 American Journal of Plant Sciences, 2013, 4, 2206-2217 Published Online November 2013 (http://www.scirp.org/journal/ajps) http://dx.doi.org/10.4236/ajps.2013.411274 Open Access AJPS Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants Fatima Alejos-Gonzalez, Kelly Perkins, Malcolm Isaiah Winston, De-Yu Xie* Department of Plant and Microbial Biology, North Carolina State University, Raleigh, USA. Email: *dxie@ncsu.edu Received September 23rd, 2013; revised October 20th, 2013; accepted October 26th, 2013 Copyright © 2013 Fatima Alejos-Gonzalez et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT To enhance the understanding of artemisinin biosynthesis, we have successfully bred self-pollination Artemisia annua plants. Here, we report efficient somatic embryogenesis and organogenesis of self-pollination plants and artemisinin formation in regenerated plants. The first through sixth nodal leaves of seedlings are used as explants. On agar-solidified MS basal medium supplemented with TDZ (0.6 mg/l) and IBA (0.1 mg/l), all explants after inoculation of less than 3 weeks start to form embryogenic calli, which further produce globular, torpedo, heart and early cotyledon embryos. In all six positional leaves, explants from the sixth leaf show the rapidest responses to induction of embryo- genic calli and somatic embryos. On this medium, somatic embryos continuously develop into adventitious buds, which can form adventitious roots on a rooting medium containing NAA (0.5 mg/l). Meanwhile, on agar-solidified MS basal medium supplemented with BAP (1 mg/l) and NAA (0.05 mg/l), approximately 100% of explants from leaves #3 - 6 form calli in less than 3 weeks of inoculation and adventitious buds via organogenesis in 3 - 4 weeks. In all six posi- tional leaves, explants from the sixth leaf exhibit the rapidest response to induction of calli and adventitious buds. Nearly 100% adventitious buds can form adventitious roots on the rooting medium. Regenerated plants from both so- matic embryogenesis and organogenesis complete self-pollination to produce seeds in 80 - 90 days of growth in growth chamber. LC-ESI-MS analysis demonstrates that regenerated plants biosynthesize artemisinin. These results show the highly efficient regeneration capacity of self-pollination A. annua plants that can form a new platform to enhance the understanding of artemisinin biosynthesis and metabolic engineering. Keywords: Artemisi a an n ua; Artemisinin; Biosynthesis; Self-Pollination; Somatic Embryogenesis; Organogenesis; HPLC-MS 1. Introduction To date, Artemisia annua is the only natural resource producing artemisinin which is the main compound used in the artemisinin-based combination therapy (ACT) fight- ing against malarial diseases caused by parasites, such as Plasmodium falciparum and P. viva [1-5]. As we know, malaria is one of the most severe infectious diseases causing life loss of approximately one million people every year. Since 1970s when artemisinin was identified to be an endoperoxide lactone sesquiterpene in A. annua by Chinese scientists [6,7], its medicinal activity helped Chinese people to effectively fight against and control malarial disease in China. Later on, this medicine was recommended to other epidemic countries and regions by World Health Organization (WHO) [1,3,8]. Over the past years, due to the low and variable content of artemisinin in plants, its yield has never met the high demanding for therapy. To fight against malaria, both institutional labo- ratories and companies globally have started to investi- gate A. annua and biosynthesis of artemisinin. Many great efforts have made multiple progresses in the areas of selection of ecotypes [9-11], genetic breeding [2,5,11,12], tissue culture [2,13-15], genetic transforma- tion [16-19], gene cloning and metabolic engineering [20-23]. Particularly, the biochemical and transgenic elucidation of biosynthetic steps from amorpha-4, *Corresponding author.  Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants 2207 11-diene to artemisinic acid [20,24,25] and dihyroar- temisinic acid [25,26] has provided a high potential for semi-synthesis of artemisinin. The introduction of these steps into yeast has allowed the production of artemisinic acid from fermentation [24]. This invention has devel- oped a promising potential approach to synthesize ar- temisinin. Currently, plant growth in the field is still the main approach to produce artemisinin for ACT of malaria. Over the past many years, breeding efforts have largely increased the yield of artemisinin [11,27] and enhanced the understanding of artemisinin biosynthesis [5,12]. However, due to the feature of A. annua preferring to cross pollination and hybridity of progenies [5,27,28], the variation problem of artemisinin yield has remained to be resolved. In addition, no success in mutagenesis has been reported to use forward genetic tools to understand artemisinin biosynthesis. To overcome this problem, we have been endeavoring to breed self-pollination plants [2]. To date, we obtained F6 progenies of plants, in which no segregation occurs. Accordingly, this self-pol- lination population allows us being able to investigate genetics and regulation of artemisinin biosynthesis. Par- ticularly, self-pollination plants will allow us continuing to use forward and reverse genetics to dissect the bio- synthetic pathway of artemisinin and to use metabolic engineering approaches for high production. As we know, a successful tissue culture system is the basis for genetic transformation. Over the past approxi- mately 30 years, numerous experiments have been per- formed to use tissue culture to regenerate and propagate A. annua clones for artemisinin production, as a good result, basal medium and phytohormone combinations have been optimized for different ecotypes [13-15,29-36]. These past endeavors greatly helped us save time and labor to avoid testing all phytohormones. Therefore, in our investigation, we only selected a few of combinations of plants hormones to test regeneration capacity of self-pollination plants and develop protocols. Young seedlings were used as material resources. Leaves from the first node to the sixth node of seedlings were used as explants to compare their regeneration efficiency. Of them, the sixth leaf showed 100% efficiency in both so- matic embryogenesis and organogenesis. This high re- generation efficiency allows us to further utilize self- pollination plants for future genetic transformation and knockout of genes to understand the biosynthetic path- way and regulation of artemisinin in the future. 2. Materials and Methods 2.1. Chemicals Indo-3-butyric acid (IBA), naphthaleneacetic acid (NAA), 6-benzylaminopurine (BAP or 6-BA), sucrose, and phy- toagar as well as chemicals of macronutrients, micronu- trients and organic nutrients used in basal MS medium [37] were purchased from Plant Media (Dublin, OH, USA). Thidiazuron (TDZ) was purchased from Sigma (St. Louis, MO, USA). 