 Advances in Bioscience and Biotechnology, 2013, 4, 30-40 ABB http://dx.doi.org/10.4236/abb.2013.411A2005 Published Online November 2013 (http://www.scirp.org/journal/abb/) Evaluation of the impact of leucocytospermia on semen oxidative status by chemiluminescence technique in infertile men* Afifa Sellami1,2#†, Nozha Chakroun1,2#, Yemna Rtaib1,2, Hajer Hdhili1,2, Riadh Ben Mansour3, Louati Dolira4, Kais Chaabene4, Leila Keskes1, Saloua Lassoued3, Tarek Rebai1,2 1Laboratory of Histology and Biology of Reproduction, Medical School, Sfax, Tunisia 2Histology Embryology Research Unit, Medical School, Sfax, Tunisia 3Laboratory of Cell Culture, Biotechnology Institute, Sfax, Tunisia 4Gynaecology and Obstetrics Department, Hedi Chaker Academic Hospital, Sfax, Tunisia Email: †sellamiafifa@yahoo.fr, nozhafeki@yahoo.fr Received 9 September 2013; revised 10 October 2013; accepted 26 October 2013 Copyright © 2013 Afifa Sellami et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The presence of high reactive oxygen species (ROS) levels in semen is a major factor involved in the de- cline of male fertility. In seminal plasma, ROS are mainly produced by activated leucocytes. Spermato- zoa were the first cell type reported to show a poten- tial susceptibility to oxidative damage. The aim of our study was to evaluate the impact of leucocyto- spermia on basal and FMLP (Formyl-Methionyl- Leucyl-Phenylalanine) induced oxidative status in semen of infertile men. We also analyzed the correla- tions of the spermatic parameters with amounts of ROS in semen. Our study included 50 semen samples of infertile men. Sperm analysis was performed using WHO standardized method. Seminal leucocytes were quantified using peroxidase technique. The meas- urement of ROS levels in semen was made by che- miluminescence assay. We measure respectively ROS amounts in neat semen and in washed sperm cells suspension from the same ejaculate. We also applied the test of provocation of leucocytes by FMLP on neat and washed samples to assess the spermatic oxidative status after leucocyte stimulation. Our results showed significant correlations between ROS levels in neat semen and many sperm parameters: motility, sperm concentration, leucocytes concentration and the rate of sperm cytoplasmic droplets. The studied samples were divided into 2 groups: (G1) composed of 36 samples without leucocytospermia and (G2) com- posed of 14 leucospermic samples. ROS levels were significantly lower in G1 than in G2 (p = 0.002). ROS production was significantly increased after applica- tion of FMLP in washed leucospermic samples (p = 0.001). The measurement of ROS in neat semen is a considerable contribution to explore the impairment of semen quality in infertile men. ROS levels in washed semen reflect the oxidative status generated by sperm preparation techniques used in assisted re- productive procedures. Levels of ROS are highly in- fluenced by the presence of leucocytes and associated with decreased seminal parameters. Keywords: Semen; Oxidative Stress; Leucocytospermia; Chemiluminescence; ROS; FMLP 1. INTRODUCTION Oxidative stress induced by reactive oxygen species (ROS) plays crucial roles in a wide range of physiologi- cal processes and is also implicated in various diseases, such as cancer, cardiovascular pathology, neurodegen- erative disorders, and other chronic conditions 1,2. In semen, the controlled generation of very low amounts of ROS appears to regulate sperm normal functions 3,4. Oxidative stress appears in semen once an imbalance between the production of ROS and their destruction by different enzymatic and non-enzymatic seminal antioxi- dant systems is created 5. Interestingly, spermatozoa were the first cell type reported to show a potential sus- ceptibility to oxidative damage 6. While the presence of high levels of ROS in the ejaculate is among the risk factors involved in reducing male fertility 7,8. It has evolved that three inter-related mechanisms account for *Disclosure: The authors declare that there are no conflicts of interest. #The first two authors contribute equally to the article. †Corresponding author. OPEN ACCESS  A. Sellami et al. / Advances in Bioscience and Biotechnology 4 (2013) 30-40 31 oxidative stress-mediated male infertility: impaired mo- tility, impaired fertilization and oxidative DNA damage 9,10. The principle sources of excessive generation of ROS in semen are activated leucocytes and abnormal sper- matozoa 11,12. Indeed, the prevalence of leucocyto- spermia in infertile men varies from 2% to 40% de- pending on the patient population, the detection method and threshold values used 13. Moreover, it has been shown that depending on their activation status, leuco- cytes are capable of producing ROS and cytokines and there seems to be a relation between ROS formation and cytokine production 14,15. The importance of seminal ROS production has been already stressed in the World Health Organization manu- als (WHO 1999 and WHO 2010) 16,17. The chemilu- minescence method is the most commonly employed technique as a direct measurement of ROS generation in semen according to the standardized method recom- mended by the WHO 1999 16. This assay is capable of quantifying both intracellular and extracellular ROS 18. Furthermore, the use of leukocyte-specific stimulant (FMLP: formyl-methionyl-leucyl-phenylalanine) can en- hance the chemiluminescent signal to measure low amounts of light generated by leucocytes 16,19. The degree of sperm damage by leucocytes products depends on the location of the inflammatory reaction, the duration of exposure of sperm to these products and the ability of spermatozoa to activate its intrinsic anti-lipop- eroxidative defense systems 20. The clinical signify- cance of both ROS and leucocytes levels continues to be debated. Some authors have found ROS levels or leuko- cyte counts to be of little prognostic help in either in vivo or in vitro reproduction 21. These findings are in dis- agreement with other in vivo 22 and in vitro studies 19,23 that reported the significant prognostic value of semen ROS levels in reproduction. Much of the contro- versy centers on the best definition of pathological leu- cocytospermia and the correlation of leucocytes with seminal oxidative stress are unclear 15,24,25. The aims of our study were to evaluate the impact of leucocytospermia on basal and FMLP induced semen oxidative status using the chemiluminescence technique in infertile men. We also analyzed the correlations of the routine spermatic parameters with amounts of ROS gen- eration in semen. 