of Recombinant Human Soluble Thrombomodulin in Patients with Acute Respiratory Distress Syndrome Open Access IJCM 491 Table 1. Characteristics on admission. Survivor (n = 11) Nonsurvivor (n = 6) P value Age (years) 73.7 ± 4.3 77.2 ± 1.2 0.66 Sex, M/F 8/3 4/2 0.40 Mild ARDS 1 0 Moderate ARDS 8 4 Severe ARDS 2 2 Direct/Indirect 7/4 5/1 0.39 Infectious/Noninfectious 8/3 6/0 0.16 WBC (/μL) 15216 ± 1232 15640 ± 1181 0.77 Platelet (x104/μL) 15.2 ± 2.4 20.1 ± 3.9 0.52 Fibrinogen (mg/dL) 397 ± 45.7 501 ± 76.8 0.57 PT(sec) 1.15 ± 0.02 1.68± 0.10 0.22 APTT (sec) 29.9 ± 0.29 41.5 ± 1.2 0.038 FDP (μg/mL) 45.2 ± 4.4 164 ± 39.1 0.16 TAT (ng/mL) 22.7 ± 2.1 49.6 ± 2.6 0.0081 LDH (IU/L) 468 ± 45.1 866 ± 109 0.16 CRP (mg/mL) 13.2 ± 2.3 17.7 ± 2.0 0.46 KL-6 (U/mL) 403 ± 62.4 656 ± 46.8 0.26 SOFA score 8.1 ± 0.3 7.8 ± 0.6 0.81 DIC score 5.5 ± 0.3 5.2 ± 0.6 0.82 PF ratio (mmHg) 145 ± 8.5 107 ± 5.3 0.14 SpO2/FIO2 (%) 155 ± 9.3 142 ± 6.6 0.44 Intubation/NIV (n) 9/2 4/2 0.40 AE-IPF, acute exacerbation of idiopathic pulmonary fibrosis; ARDS, acute respiratory distress syndrome; M, male; F, female; WBC, w8hite blood cell; PT, prothrombin time; APTT, activated partial thromboplastin time; FDP, fibrinogen degradation products; TAT, thrombin-antithrombin complex; LDH, lactate dehydrogenase; CRP, C-reactive protein; KL-6, Krebs von den Lungen-6; SOFA, Sequential Organ Failure Assessment; DIC, disseminated intravascular co- agulation; PF, PaO2/FIO2; NIV, noninvasive ventilation. Figure 1. A comparison of the survival curves. The survival curves of normal KL-6 ARDS and high KL-6 ARDS pa- tients. The ARDS patients were classified into high KL-6 and normal KL-6 groups on the basis of their initial serum KL-6 levels. There was a significant difference in the mor- tality rate between normal KL-6 and high KL-6 patients (P = 0.045). *P < 0.05 compared to the high KL-6 ARDS group. KL-6, Krebs von den Lungen-6. day 3 in survivors, and continued to improve until day 7. There was a significantly higher SpO2/FIO2 in survivor compared to that of nonsurvivors on day 5 and 7 after treatment (P = 0.049 and 0.046, respectively). 9.4. The Effect of Treatment on the Coagulation Data in the Patients with ARDS The serial changes of coagulation data in the patients with ARDS are shown in Figure 3. There were no sig- nificant differences in the serial changes in the platelet counts (Figure 3(a)) and fibrinogen (Figure 3(b)) be- tween the two groups. The serial changes of the FDP in nonsurvivors were tended to be higher than those of sur- vivors (Figure 3(c)).There was a significant difference in the serial change of the fibrinogen and TAT in survivors (Figures 3(b) and 3(d)) (P = 0.015 and 0.039, respec- tively) on day 7 compared to the initial data. In survivors, TAT showed significantly lower levels in comparison to that of nonsurvivors on admission and on day 7 after treatment of rhTM (P = 0.0081 and 0.0089, respectively). 9.5. Cytokine Levels after the Administration of rhTM The serum IL-6 (Figure 4(a) decreased in survivors, and )  The Concept Study of Recombinant Human Soluble Thrombomodulin in Patients with Acute Respiratory Distress Syndrome 492 Figure 2. The serial changes of the WBC count, CRP, LDH and SpO2/FIO2. The data are expressed as the group means ± standard error of the mean. (a) The serial changes of the WBC count in survivor were significant difference on day 5 and 7 in comparison to nonsurvivor. (b) There were not significant differences in the change of the CRP level between two groups. (c) There were no significant differences in the changes in the LDH level between the two groups. (d) The SpO2/FIO2 increased from day 3 in survivor, and continued to improve until day 7. There was a significantly higher SpO2/FIO2 in survivor com- pared to that of nonsurvivor on day 5 and 7 after treatment. *P < 0.05 compared to the nonsurvivor, †P < 0.05 compared to before treatment. WBC, white blood cell; CRP, c-reactive protein; LDH, lactate dehydrogenase; SpO2/FIO2, oxygen satura- tion/the fraction of inspired oxygen. was closer to the initial levels in nonsurvivors. The IL-6 level in survivor on day 7 was significantly decreased compared to the initial level (P = 0.019). The serial changes of HMG-1 (Figure 4(b)) showed increased lev- els in nonsurvivors until day 7 after administration of rhTM. On day 7, the HMG-1 level in nonsurvivors was significantly higher compared to the initial level in non- survivosr and the level on day 7 in survivors (P = 0.011 and 0.0002, respectively). Serum KL-6 on day 7 in sur- vivor was a significantly lower level than that of nonsur- vivor (P = 0.027), and serum KL-6 on day 7 in nonsur- vivors was a significant higher levels than that on admis- sion (P = 0.049). 