2.2. Medium Preparation and Photoperiod The basal MS medium was used in our experiments. Phytohormones used were sterilized using a filtration through a 0.2 µm membrane. All media used in experi- ments were added 2% (W/V) sucrose and 0.45% (W/V) phytoagar, adjusted to pH 5.7 with 1 N NaOH and then autoclaved 35 min at 121˚C. After autoclaved, media were cooled down to 50˚C - 60˚C, necessary phytohor- mones were added to reach working concentrations used in each medium described below. Twenty milliliters of liquefied agar medium was poured into one petri dish (15 × 100 mm, height × diameter) and then solidified at room temperature. The photoperiod and temperature for callus induction and regeneration were set up at light/dark (16/8 hrs) and 24˚C - 25˚C, respectively. The light intensity was set up at 50 µmol·m−1·s−1. 2.3. Seed Germination and Selection of Explants Seeds from self-pollination progeny plants (F5 and F6) of A. annua grown in phytotron were used in this ex- periment. Seeds were treated 1 min in 0.5 ml of 70% ethanol contained in a sterile Eppendorf tube. During this treatment, the tube was vortexed thoroughly. Ethanol was then removed to a waste container. Seeds were subse- quently washed four times using autoclaved deionized H2O. These surface-sterilized seeds were then treated 5 min using 10% Clorox in a sterile Eppendorf tube, during which the tube was vortexed 1 min thoroughly. After Clorox was disposed into a waste container, seeds were washed four times using autoclaved deionized H2O. Ster- ilized seeds were placed on phytoagar-solidified MS me- dium contained in petri dishes, which were then placed in an incubator with necessary photoperiod and temperature described above. After three weeks of seed germination, seedlings (Fig- ure 1(a)) developed the first two true simple leaves (#1 and #2) in addition to the two cotyledons. The size of two leaves was approximately 0.8 - 1 cm in length. The first and second leaves of these three-week old seedlings were excised for explant materials. The 3rd, 4th, 5th and 6th leaves (Figure 1(b)) of 35-old seedlings were excised as explant materials. For explant preparation, the first and second leaves were wounded on both adaxial and abaxial surfaces with Open Access AJPS  Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants Open Access AJPS 2208 Figure 1. Growth of seedlings and leaves used for explants. (a) Seedlings (three-week old) grown on agar-solidified ME1 (basal MS) medium in a petri dish; (b) Morphologies of the 1st and 2nd leaves from three-week old seedlings and the 3rd through 6th leaves of seedlings from 35-day old seedlings. 2.6. Induction of Adventitious Roots to Obtain Plantlets a sterilized razor. The 3rd - 6th leaves were cut into ap- proximately 0.8 × 1 cm size pieces. Wounded leaf pieces were used as explants for induction of calli and adventi- tious buds described below. Based on many optimized media for rooting of adventi- tious buds/shoots reported previously (seeing discussion), we selected one medium consisting of basal MS medium supplemented with 0.05 mg/l NAA (Table 1). Agar-so- lidified rooting medium was contained in 15 cm long glass tubes. 