2. MATERIAL AND METHODS 2.1. Patients Our study was carried out in 50 semen samples from male partner of infertile couple attending the Histology- Embryology laboratory of Sfax medical school (Tunisia) for semen investigations. The patients were aged be- tween 27 and 51 years old with a mean age (± Standard Deviation (SD)) of 36.16 ± 0.57 years. 2.2. Collection of Semen Samples Semen samples were collected by masturbation after 3 - 5 days of sexual abstinence and allowed to liquefy for 30 minutes at 37˚C. 2.3. Semen Analysis Basic semen analysis consisted in the measurement of the following semen parameters: volume, sperm concen- tration, percentage of motile spermatozoa, sperm vitality and percentage of normal spermatozoa. For sperm con- centration, diluted semen samples were mixed before transferring a drop to the chamber of the hemocytometer. The spermatozoa were counted under a light microscope at 400× magnification. To determine the percentage of motile spermatozoa, a 10 µl drop of mixed semen was placed on a heated glass slide (37˚C) under a square cover glass (22 mm) and observed at 400× magnifica- tion. The percentage of motile spermatozoa was evalu- ated immediately and four hours after semen liquefaction, we evaluated total motility, rapid progressive motility (type a), slow progressive motility (type b), and no pro- gressive motility (type c) according to WHO guidelines 16. Sperm vitality was assessed using eosin-nigrosin staining technique. A 20 µl of liquefied semen was mixed with 20 µl of eosin (1%) and 20 µl of nigrosin (10%). The suspension was incubated for 30 s at room tempera- ture. Then, a 20 µl of the solution was smeared on a mi- croscope slide. The smear was air dried and examined at 1000× magnification under oil immersion. Unstained sperm (white) were classified as viable and those that showed any pink or red coloration were classified as dead. Sperm morphology was assessed in Shorr-stained semen smears. All parameters were carried out according to the standardized methods recommended by the WHO 16. 2.4. Assessment of Leucocytes in Semen by Peroxidase Method A leukocyte count was carried out by using the cyto- chemical peroxidase method, as described in the WHO laboratory manual 16. This assay identifies polymor- phonuclear granulocytes, the most prevalent leucocyte type in semen, as peroxidase-positive cells. A working solution was prepared by combining 250 µl of Benzidine with 50 µl of Hydrogen peroxide (H2O2). The procedure consisted of mixing 50 µl of neat semen with 50 µl of the working solution. This mixture was allowed for 30 min- utes at room temperature. We transferred 20 µl of the mixture onto a hemocytometer chamber and the number of peroxidase-positive leucocytes which stained brown Copyright © 2013 SciRes. OPEN ACCESS  A. Sellami et al. / Advances in Bioscience and Biotechnology 4 (2013) 30-40 32 was counted at 400× magnification. Leucocytospermia was defined as the presence of more than 1 × 106 leuco- cytes per milliliter of semen 16. 2.5. Semen Preparation From each sample, one aliquot of 0.5 ml of liquefied neat semen was used for immediate ROS measurement and another 0.5 ml aliquot of semen was centrifuged at 300 g for 7 minutes. Seminal plasma was removed and the pel- let of cells was washed twice in 3 ml of PBS (Phosphate Buffer Saline, isotonic solution, pH = 7.4). The super- natant was then removed and the cells pellet was sus- pended in a volume of 0.5 ml of PBS. Sperm concentra- tion in the final solution was evaluated before ROS measurement. 2.6. Measurement of Reactive Oxygen Species (ROS) in Semen by Chemiluminescence Technique The measurement of ROS in semen was made by che- miluminescence using a microplate luminometer (Lumi- noskan Ascent, Thermo Electron Corporation). Chemilu- minescence probe used is luminol (5-amino-2, 3-dihydro 1, 4-phthalazinedione; Sigma chemical Co) which is a redox-sensitive, light emitting probe 26. A 10 mM stock solution of Luminol was prepared in DMSO (Di- methyl Sulfoxide; Sigma-Aldrich). For each semen sam- ple, we measured the basal levels of ROS in neat semen and in a suspension of washed semen cells obtained from the same ejaculate. The basal ROS production was measured respectively in 200 µl of liquefied neat semen and 200 µl of washed cells suspension after addition of 5 µl of the working solution of Luminol (0.1 mM) obtained by dilution of the stock solution with the HBSS (Hank’s Balanced Salt Solution). Negative controls for estimation of back- ground signals were prepared for each assay by adding 5 µl of 0.1 mM Luminol to 200 µl of PBS. Also, 200 µl of PBS served as a blank. We also applied the test of specific provocation of leucocytes by FMLP (Formyl-Methionyl-Leucyl-Pheny- lalanine; Sigma Aldrich) on all our samples (neat and washed). The FMLP is used to stimulate a chemilu- minescent signal from any polymorphonuclear granulo- cytes that are present in the sperm suspension 16. Since FMLP receptors are not present on the surface of human spermatozoa, this signal is specific for the leucocyte population 16. A 0.1 mM stock solution of FMLP in DMSO was prepared. The ROS production was measured respectively in 200 µl of liquefied neat semen and 200 µl of washed cells suspension after addition of 5 µl of the working solution of Luminol (0.1 mM) and stimulation with 2 µl of FMLP working solution (0.2 µM) obtained by dilution of the FMLP stock solution with the HBSS. The che- miluminescence signal was monitored for 15 minutes. Results were expressed as relative light units (RLU) per minute and per 20 × 106 spermatozoa. 