9.6. Adverse Events No adverse events according to bleeding had after the administration of rhTM. There were no other potential adverse events, such as liver, renal function, or cardio- vascular systems. 10. Discussion Our present study suggests that activated intravascular coagulation disturbances occurred in ARDS patients be- cause of elevated levels of plasma FDP and TAT. The modulation of the coagulation disturbance by our con- ventional therapy with rhTM administration may be re- lated to the mortality of ARDS patients, because the se- rial changes in the fibrinogen and plasma TAT, and the SpO2/FIO2 level showing the respiratory conditions sig- nificantly increased in ARDS patients. We found addi- tional rhTM administration appeared to be safe, without any major adverse effects. TF expression, a key mediator of the activation of co- agulation in the lungs, and fibrin deposition, were de- tected by the presence of specific antibodies in the al- veolar space and the pathogeesis of the early phase of n Open Access IJCM  The Concept Study of Recombinant Human Soluble Thrombomodulin in Patients with Acute Respiratory Distress Syndrome 493 Figure 3. The serial changes in the coagulation data. The data are expressed as the group means ± standard error of the mean. (a) There were no differences in the serial changes in the platelet counts between the two groups. (b) There was a significantly lower level in nonsurvivor compared to that on day 7 after treatment. (c) There were no differences in the serial changes in the plasma FDP between the two groups. (d) The plasma TAT showed significantly lower levels in survivors before the ad- ministration of rhTM. On day 7, the plasma TAT level in survivors was significantly lower that the initial level and the level of nonsurvivors *P < 0.05 compared to the nonsurvivor, †P < 0.05 compared to before treatment. FDP, fibrinogen degradation products; TAT, thrombin-antithrombin comple x. ARDS [3]. The imbalance between the activation of co- agulation and inhibition of fibrinolysis in patients with ARDS appears to occur both systemically and in the al- veolar space. Therefore, rhTM, which can modulate the coagulation pathway, such as the protein C pathway and the antithrombin pathway, may be useful for the treat- ment of ARDS. The use of activated protein C for ARDS patients, however, was a negative trial [8]. The rhTM plays an important role in regulating not only coagulation, but also inflammation, under conditions of sepsis. Two dif- ferent mechanisms have been described for the antico- agulant effects of rhTM [1,9]. Thrombin is one of the key molecules involved in coagulation, and has several bio- logical functions, including augmentation of vascular permeability [5] and enhancement of inflammation [6]. Thrombin formation leads to fibrin polymerization and deposition, with resultant formation of hyaline mem- branes, a pathological hallmark of ARDS. Direct binding of rhTM to thrombin can lead to its disruption. Therefore, the major mechanism of rhTM binding to thrombin is a pathway through the production of activated protein C. Recently, another mechanism underlying the anti-in- flammatory effect has been reported, wherein the N-ter- minal lectin-like domain of rhTM sequesters and cleaves HMG-1, which is released from necrotic cells and modulates several signals that induce a proinflammatory response leading to severe cell damage [10-12] and by lipopolysaccharide in the plasma in an experimental en- dotoxin model [11]. Nagato et al. reported that rhTM inhibits the production of inflammatory cytokines, de- creases