2.4. Treatment of Explants with TDZ and IBA Combinations Three different concentrations of TDZ and 0.1 mg/l IBA were selected to form three combinations (Table 1). Meanwhile, MS basal medium was used as a control. Thirty explants from the 1st and 2nd leaves (Figure 1(b)) were inoculated onto agar-solidified medium contained in one petri dish (100 × 15 mm, diameter × height, in size). The other petri dish was performed as a technical repeat for each medium. Petri dishes were sealed with parafilm and placed under the culture condition described above. Explants were examined every day and taken pictures at different days (e.g. 2, 7, 17 and 30 days) after inoculation. The dates of callus and adventitious bud formation were recorded in detail. This experiment was repeated 4 times. In addition, this experiment was tested with both F5 and F6 progeny plants, respectively. Adventitious buds (0.3 - 0.5 cm in length) from so- matic embryos induced by TDZ and IBA combinations were excised from calli for root induction. Adventitious buds (0.5 - 1 cm in length with 2 - 3 leaves) induced by BAP and NAA were separated from explants or calli for root induction. Adventitious buds were inoculated on rooting medium (ME5, Table 1) contained in glass tubes. All tubes were sealed with parafilm and placed under the culture condition described above. 2.7. Plant Growth in Soil, Self-Pollination and Seed Germination After one month of root induction, plantlets were trans- planted to small pots (15 × 15 cm, diameter by height) filled with premier Pro-Mix-PGX (fine granulated) soil. One pot was planted with one plantlet and then was placed on a nursery bed facilitated with a photoperiod of 12/12 (light/dark) at 25˚C in the Phytotron. The light intensity was set at 50 µE/m2/sec. During the period in the nursery bed, plants were misted with tap water one time per 3 sec during the light cycle and one time per 3 min during the dark cycle. After two weeks of growth, each regenerated plant was transplanted into a 10 cm pot filled with premier Pro-Mix-PGX (fine granulated) soil. All plants were then placed in a growth chamber facili- tated with a photoperiod of 9/15 hrs (light/dark). A tem- perature cycle was set at 26˚C/22˚C (light/dark) as re- ported previously [2]. Plants were watered every other day with nutrients and alternate days with tap water. 2.5. Test of Regeneration Capacity among Leaves #1 through #6 In this study, we selected two combinations of plant hormones to test regeneration capacity of explants from different positional leaves. One was 0.6 mg/L TDZ and 0.1 mg/L IBA (ME4, Table 1) and the other was 1 mg/l BAP and 0.05 mg/l NAA. Explants were obtained from 1st, 2nd, 3rd, 4th, 5th and 6th leaves (Figure 1(b)) respectively, of which explants from leaves #1 and 2 were considered as one group, while each of others was as an individual group, respec- tively. Fifteen explants from each group were inoculated onto agar-solidified medium contained in one petri dish. Two petri dishes were tested as a technical repeat for each group of explants. Inoculation, observation and taking picture were as described above. This experiment was repeated 4 times and tested using both F5 and F6 progenies of plant, respectively. To test self-pollination, each plant was covered using a plastic bag with an opening of the top and management of flowering and seed harvest were the same as reported previously [2]. 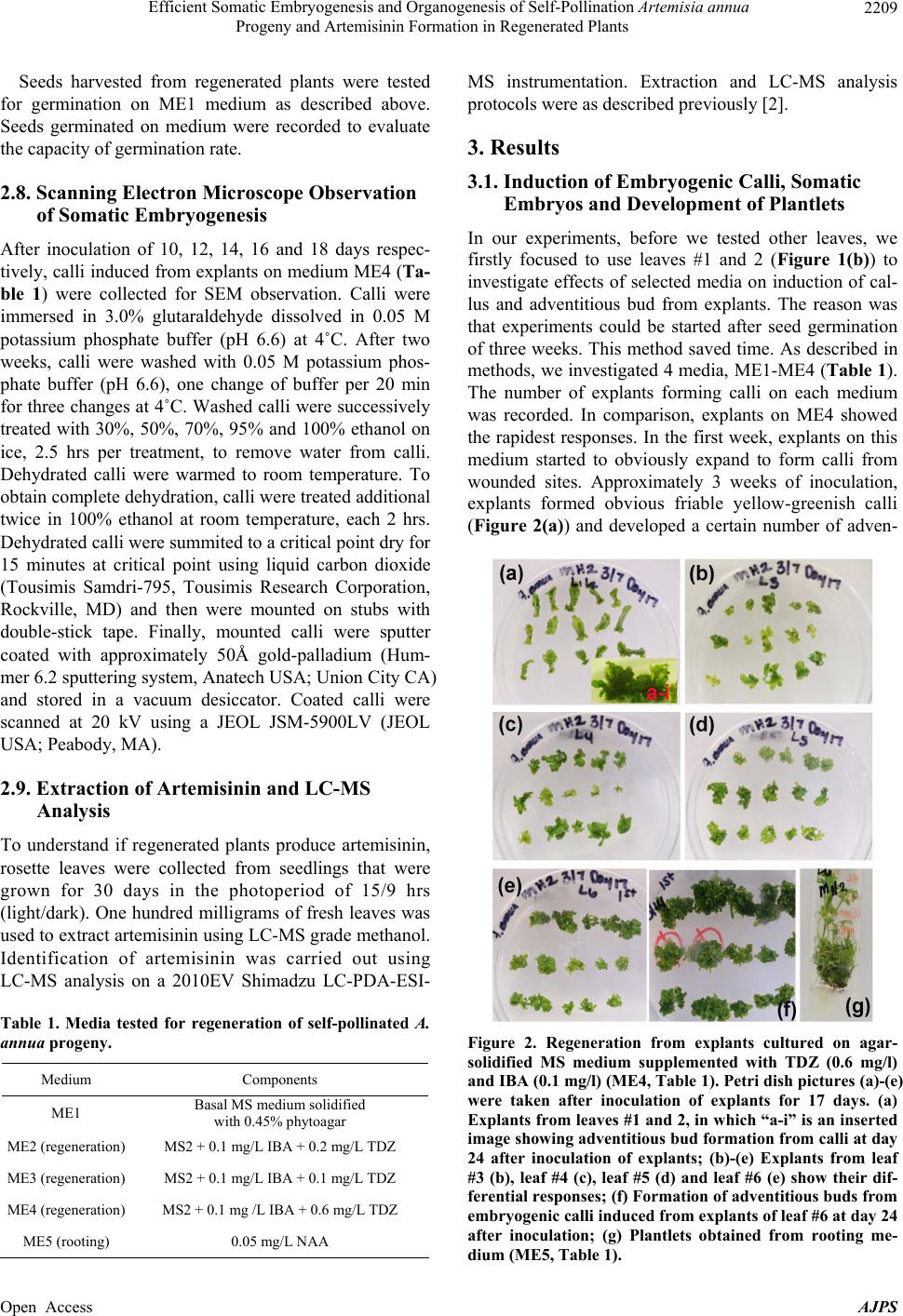 Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants 2209 Seeds harvested from regenerated plants were tested for germination on ME1 medium as described above. Seeds germinated on medium were recorded to evaluate the capacity of germination rate. 2.8. Scanning Electron Microscope Observation of Somatic Embryogenesis After inoculation of 10, 12, 14, 16 and 18 days respec- tively, calli induced from explants on medium ME4 (Ta- ble 1) were collected for SEM observation. Calli were immersed in 3.0% glutaraldehyde dissolved in 0.05 M potassium phosphate buffer (pH 6.6) at 4˚C. After two weeks, calli were washed with 0.05 M potassium phos- phate buffer (pH 6.6), one change of buffer per 20 min for three changes at 4˚C. Washed calli were successively treated with 30%, 50%, 70%, 95% and 100% ethanol on ice, 2.5 hrs per treatment, to remove water from calli. Dehydrated calli were warmed to room temperature. To obtain complete dehydration, calli were treated additional twice in 100% ethanol at room temperature, each 2 hrs. Dehydrated calli were summited to a critical point dry for 15 minutes at critical point using liquid carbon dioxide (Tousimis Samdri-795, Tousimis Research Corporation, Rockville, MD) and then were mounted on stubs with double-stick tape. Finally, mounted calli were sputter coated with approximately 50Å gold-palladium (Hum- mer 6.2 sputtering system, Anatech USA; Union City CA) and stored in a vacuum desiccator. Coated calli were scanned at 20 kV using a JEOL JSM-5900LV (JEOL USA; Peabody, MA). 2.9. Extraction of Artemisinin and LC-MS Analysis To understand if regenerated plants produce artemisinin, rosette leaves were collected from seedlings that were grown for 30 days in the photoperiod of 15/9 hrs (light/dark). One hundred milligrams of fresh leaves was used to extract artemisinin using LC-MS grade methanol. Identification of artemisinin was carried out using LC-MS analysis on a 2010EV Shimadzu LC-PDA-ESI- Table 1. Media tested for regeneration of self-pollinated A. annua progeny. Medium Components ME1 Basal MS medium solidified with 0.45% phytoagar ME2 (regeneration) MS2 + 0.1 mg/L IBA + 0.2 mg/L TDZ ME3 (regeneration) MS2 + 0.1 mg/L IBA + 0.1 mg/L TDZ ME4 (regeneration) MS2 + 0.1 mg /L IBA + 0.6 mg/L TDZ ME5 (rooting) 0.05 mg/L NAA MS instrumentation. Extraction and LC-MS analysis protocols were as described previously [2]. 3. Results 3.1. Induction of Embryogenic Calli, Somatic Embryos and Development of Plantlets In our experiments, before we tested other leaves, we firstly focused to use leaves #1 and 2 (Figure 1(b)) to investigate effects of selected media on induction of cal- lus and adventitious bud from explants. The reason was that experiments could be started after seed germination of three weeks. This method saved time. As described in methods, we investigated 4 media, ME1-ME4 (Table 1). The number of explants forming calli on each medium was recorded. In comparison, explants on ME4 showed the rapidest responses. In the first week, explants on this medium started to obviously expand to form calli from wounded sites. Approximately 3 weeks of inoculation, explants formed obvious friable yellow-greenish calli (Figure 2(a)) and developed a certain number of adven- Figure 2. Regeneration from explants cultured on agar- solidified MS medium supplemented with TDZ (0.6 mg/l) and IBA (0.1 mg/l) (ME4, Table 1). Petri dish pictures (a)-(e) were taken after inoculation of explants for 17 days. (a) Explants from leaves #1 and 2, in which “a-i” is an inserted image showing adventitious bud formation from calli at day 24 after inoculation of explants; (b)-(e) Explants from leaf #3 (b), leaf #4 (c), leaf #5 (d) and leaf #6 (e) show their dif- ferential responses; (f) Formation of adventitious buds from embryogenic calli induced from explants of leaf #6 at day 24 after inoculation; (g) Plantlets obtained from rooting me- dium (ME5, Table 1). Open Access AJPS  Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants 2210 titious buds, which continuously developed leaf struc- tures in the following 4th and 5th weeks of culture (Figure 