3. STATISTICAL ANALYSIS A statistical analysis was performed using SPSS 13.0 software. Statistical tests including Student’s t test, Pear- son’s and Spearman’s correlations, linear regression were used. The statistical significance was considered for p values < 0.05. 4. RESULTS The mean values (± SD) and ranges of semen parameters, basal and after FMLP stimulation ROS levels in neat and washed semen are summarized in Table 1. The levels of basal ROS in neat semen was negatively correlated with the immediate and late total motility, the immediate and late rapid progressive motility and the sperm concentration (Table 2; Figures 1(a)-(c)). We also found significant and positive correlations of levels of basal ROS in neat semen with the leucocytes concen- tration in semen firstly and with the rates of cytoplasmic droplets in the mid piece area of spermatozoa secondly (Table 2; Figures 1(d) and (e)). There was a marked increase of ROS in semen after washing (Table 1) with significant correlations of the Table 1. Means and ranges of semen parameters, basal ROS levels in neat and washed semen and ROS levels in neat and washed semen after FMLP stimulation (n = 50). Means ± SD Ranges Volume (ml) 3.9 ± 1.5 1.9 - 8.5 Total motility (%) 38.7 ± 14.6 0 - 60 Rapid progressive mobility “a” (%) 10.6 ± 7.1 0 - 25 Sperm concentration (Millions/ml) 45.9 ± 39.8 0.02 - 189.6 Vitality (%) 68.9 ± 14.9 26 - 89 Leucocytes (millions/ml) 0.9 ± 2.1 0.01 - 9.5 Normal morphology (%) 7.2 ± 5.3 0 - 22 Cytoplasmic droplets (%) 5.1 ± 4.9 0 - 20 Basal ROS (RLU/mn/ 20.106 spz) In neat semen 3.9 ± 21.4 0.01 - 150.5 In washed semen 164.6 ± 1136.2 0.03 - 8037 ROS after FMLP stimulation (RLU/mn/20 × 106 spz) In neat semen 3.9 ± 21.7 0.01 - 153.0 In washed semen 222.7 ± 1542.7 0.02 - 10912.5 Copyright © 2013 SciRes. OPEN ACCESS 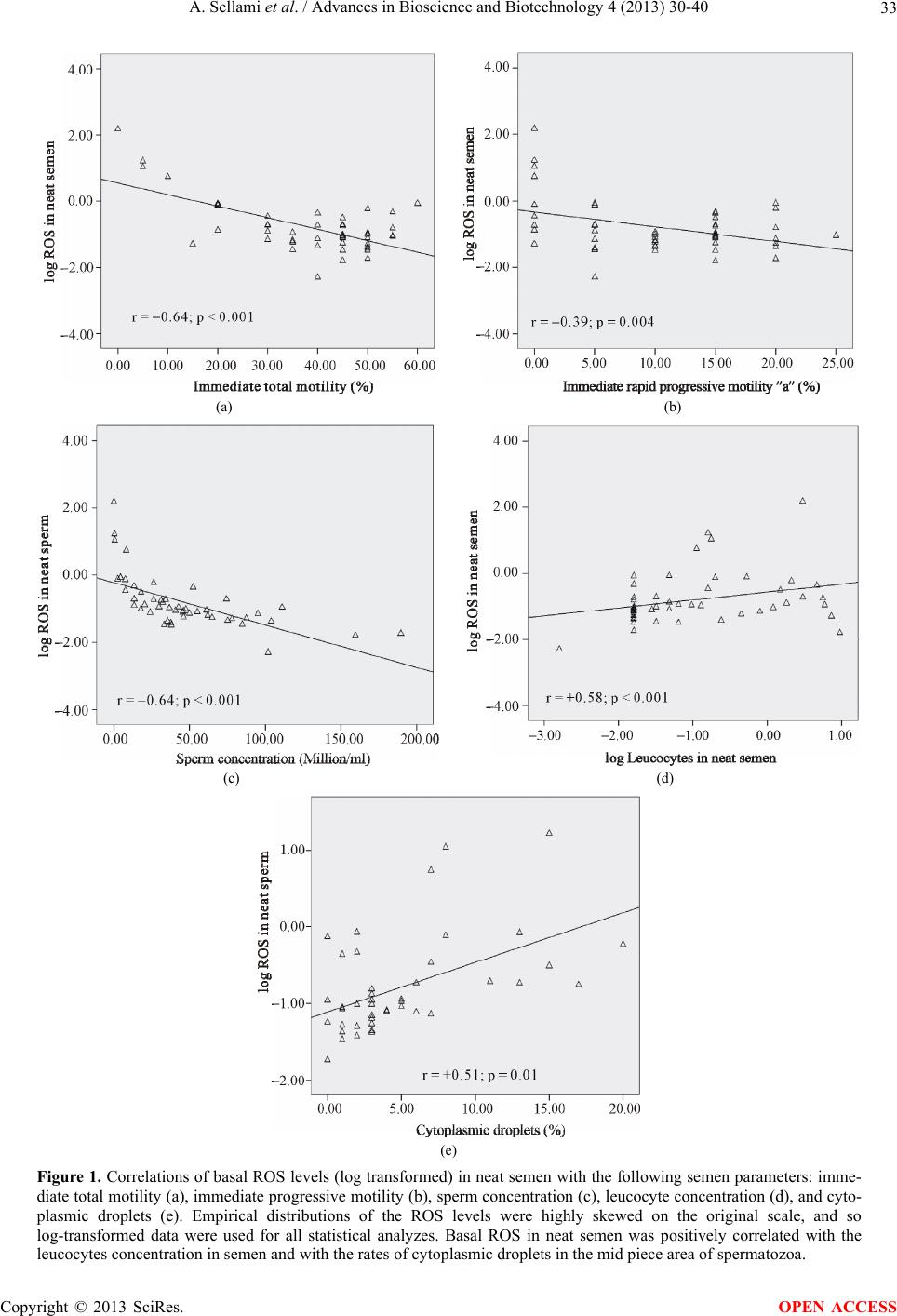 A. Sellami et al. / Advances in Bioscience and Biotechnology 4 (2013) 30-40 Copyright © 2013 SciRes. 33 (a) (b) (c) (d) (e) Figure 1. Correlations of basal ROS levels (log transformed) in neat semen with the following semen parameters: imme- diate total motility (a), immediate progressive motility (b), sperm concentration (c), leucocyte concentration (d), and cyto- plasmic droplets (e). Empirical distributions of the ROS levels were highly skewed on the original scale, and so log-transformed data were used for all statistical analyzes. Basal ROS in neat semen was positively correlated with the leucocytes concentration in semen and with the rates of cytoplasmic droplets in the mid piece area of spermatozoa. OPEN ACCESS 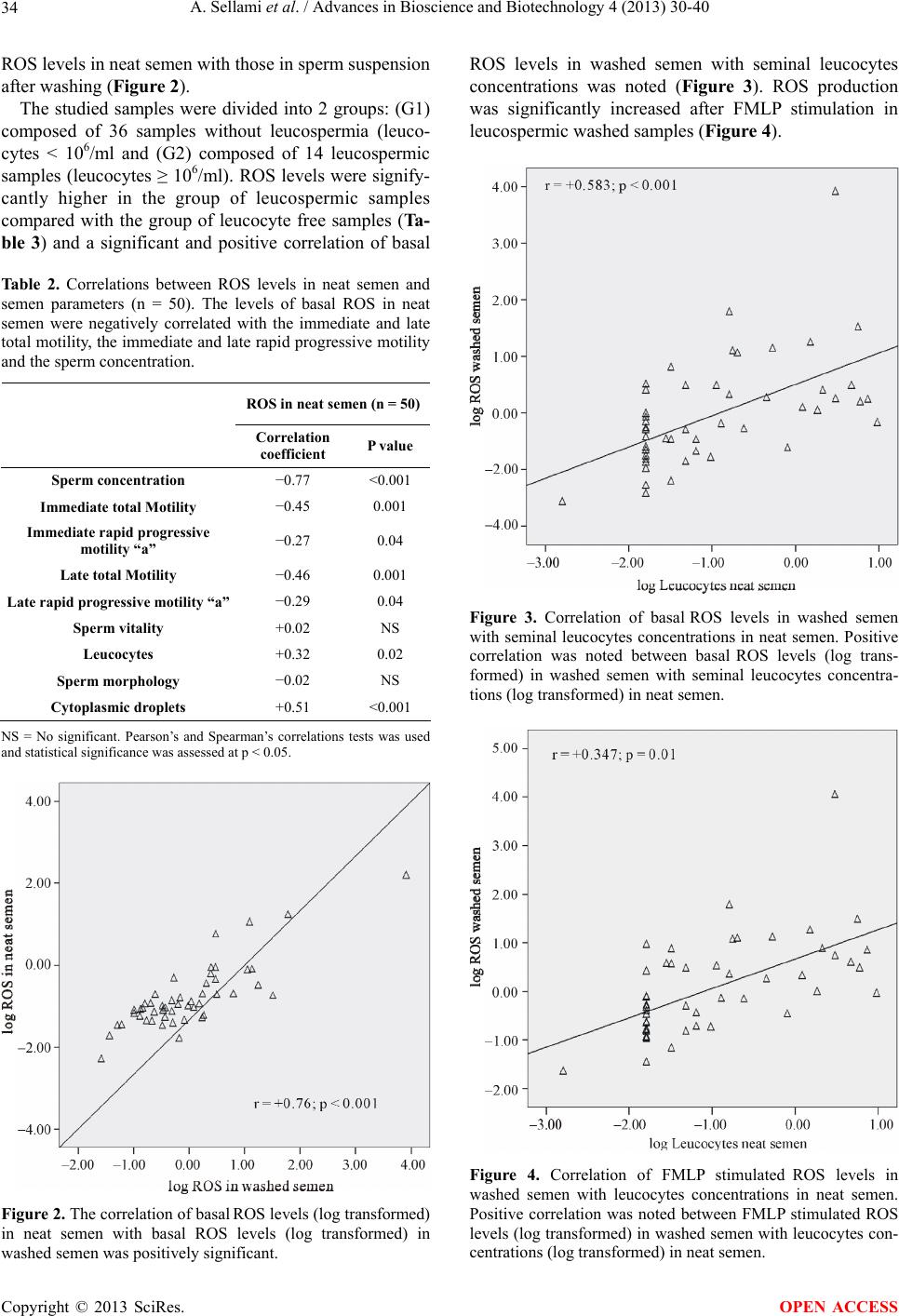 A. Sellami et al. / Advances in Bioscience and Biotechnology 4 (2013) 30-40 34 ROS levels in neat semen with those in sperm suspension after washing (Figure 2). The studied samples were divided into 2 groups: (G1) composed of 36 samples without leucospermia (leuco- cytes ˂ 106/ml and (G2) composed of 14 leucospermic samples (leucocytes ≥ 106/ml). ROS levels were signify- cantly higher in the group of leucospermic samples compared with the group of leucocyte free samples (Ta- ble 3) and a significant and positive correlation of basal Table 2. Correlations between ROS levels in neat semen and semen parameters (n = 50). The levels of basal ROS in neat semen were negatively correlated with the immediate and late total motility, the immediate and late rapid progressive motility and the sperm concentration. ROS in neat semen (n = 50) Correlation coefficient P value Sperm concentration −0.77 <0.001 Immediate total Motility −0.45 0.001 Immediate rapid progressive motility “a” −0.27 0.04 Late total Motility −0.46 0.001 Late rapid progressive motility “a”−0.29 0.04 Sperm vitality +0.02 NS Leucocytes +0.32 0.02 Sperm morphology −0.02 NS Cytoplasmic droplets +0.51 <0.001 NS = No significant. Pearson’s and Spearman’s correlations tests was used and statistical significance was assessed at p < 0.05. Figure 2. The correlation of basal ROS levels (log transformed) in neat semen with basal ROS levels (log transformed) in washed semen was positively significant. ROS levels in washed semen with seminal leucocytes concentrations was noted (Figure 3). ROS production was significantly increased after FMLP stimulation in leucospermic washed samples (Figure 4). Figure 3. Correlation of basal ROS levels in washed semen with seminal leucocytes concentrations in neat semen. Positive correlation was noted between basal ROS levels (log trans- formed) in washed semen with seminal leucocytes concentra- tions (log transformed) in neat semen. Figure 4. Correlation of FMLP stimulated ROS levels in washed semen with leucocytes concentrations in neat semen. Positive correlation was noted between FMLP stimulated ROS levels (log transformed) in washed semen with leucocytes con- entrations (log transformed) in neat semen. c Copyright © 2013 SciRes. OPEN ACCESS  A. Sellami et al. / Advances in Bioscience and Biotechnology 4 (2013) 30-40 35 Table 3. Comparison of semen parameters, basal and FMLP induced semen oxidative status between leucocyte free samples (G1) and leucospermic samples (G2); the ROS levels were significantly enhanced in washed semen and after FMLP application in leucospermic group. Means ± SD G1 (n = 36) Means ± SD G2 (n = 14) P value Volume (ml) 4.2 ± 1.6 3.6 ± 1.2 NS Sperm concentration (Millions/ml) 42.3 ± 37.4 54.9 ± 45.6 NS Immediate total Motility (%) 39.7 ± 14.3 36.1 ± 15.6 NS Immediate rapid progressive motility “a” (%) 10.4 ± 6.8 11.1 ± 7.9 NS Late total Motility (%) 35.1 ± 15.8 28.5 ± 16.5 NS Late rapid progressive motility “a” (%) 7.7 ± 6.2 7.3 ± 6.9 NS ROS (RLU/20.106 spz) Neat semen FMLP (−) 1.06 10.96 0.002 Neat semen FMLP (+) 0.95 11.22 0.002 Wasched semen FMLP (−) 3.12 579.72 0.001 Wasched semen FMLP (+) 3.49 786.11 0.001 FMLP (−): without FMLP stimulation; FMLP (+): after FMLP stimulation; NS: No significant. Student’s t test was used for comparison and statistical signifi- cance was assessed at p < 0.05. 