the plasma HMG-1 levels and reduces the mor- tality in experimental endotoxemia in rats [13]. In the present study, the levels of serum IL-6, as inflammatory cytokines, were significantly increased in non-survivors on day 7 after administration of rhTM, and the HMG-1 in non-survivors could not be suppressed by increasing the administration of rhTM. Supporting their poorer progno- sis, Abraham et al. described that an increase in HMG-1 may be related to the development of lung inflammation, resulting in mortality [14]. Moreover, the mortality of ARDS patients was related to the development of fibrosis related to the increased levels of serum KL-6 [15]. In our study, serum KL-6 level was related to the mortality and Open Access IJCM  The Concept Study of Recombinant Human Soluble Thrombomodulin in Patients with Acute Respiratory Distress Syndrome 494 Figure 4. The serial changes in the inflammatory cytokines and serum KL-6 in survivors and nonsurvivors. The data are expressed as the group means ± standard error of the mean. The serum IL-6 (a) decreased until day 7 in survivor, and was close to the initial levels in nonsurvivor compared to survivors. (b) The serial changes of HMG-1 showed increased levels in nonsurvivor until day 7 after the administra tion of rhTM. On day 7, the HMG-1 level in nonsurvivor was significantly higher that the initial level and the level of survivors. (c) on day 7, serum KL-6 levels in survivor showed significant lower compared to that of nonsurvivor and serum KL-6 levels in nonsurvivor showed significant higher compared to before the administra- tion of rhTM. *P < 0.05 compared to the nonsurvivor, †P < 0.05 compared to before treatment. IL-6, interleukin-6; HMG-1, high-mobility group-1 protein; KL-6, Krebs von den Lungen-6. therapeutic effects (Figures 1 and 4(c)). The six patients who died in our study showed worse results in most of the biomarker levels although they received the same treatment as the survival ARDS patients. We interpret them as a phenomenon of non-responders. As shown in Figure 1, the oxygenation has been shown to be a sig- nificant predictor of responders because of the deteriora- tion of their lung parenchyma [16]. There were a few limitations to our preliminary study. First, we could not obtain lung tissue specimens to con- firm the existence of microvascular thrombus formation in the lungs of ARDS patients before and after treatment. We speculated that intra-alveolar thrombin formation and fibrin deposition in the local alveoli occurs at the same time as when the systemic thrombin formation develops in blood. Second, the study involved a small number of patients, and it was also a prospective study, not a ran- domized trial. A double blind prospective study with a substantially larger sample size is therefore needed to further validate our findings to evaluate clinical outcome. 11. Conclusion In conclusion, the additional administration of rhTM improved the biomarker levels in patients with survival ARDS, as demonstrated by significant improvements in the SpO2/FIO2, HMG-1 and microvascular coagulation and there was no adverse event. Further clinical investi- gations such as survival outcome are necessary to evalu- ate the effect of rhTM on ARDS patients. REFERENCES [1] M. Van de Wouwer, D. Collen and E. M. Conway, “Thrombomodulin-Protein C-EPCR System: Integrated to Regulate Coagulation and Inflammation,” Arteriosclero- sis, Thrombosis, and Vascular Biology, Vol. 24, 2004, pp. Open Access IJCM  The Concept Study of Recombinant Human Soluble Thrombomodulin in Patients with Acute Respiratory Distress Syndrome 495 1374-1383. http://dx.doi.org/10.1161/01.ATV.0000134298.25489.92 [2] H. Saito, I. Maruyama, S. Shimazaki, Y. Yamamoto, N. Akikawa, R. Ohno, A. Hirayama, T. Matsuda, H. Asakura, M. Nakashima and N. Aoki, “Efficacy and Safety of Re- combinant Human Soluble Thrombomodulin (ART-123) in Disseminated Intravascular Coagulation: Results of a Phase III, Randomized, Double-Blind Clinical Trial,” Journal of Thrombosis and Haemostasis, Vol. 5, No. 1, 2007, pp. 31-41. http://dx.doi.org/10.1111/j.1538-7836.2006.02267.x [3] S. C. Sebag, J. A. Bastarache