5. DISCUSSION It is well established that the production of excessive quantities of reactive oxygen species in the male genital tract, essentially by leucocytes, can overwhelm the semi- nal antioxidant defenses and give rise to a harmful oxi- dative stress altering the microenvironment in which spermatozoa develop and mature 5,18,27. Currently, there are growing evidences that genital oxidative stress is involved in the pathogenesis of many reproductive processes through sperm damage, deformity and eventu- ally male infertility 28,29. We used chemiluminescence technique in our study because it is the most commonly described assay to detect ROS production within semen 30. It is very sensitive and has the advantage of rela- tively well established reported ranges for both the fertile and infertile population 31-33. As regards the relationship between semen quality and its oxidative status evaluated by chemiluminescence as- say, we found significant correlations between basal ROS levels produced in neat semen of infertile men and conventional sperm parameters such as motility, sperm concentration and one of sperm morphological abnor- malities (cytoplasmic droplets in mid piece area of sper- matozoa). The decrease of sperm motility associated to an oxida- tive stress in infertile men semen was reported previ- ously 23,34-36 and was related to the negative effect of ROS on sperm mitochondrial membrane potential and the reduction of ATP production 37-39. In addition, the sperm membranes are characterized by the abundance of unsaturated fatty acids having a double-bonded nature and predisposing them to lipid peroxidation 26,40. This peroxidation reaction affects membrane structure and fluidity and causes damage to axonemal proteins leading to a permanent impairment in sperm motility 41-43. In the present study, we founded a significant negative correlation between sperm concentration and the levels of ROS in semen. Indeed, it was widely reported that seminal concentrations of ROS in oligospermic patients were higher than that of normospermic patients 31, 44-46. ROS and their metabolites attack DNA, lipids and proteins, alter enzymatic systems and cell signaling pathways, producing irreparable alterations and may ac- celerate the process of germ cell apoptosis 47. The lat- ter process can lead to decline in sperm counts and may explain the apparent deterioration of semen quality 39, 48. Also, the decrease in the sperm concentration could be explained by the presence of a genital inflammation process which often results in the presence of high number of leucocytes, principle source of ROS in semen 49,50. Indeed, our results showed a significant in- crease of ROS in leucospermic samples compared with the samples without leucospermia. These results are con- sistent with other reports indicating that seminal leuco- cytes have the potential to cause oxidative stress through their degranulation and the formation of free radicals 9. In fact, it was also reported that the presence of leu- cocytes in semen is associated with a high rate of pro- duction of ROS with reduction of sperm motility and its fertilizing ability 34. The activation state of leuco- cytes must play an important role in determining final ROS output 51. Pro-inflammatory seminal plasma cy- tokines such as IL-6, IL-8 and tumour necrosis factor TNF α seem to be produced locally by activated leuco- cytes in semen and a marked relationship between these cytokines, leucocytes and seminal ROS production was Copyright © 2013 SciRes. OPEN ACCESS  A. Sellami et al. / Advances in Bioscience and Biotechnology 4 (2013) 30-40 36 reported 14,50,52. During epididymal transit, the sperm are not in contact with seminal plasma antioxidants, and are therefore vulnerable to oxidative damage, especially when there is an epididymal inflammation 49. It was also demonstrated that leucocytospermia increased the production of ROS by human spermatozoa 53. The application of a leucocyte-specific stimulant (FMLP) test showed an increase in ROS production especially in semen samples of leucospermic group. Leucocytes are the only cells present in the human ejaculate possessing detectable receptors for FMLP and capable of generating reactive oxygen species in re- sponse to this reagent 54. Our results confirm that leucocytes are the major source of ROS in semen and present an additional argument that the presence of leucocytes in semen is associated with a high rate of production of ROS. Moreover, the results of the FMLP provocation test have an important bearing on the fertile- izing capacity of the spermatozoa in vitro 19,55. The leucocytes are the principle but not the exclusive source of ROS in semen and in concordance with our findings, Aitken 44 and Gomez 56 showed a signify- cant production of free radicals by cytosplasmic droplets present at the midpiece of the defective morphologically abnormal spermatozoa. These residues are rich in Glu- cose-6-phosphate dehydrogenase (G6PD), an enzyme which controls the rate of glucose flux and intracellular production of Nicotinamide Adenine Dinucleotide Phos- phate (NADPH) 57. The latter is used to fuel the gen- eration of ROS via NADPH oxidase located within the sperm membrane 56-58. As a result, teratozoospermic sperm produce increased amounts of ROS with a reduced antioxidant capacity compared with morphologically normal sperm 9,59. All these relationships underpin the well established observation that higher ROS have a negative impact on sperm quality but even men with normozoospermic idiopathic infertility exhibit signifi- cantly higher seminal ROS production and lower anti- oxidant capacity than fertile men for as yet unknown reasons 28,60. Besides the measure of ROS level in neat semen, we quantified ROS in semen after washing and removal of seminal plasma. We apply this assay in our study to evaluate the oxidative status of semen during sperm preparation techniques for assisted reproductive tech- nologies. Our results are similar to those reported in other studies 61-63 that showed a significant increase of ROS production in semen by repeated cycles of cen- trifugation and aggravated by the ablation of the natural antioxidant environment. Some studies 64-66 showed that the addition of antioxidants such as EDTA, catalase, ascorbate and tocopherol in sperm preparation medium may scavenge ROS and decrease their deleterious effects on spermatozoa but these findings are discussed 67,68. Another point for consideration is the implication of genital oxidative stress in the sperm DNA fragmentation commonly observed in spermatozoa of infertile men 69. In fact, significant correlations of seminal ROS levels with DNA damage were reported in many studies 43,70, 71. Moreover, other studies reported an increase in DNA damage in sperm from leucocytospermic samples 53, 72,73. Recently, it was suggested a direct implication of leucocytes, as an exogenous factor, in generating DNA base modifications evaluated by the formation of 8- oxoguanine, a key biomarker of the oxidative DNA damage 74. Oxidized DNA bases have increased sus- ceptibility to ROS action which reflects a direct and spe- cific action of ROS on sperm DNA 9,75. The most re- cent studies on the origin of sperm DNA damage sug- gested that there might be a cascade of changes that pro- gress from the induction of oxidative stress and DNA base-pair oxidation to DNA fragmentation and cell death 76. Nevertheless, it seems that the plasma membrane is less vulnerable to oxidative damage than DNA and sperm with significant oxidative DNA damage and intact membranes could preserve its ability to fertilize oocyte 70. Many of these embryos developed from spermato- zoa with fragmented DNA will unfortunately fail at the blastocyst or early fetal stage 77,78. Thereby, the cur- rently use of mechanical techniques such as Intra Cyto- plasmic Sperm Injection (ICSI) to bypass some male factor infertility is unlikely to be the ‘best practice’ since ROS damaged DNA, frequently induced by seminal leucocytes, will result in poor quality blastocysts and an increase in miscarriage 9,25. Additionally, the patients may be urged to consider antioxidant therapy before un- dergoing reproductive assistance technique that may re- duce DNA damage levels and improve sperm fertility potential 9. 6. CONCLUSIONS Oxidative stress is a major factor in male reproductive disorders because it may impair the physiological func- tion of spermatozoa at the molecular level. Understand- ing the exact mechanisms, by which oxidative stress de- velops in semen, will improve the management of semen quality impairment by potential toxic ROS. To establish a treatment strategy of genital oxidative stress in infertile men, we must first specify its origin: seminal leucocytes and/or sperm cells themselves, to guide thereby the op- timum therapeutic modalities for male infertility particu- larly in the context of leucocytospermia. Progress in assisted reproductive technologies contin- ues to be offered to infertile men, who would otherwise be unable to conceive chances to have their own off- spring. However, these “mechanical” procedures are un- able to compensate for oxidative damage to sperm DNA. Copyright © 2013 SciRes. OPEN ACCESS  A. Sellami et al. / Advances in Bioscience and Biotechnology 4 (2013) 30-40 37 In addition, direct treatment of oxidative stress and its source may allow for natural conception. 7. ACKNOWLEDGEMENTS We would like to thank the technicians of the laboratory of Histol- ogy-Embryology, Medical school Sfax for their help in semen process- ing and their technical assistance. We express also our gratitude to the staff of the laboratory of cell culture; Biotechnology institute, Sfax, Tunisia for their help. This work was supported by the Histology Em- bryology research unit, Medical school Sfax and the Tunisian Ministry of Higher Education, Scientific Research, and Technology. REFERENCES [1] Rahman, K. (2007) Studies on free radicals, antioxidants, and co-factors. Clinical Interventions in Aging, 2, 219- 236. [2] Brieger, K., Schiavone, S., Miller Jr., F.J. and Krause, K.H. (2102) Reactive oxygen species: From health to dis- ease. Swiss Medical Weekly, 142, w13659. http://dx.doi.org/10.4414/smw.2012.13659 [3] Agarwal, A. and Saleh, R.A. (2002) Role of oxidants in male infertility: Rationale, significance, and treatment. Urologic Clinics of North America, 29, 817-827. http://dx.doi.org/10.1016/S0094-0143(02)00081-2 [4] Cocuzza, M., Sikka, S.C., Athayde, K.S. and Agarwal, A. (2007) Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence based analysis. International braz j urol, 33, 603-621. http://dx.doi.org/10.1590/S1677-55382007000500002 [5] Agarwal, A., Makker, K. and Sharma, R. (2008) Clinical relevance of oxidative stress in male factor infertility: An update. American Journal of Reproductive Immunology, 59, 2-11. http :/ /d x. do i.o rg/10 .1111 /j .1600-0897.2007.00559.x [6] Gharagozloo, P. and Aitken, R.J. (2011) The role of sperm oxidative stress in male infertility and the signifi- cance of oral antioxidant therapy. Human Reproduction, 26, 1628-1640. http://dx.doi.org/10.1093/humrep/der132 [7] Agarwal, A. and Said, T.M. (2005) Oxidative stress, DNA damage and apoptosis in male infertility: A clinical ap- proach. BJU International, 95, 503-507. http :/ /d x. do i.o rg/10 .1111 /j .1464-410X.2005.05328.x [8] Saalu, L.C. (2010) The incriminating role of reactive oxygen species in idiopathic male infertility: An evidence based evaluation. Pakistan Journal of Biological Sciences, 13, 413-422. http://dx.doi.org/10.3923/pjbs.2010.413.422 [9] Tremellen, K. (2008) Oxidative stress and male infertile- ity—A