and L. B. Ware, “Therapeu- tic Modulation of Coagulation and Fibrinolysis in Acute Lung Injury and the Acute Respiratory Distress Syn- drome,” Current Pharmaceutical Biotechnology, Vol. 12, No. 9, 2011, pp. 1481-1496. http://dx.doi.org/10.2174/138920111798281171 [4] J. H. Finigan, “The Coagulation System and Pulmonary Endothelial Function in Actre Lung Injury,” Microvascu- lar Research, Vol. 77, No. 1, 2009, pp. 35-38. http://dx.doi.org/10.1016/j.mvr.2008.09.002 [5] H. Lum and A. B. Malik, “Regulation of Vascular Endo- thelial Barrier Function,” American Journal of Physiology —Lung Cellular and Molecular Physiology, Vol. 267, 1994, pp. L233-241. [6] G. Cirino, C. Cicala, M. R. Bucci, L. Sorrentino, J. M. Maraganore, and S. R. Stone, “Thrombin Functions as an Inflammatory Mediator through Activation of Its Recep- tor,” The Journal of Experimental Medicine, Vol. 183, No. 3, 1996, pp. 821-827. http://dx.doi.org/10.1084/jem.183.3.821 [7] The ARDS Definition Task Force, “Acute Respiratory Distress Syndrome Berlin Definition,” JAMA, Vol. 307, No. 23, 2012, pp. 1-8. http://dx.doi.org/10.1001/jama.2012.5669 [8] K. D. Liu, J. Levitt, H. Zhuo, R. H. Kallet, S. Brady, J. Steingrub, M. Tidswell, M. D. Siegel, G. Soto, M. W. Peterson, M. S. Chesnutt, C. Phillips, A. Weinacker, B. T. Thompson, M. D. Eisner and M. A. Matthay, “Random- ized Clinical Trial of Activated Protein C for the Treat- ment of Acute Lung Injury,” American Journal of Respi- ratory and Critical Care Medicine, Vol. 178, No. 6, 2008, pp. 618-623. http://dx.doi.org/10.1164/rccm.200803-419OC [9] M. Mohri, Y. Gonda, M. Oka, Y. Aoki, K. Gomi, T. Ki- yota, T. Sugihara, S. Yamamoto, T. Ishida and I. Maru- yama, “The Antithrombotic Effects of Recombinant Hu- man Soluble Thrombomodulin (rhsTM) on Tissue Factor- Induced Disseminated Intravascular Coagulation in Crab- Eating Monkeys (Macaca fascicularis),” Blood Coagul Fibrinolysis, Vol. 8, 1997, pp. 274-283. http://dx.doi.org/10.1097/00001721-199707000-00003 [10] K. Abeyama, D. M. Stern, Y. Ito, K. Kawahara, Y. Yo- shimoto, M. Tanaka, T. Uchimura, N. Ida, Y. Yamazaki, S. Yamada, Y. Yamamoto, H. Yamamoto, S. Iino, N. Ta- niguchi and I. Maruyama, “The N-Terminal Domain of Thrombomodulin Sequesters High-Mobility Group-B1 Protein, a Novel Antiinflammatory Mechanism,” Journal of Clinical Investigation, Vol. 115, No. 5, 2005, pp. 1267- 1274. [11] C. S. Shi, G. Y. Shi, S. M. Hsiao, Y. C. Kao, K. L. Kuo, C. Y. Ma, C. H. Kuo, B. I. Chang, C. F. Chang, C. H. Lin, C. H. Wong and H. L. Wu, “Lectin-Like Domain of Thrombomodulin Binds to Its Specific Ligand Lewis Y Antigen and Neutralizes Lipopolysaccharide-Induced In- flammatory Response,” Blood, Vol. 112, No. 9, 2008, pp. 3661-3670. [12] M. Van de Wouwer, S. Plaisance, A. De Vriese, E. Waelkens, D. Collen, J. Persson, M. R. Daha and E. M. Conway, “The Lectin-Like Domain of Thrombomodulin Interferes with Complement Activation and Protects against Arthritis,” Journal of Thrombosis and Haemosta- sis, Vol. 4, No. 8, 2006, pp. 1813-1824. [13] M. Nagato, K. Okamoto, Y. Abe, A. Higure and K. Ya- maguchi, “Recombinant Human Soluble Thrombo- modulin Decreases the Plasma High-Mobility Group Box-1 Protein Levels, Whereas Improving the Acute Liver Injury and Survival Rates in Experimental En- dotoxemia,” Critical Care Medicine, Vol. 37, No. 7, 2009, pp. 2181-2186. [14] E. Abraham, J. Arcaroli, A. Carmody, H. Wang and K. J. Tracey, “HMG-1 as Mediator of Acute Lung Inflamma- tion,” The Journal of Immunology, Vol. 165, No. 6, 2000, pp. 2950-2954. [15] A. Ishizaka, T. Matsuda, K. H. Albertine, H. Koh, S. Ta- saka, N. Hasegawa, N. Kohno, T. Kotani, H. Morisaki, J. Takeda, M. Nakamura, X. Fang, T. R. Martin, M. A. Matthay and S. Hashimoto, “Elevation of KL-6, a Lung Epithelial Cell Marker, in Plasma and Epithelial Lining Fluid in Acute Respiratory Distress Syndrome,” Ameri- can Journal of Physiology—Lung Cellular and Molecular Physiology, Vol. 286, 2004, pp. L1088-1094. [16] P. J. Offner and E. E. Moore, “Lung Injury Severity Scor- ing in the Era of Lung Protective Mechanical Ventilation: The PaO2/FIO2 Ratio,” Journal of Trauma, Vol. 55, No. 2, 2003, pp. 285-289. Open Access IJCM
|