clinical perspective. Human Reproduction Update, 14, 243-258. http://dx.doi.org/10.1093/humupd/dmn004 [10] Aitken, R.J. and Koppers, A.J. (2011) Apoptosis and DNA damage in human spermatozoa. Asian Journal of Andrology, 13, 36-42. http://dx.doi.org/10.1038/aja.2010.68 [11] Kessopoulou, E., Tomlinson, M.J., Barratt, C.L., Bolton, A.E. and Cooke, I.D. (1992) Origin of reactive oxygen species in human semen: Spermatozoa or leucocytes? The Journal of the Society for Reproduction and Fertility, 94, 463-470. http://dx.doi.org/10.1530/jrf.0.0940463 [12] Sharma, R.K. and Agarwal, A. (1996) Role of reactive oxygen species in male infertility. Urology, 48, 835-850. http://dx.doi.org/10.1016/S0090-4295(96)00313-5 [13] Anderson, D.J. and Politch, J.A. (1996) Evaluation and treatment of the infertile male. In: Centola, G.M. and Ginsburg, K.A., Eds., Cambridge University Press, New York, 263-277. [14] Depuydt, C.E., Bosmans, E., Zalata, A., Schoonjans, F. and Comhaire, F.H. (1996) The relation between reactive oxygen species and cytokines in andrological patients with or without male accessory gland infection. Journal of Andrology, 17, 699-707. [15] Keck, C., Gerber-Schäfer, C., Clad, A., Wilhelm, C. and Breckwoldt, M. (1998) Seminal tract infections: Impact on male fertility and treatment options. Human Repro- duction Update, 4, 891-903. http://dx.doi.org/10.1093/humupd/4.6.891 [16] World Health Organization (1999) Laboratory manual for the examination of human semen and sperm-cervical mu- cus interaction. Cambridge University Press, Cambridge. [17] World Health Organization (2010) Laboratory manual for the examination and processing of human semen. WHO Press, Geneva. [18] Kashou, A.H., Sharma, R. and Agarwal, A. (2013) As- sessment of oxidative stress in sperm and semen. In: Carrell, D.T. and Aston, K.I., Eds., Spermatogenesis: Me- thods and protocols, Methods in Molecular Biology, Hu- mana Press, New York, 351-361. [19] Krausz, C., Mills, C., Rogers, S., Tan, S.L. and Aitken, R.J. (1994) Stimulation of oxidant generation by human sperm suspensions using phorbol esters and formyl pep- tides: Relationships with motility and fertilization in vitro. Fertility and Sterility, 62, 599-605. [20] Storey, B.T. (1997) Biochemistry of the induction and prevention of lipoperoxidative damage in human sper- matozoa. Molecular Human Reproduction, 3, 203-213. http://dx.doi.org/10.1093/molehr/3.3.203 [21] Tomlinson, M.J., Barratt, C.L. and Cooke, I.D. (1993) Prospective study of leukocytes and leukocyte subpopu- lations in semen suggests they are not a cause of male in- fertility. Fertility and Sterility, 60, 1069-1075. [22] Aitken, R.J., Irvine, D.S. and Wu, F.C. (1991) Prospec- tive analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertil- ity. American Journal of Obstetrics & Gynecology, 164, 542-551. http://dx.doi.org/10.1016/S0002-9378(11)80017-7 [23] du Plessis, S.S., McAllister, D.A., Luu, A., Savia, J., Agarwal, A. and Lampiao, F. (2010) Effects of H2O2 ex- posure on human sperm motility parameters, reactive oxy- gen species levels and nitric oxide levels. Andrologia, 42, 206-210. http://dx.doi.org/10.1111/j.1439-0272.2009.00980.x [24] Sharma, R.K., Pasqualotto, A.E., Nelson, D.R., Thomas Jr., A.J. and Agarwal A. (2001) Relationship between seminal white blood cell counts and oxidative stress in Copyright © 2013 SciRes. OPEN ACCESS  A. Sellami et al. / Advances in Bioscience and Biotechnology 4 (2013) 30-40 38 men treated at an infertility clinic. Journal of Andrology, 22, 575-583. [25] Barraud-Lange, V., Pont, J.C., Ziyyat, A., Pocate, K., Si- fer, C., Cedrin-Durnerin, I., Fechtali, B., Ducot, B. and Wolf, J.P. (2011) Seminal leukocytes are good samaritans for spermatozoa. Fertility and Sterility, 96, 1315-1319. http://dx.doi.org/10.1016/j.fertnstert.2011.09.035 [26] Zini, A. and Sigman, M. (2009) Evaluation of sperm function. In: Lipshultz, L.I., Howards, S.S. and Nieder- berger, C.S., Eds., Infertility in the Male, Cambridge Uni- versity Press, New York, 177-198. http://dx.doi.org/10.1017/CBO9780511635656.012 [27] Agarwal, A. and Prabakaran, S.A. (2005) Oxidative stress and antioxidants in male infertility: A difficult balance. Iranian Journal of Reproductive Medicine, 3, 1-8. [28] Agarwal, A., Sharma, R.K., Nallella, K.P., Thomas Jr., A.J., Alvarez, J.G. and Sikka, S.C. (2006) Reactive oxy- gen species as an independent marker of male factor in- fertility. Fertility and Sterility, 86, 878-885. http://dx.doi.org/10.1016/j.fertnstert.2006.02.111 [29] Doshi, S.B., Khullar, K., Sharma, R.K. and Agarwal, A. (2012) Role of reactive nitrogen species in male infertile- ity. Reproductive Biology and Endocrinology, 10, 109. http://dx.doi.org/10.1186/1477-7827-10-109 [30] Sikka, S.C. (2004) Role of oxidative stress and antioxi- dants in andrology and assisted reproductive technology. Journal of Andrology, 25, 5-18. [31] Ochsendorf, F.R., Thiele, J., Fuchs, J., Schuttau, H., Freis- leben, H.J., Buslau, M. and Milbradt, R. (1994) Chemilu- minescence in semen of infertile men. Andrologia, 26, 289-293. http :/ /d x. do i.o rg/10 .1111 /j .1439-0272.1994.tb00804.x [32] Williams, A.C. and Ford, W.C. (2005) Relationship be- tween reactive oxygen species production and lipid per- oxidation in human sperm suspensions and their associa- tion with sperm function. Fertility and Sterility, 83, 929- 936. http://dx.doi.org/10.1016/j.fertnstert.2004.11.031 [33] Athayde, K.S., Cocuzza, M., Agarwal, A., Krajcir, N., Lucon, A.M., Srougi, M. and Hallak, J. (2007) Develop- ment of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. Journal of An- drology, 28, 613-620. http://dx.doi.org/10.2164/jandrol.106.001966 [34] Plante, M., de Lamirande, E. and Gagnon, C. (1994) Re- active oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertility and Sterility, 62, 387- 393. [35] Griveau, J.F. and Le Lannou, D. (1997) Reactive oxygen species and human spermatozoa: Physiology and pathol- ogy. International Journal of Andrology, 20, 61-69. http://dx.doi.org/10.1046/j.1365-2605.1997.00044.x [36] Armstrong, J.S., Rajasekaran, M., Chamulitrat, W., Gatti, P., Hellstrom, W.J. and Sikka, S.C. (1999) Characteriza- tion of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Free Radical Biology and Medicine, 26, 869-880. http://dx.doi.org/10.1016/S0891-5849(98)00275-5 [37] Baumber, J., Ball, B.A., Gravance, C.G., Medina, V. and Davies-Morel, M.C. (2000) The effect of reactive oxygen species on equine sperm motility, viability, acrosomal in- tegrity, mitochondrial membrane potential, and mem- brane lipid peroxidation. Journal of Andrology, 21, 895- 902. [38] Wang X., Sharma, R.K., Gupta, A., George, V., Thomas, A.J., Falcone, T. and Agarwal, A. (2003) Alterations in mitochondria membrane potential and oxidative stress in infertile men: A prospective observational study. Fertility and Sterility, 80, 844-850. http://dx.doi.org/10.1016/S0015-0282(03)00983-X [39] Agarwal, A., Saleh, R.A. and Bedaiwy, M.A. (2003) Role of reactive oxygen species in the pathophysiology of hu- man reproduction. Fertility and Sterility, 79, 829-843. http://dx.doi.org/10.1016/S0015-0282(02)04948-8 [40] Aitken, R.J., Wingate, J.K., De Iuliis, G.N., Koppers, A.J. and McLaughlin, E.A. (2006) Cis-unsaturated fatty acids stimulate reactive oxygen species generation and lipid peroxidation in human spermatozoa. The Journal of Cli- nical Endocrinology & Metabolism, 91, 4154-4163. http://dx.doi.org/10.1210/jc.2006-1309 [41] de Lamirande, E. and Gagnon, C. (1992) Reactive oxy- gen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. Journal of Andrology, 13, 368-378. [42] Tavilani, H., Doosti, M. and Saeidi, H. (2005) Malondial- dehyde levels in sperm and seminal plasma of astheno- zoospermic and its relationship with semen parameters. Clinica Chimica Acta, 356, 199-203. http://dx.doi.org/10.1016/j.cccn.2005.01.017 [43] Kothari, S., Thompson, A., Agarwal, A. and du Plessis, S.S. (2010) Free radicals: Their beneficial and detrimen- tal effects on sperm function. Indian Journal of Experi- mental Biology, 48, 425-435. [44] Aitken, R.J., Buckingham, D., West, K.M., Wu, F.C., Zi- kopoulos, K. and Richardson, D.W. (1992) Differential contribution of leucocytes and spermatozoa to the gene- ration of reactive oxygen species in the ejaculates of oli- gozoospermic patients and fertile donors. The Journal of the Society for Reproduction and Fertility, 94, 451-462. http://dx.doi.org/10.1530/jrf.0.0940451 [45] Zalata, A., Hafez, T. and Comhaire, F. (1995) Evaluation of the role of reactive oxygen species in male infertility. Human Reproduction, 10, 1444-1451. http://dx.doi.org/10.1093/HUMREP/10.6.1444 [46] Pasqualotto, F.F., Sharma, R.K., Nelson, D.R., Thomas, A.J. and Agarwal, A. (2000) Relationship between oxida- tive stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertility and Sterility, 73, 459-464. http://dx.doi.org/10.1016/S0015-0282(99)00567-1 [47] Boonstra, J. and Post, J.A. (2004) Molecular events asso- ciated with reactive oxygen species and cell cycle pro- gression in mammalian cells. Gene, 337, 1-13. http://dx.doi.org/10.1016/j.gene.2004.04.032 [48] Tvrdá, E., Kňažická, Z., Bárdos, L., Massányi, P. and Lukáč, N. (2011) Impact of oxidative stress on male ferti- lity—A review. Acta Veterinaria Hungarica, 59, 465-484. Copyright © 2013 SciRes. OPEN ACCESS  A. Sellami et al. / Advances in Bioscience and Biotechnology 4 (2013) 30-40 39 http://dx.doi.org/10.1556/AVet.2011.034 [49] Ochsendorf, F.R. (1999) Infections in the male genital tract and reactive oxygen species. Human Reproduction Update, 5, 399-420. http://dx.doi.org/10.1093/humupd/5.5.399 [50] Fraczek, M. and Kurpisz, M. (2007) Inflammatory medi- ators exert toxic effects of oxidative stress on human spermatozoa. Journal of Andrology, 28, 325-333. http://dx.doi.org/10.2164/jandrol.106.001149 [51] Villegas, J., Schulz, M., Soto, L., Iglesias, T., Miska, W. and Sánchez, R. (2005) Influence of reactive oxygen spe- cies produced by activated leukocytes at the level of apo- ptosis in mature human spermatozoa. Fertility and Steril- ity, 83, 808-810. http://dx.doi.org/10.1016/j.fertnstert.2004.09.022 [52] Wang, A., Fanning, L., Anderson, D.J. and Loughlin, K.R. (1997) Generation of reactive oxygen species by leukocy- tes and sperm following exposure to urogenital tract in- fection. Archives of Andrology, 39, 11-17. http://dx.doi.org/10.3109/01485019708987896 [53] Saleh, R.A., Agarwal, A., Kandirali, E., Sharma, R.K., Thomas, A.J., Nada, E.A., Evenson, D.P. and Alvarez, J.G. (2002) Leukocytospermia is associated with in- creased reactive oxygen species production by human spermatozoa. Fertility and Sterility, 78, 1215-1224. http://dx.doi.org/10.1016/S0015-0282(02)04237-1 [54] Krausz, C., West, K., Buckingham, D. and Aitken, R.J. (1992) Development of a technique for monitoring the contamination of human semen samples with leukocytes. Fertility and Sterility, 57, 1317-1325. [55] Sukcharoen, N., Keith, J., Irvine, D.S. and Aitken, R.J. (1996) Prediction of the in-vitro fertilization (IVF) poten- tial of human spermatozoa using sperm function tests: The effect of the delay between testing and IVF. Human Reproduction, 11, 1030-1034. http://dx.doi.org/10.1093/oxfordjournals.humrep.a019291 [56] Gomez, E., Buckingham, D.W., Brindle, J., Lanzafame, F., Irvine, D.S. and Aitken, R.J. (1996) Development of an image analysis system to monitor the retention of re- sidual cytoplasm by human spermatozoa: Correlation with biochemical markers of the cytoplasmic space, oxi- dative stress, and sperm function. Journal of Andrology, 17, 276-287. [57] Said, T.M., Agarwal, A., Sharma, R.K., Thomas Jr., A.J. and Sikka, S.C. (2005) Impact of sperm morphology on DNA damage caused by oxidative stress induced by beta- nicotinamide adenine dinucleotide phosphate. Fertility and Sterility, 83, 95-103. http://dx.doi.org/10.1016/j.fertnstert.2004.06.056 [58] Fisher, H.M. and Aitken, R.J. (1997) Comparative analy- sis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. Journal of Experimental Zoology, 277, 390-400. http://dx.doi.org/10.1002/(SICI)1097-010X(19970401)27 7:5<390::AID-JEZ5>3.0.CO;2-K [59] Said, T.M., Agarwal, A., Sharma, R.K., Mascha, E., Sik- ka, S.C. and Thomas Jr., A.J. (2004) Human sperm su- peroxide anion generation and correlation with semen quality in patients with male infertility. Fertility and Ste- rility, 82, 871-877. http://dx.doi.org/10.1016/j.fertnstert.2004.02.132 [60] Pasqualotto, F.F., Sharma, R.K., Kobayashi, H., Nelson, D.R., Thomas Jr., A.J. and Agarwal, A. (2001) Oxidative stress in normospermic men undergoing infertility eva- luation. Journal of Andrology, 22, 316-322. [61] Agarwal, A., Ikemoto, I. and Loughlin, K.R. (1994) Ef- fect of sperm washing on levels of reactive oxygen spe- cies in semen. Archives of Andrology, 33, 157-162. http://dx.doi.org/10.3109/01485019408987819 [62] Fingerova, H., Oborna, I., Novotny, J., Svobodova, M., Brezinova, J. and Radova, L. (2009) The measurement of reactive oxygen species in human neat semen and in sus- pended spermatozoa: A comparison. Reproductive Biol- ogy and Endocrinology, 7, 118. http://dx.doi.org/10.1186/1477-7827-7-118 [63] Venkatesh, S., Shamsi, M.B., Dudeja, S., Kumar, R. and Dada, R. (2011) Reactive oxygen species measurement in neat and washed semen: Comparative analysis and its sig- nificance in male infertility assessment. Archives of Gy- necology and Obstetrics, 283, 121-126. http://dx.doi.org/10.1007/s00404-010-1645-4 [64] Hughes, C.M., Lewis, S.E., McKelvey-Martin V.J. and Thompson, W. (1998) The effects of antioxidant supple- mentation during percoll preparation on human sperm DNA integrity. Human Reproduction, 13, 1240-1247. http://dx.doi.org/10.1093/humrep/13.5.1240 [65] Donnelly, E.T., McClure, N. and Lewis, S.E. (1999) The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide-induced DNA damage in human spermatozoa. Mutagenesis, 14, 505-512. http://dx.doi.org/10.1093/mutage/14.5.505 [66] Chi, H.J., Kim, J.H., Ryu, C.S., Lee, J.Y., Park, J.S., Chung, D.Y., Choi, S.Y., Kim, M.H., Chun, E.K. and Roh, S.I. (2008) Protective effect of antioxidant supple- mentation in sperm-preparation medium against oxidative stress in human spermatozoa. Human Reproduction, 23, 1023-1028. http://dx.doi.org/10.1093/humrep/den060 [67] Donnelly, E.T., McClure, N. and Lewis, S.E. (2000) Glu- tathione and hypotaurine in vitro: Effects on human sperm motility, DNA integrity and production of reactive oxygen species. Mutagenesis, 15, 61-68. http://dx.doi.org/10.1093/mutage/15.1.61 [68] Baumber, J., Ball, B.A. and Linfor, J.J. (2005) Assess- ment of the cryopreservation of equine spermatozoa in the presence of enzyme scavengers and antioxidants. American Journal of Veterinary Research, 66, 772-779. http://dx.doi.org/10.2460/ajvr.2005.66.772 [69] Mahfouz, R., Sharma, R., Thiyagarajan, A., Kale, V., Gu- pta, S., Sabanegh, E. and Agarwal, A. (2010) Semen cha- racterristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertility and Sterility, 94, 2141-2146. http://dx.doi.org/10.1016/j.fertnstert.2009.12.030 [70] Aitken, R.J. and Krausz, C. (2001) Oxidative stress, DNA damage and the Y chromosome. Reproduction, 122, 497- 506. http://dx.doi.org/10.1530/rep.0.1220497 [71] Aitken, R.J. and De Iuliis, G.N. (2007) Origins and con- sequences of DNA damage in male germ cells. Repro- Copyright © 2013 SciRes. OPEN ACCESS  A. Sellami et al. / Advances in Bioscience and Biotechnology 4 (2013) 30-40 Copyright © 2013 SciRes. 40 OPEN ACCESS ductive Bio Medicine Online, 14, 727-733. http://dx.doi.org/10.1016/S1472-6483(10)60676-1 [72] Alvarez, J.G., Sharma, R.K., Ollero, M., Saleh, R.A., Lopez, M.C., Thomas, A.J. Jr., Evenson, D.P. and Agar- wal, A. (2002) Increased DNA damage in sperm from leukocytospermic semen samples as determined by the sperm chromatin structure assay. Fertility and Sterility, 78, 319-329. http://dx.doi.org/10.1016/S0015-0282(02)03201-6 [73] Erenpreiss, J., Hlevicka, S., Zalkalns, J. and Erenpreisa J. (2002) Effect of leukocytospermia on sperm DNA integ- rity: A negative effect in abnormal semen samples. Jour- nal of Andrology, 23, 717-723. [74] Zribi, N., Chakroun, N.F., Elleuch, H., Abdallah, F.B., Ben Hamida, A.S., Gargouri, J., Fakhfakh, F. and Keskes, L.A. (2011) Sperm DNA fragmentation and oxidation are independent of malondialdheyde. Reproductive Biology and Endocrinology, 9, 47-54. http://dx.doi.org/10.1186/1477-7827-9-47 [75] Makker, K., Agarwal, A. and Sharma, R. (2009) Oxida- tive stress and male infertility. Indian Journal of Medical Research, 129, 357-367. [76] Aitken, R.J., De Iuliis, G.N., Finnie, J.M., Hedges, A. and McLachlan, R.I. (2010) Analysis of the relationships be- tween oxidative stress, DNA damage and sperm vitality in a patient population: Development of diagnostic crite- ria. Human Reproduction, 25, 2415-2426. http://dx.doi.org/10.1093/humrep/deq214 [77] Muriel, L., Garrido, N., Fernández, J.L., Remohí, J., Pel- licer, A., de los Santos, M.J. and Meseguer, M. (2006) Value of the sperm deoxyribonucleic acid fragmentation level, as measured by the sperm chromatin dispersion test, in the outcome of in vitro fertilization and intracytoplas- mic sperm injection. Fertility and Sterility, 85, 371-383. http://dx.doi.org/10.1016/j.fertnstert.2005.07.1327 [78] Avendaño, C., Franchi, A., Duran, H. and Oehninger S. (2010) DNA fragmentation of normal spermatozoa nega- tively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertility and Sterility, 94, 549-557. http://dx.doi.org/10.1016/j.fertnstert.2009.02.050 ABBREVIATIONS ROS: Reactive Oxygen Species DNA: Deoxyribonucleic Acid FMLP: Formyl-Methionyl-Leucyl-Phenylalanine WHO: World Health Organisation H2O2: Hydrogen Peroxide SD: Standard Deviation RLU: Relative Light Units HBSS: Hank’s Balanced Salt Solution NADPH: Nicotinamide Adenine Dinucleotide Phosphate G6PD: Glucose-6-Phosphate Dehydrogenase ICSI: Intra Cytoplasmic Sperm Injection
|