2(a-i)). In addition, although unlike the rapid responses on ME4, yellow-greenish calli and adventitious buds were induced from explants on ME2 and ME3, respec- tively. In contrast, explants neither formed calli nor ad- ventitious buds on ME1, the basal MS medium. Among three combinations of TDZ and IBA (Table 1, ME2-ME3), the percentage of explants forming calli was similar on ME2, ME3 and ME4 after nearly 3 weeks of inoculation (Figure 3(a)). In contrast, the percentage of adventitious bud formation from calli was significantly lower on ME2 than on ME3 and ME4, between which the average value on ME4 was higher (Figure 3(a)). As a result, we used ME4 to compare regeneration capacities of leaves #1 through 6. In contrast, neither calli nor ad- ventitious buds were formed from explants on ME1 (Figure 3(a)). To understand the features of calli, we collected callus samples induced on ME4 at different dates and then im- mediately fixed them for SEM observation. Under SEM, different stages of somatic embryo structures were ob- served, including globular, torpedo, heart and early coty- ledon embryos (Figures 4(a)-(d)). These results demon- strated that TDZ and IBA tested induced embryogenic calli. The formation of adventitious buds on ME2-ME4 was via a procedure of somatic embryogenesis. On ME4, somatic embryos could continuously develop into vegetative structures, such as buds and leaves (Fig- ures 2(a-i) and (f)). Many adventitious buds with leaves were formed from calli after three weeks of induction. As culture continued, multiple independent adventitious shoots (elongated adventitious buds) became highly ob- vious. This result showed that on this medium, somatic embryos could further develop to form shoot apical mer- istems and leaves. Furthermore, this observation was highly obvious on explants from leaves #3 - 6 described below. However, no plantlets were obtained on ME4. Neither was a plantlet formed on ME2 and ME3. To obtain plantlets, adventurous shoots were inocu- lated onto a rooting medium (ME5, Table 1), which was supplemented with 0.1 mg/l NAA. On this medium, nearly 100% of shoots developed roots to form plantlets (Figure 2(g)). Therefore, the use of ME4 and ME5 were effective to induce regeneration via somatic embryo- genesis. 3.2. Comparison of Leaves #1 through #6 Responding to TDZ and IBA To understand regeneration capacity of different leaves from seedlings, we compared six positional leaves, in- cluding the 1st and 2nd leaves (leaves #1 and 2) from three-week old seedlings and the 3rd - 6th leaves (leaves #3 - 6) from five-week old seedlings. For this compari- son, we tested explants on ME4. Responses of explants were recorded in detail at different dates. After inocula- tion of 17 days, almost all explants from different leaves formed embryogenic calli (Figures 2(a)-(e)); although the average percentage value of induction from leaves #1 and 2 was slightly lower (Figure 3(b)). The formation of adventitious buds (from somatic em- bryogenesis) was also obvious at day 17 after explant inoculation (Figures 2(a)-(e)). After three weeks of in- duction, multiple adventitious buds developed from so- matic embryos were characterized with one-two leaves (Figures 2(a-i) and (f)) but without roots. In the six posi- tional leaves, explants from the 6th leaf exhibited the highest average percentage value (Figure 3(b)). As cul- ture was extended to 3 - 4 weeks, embryogenic calli in- duced from all explants of the 6th leaf formed multiple adventitious buds (Figure 2(f)). Somatic embryos induced from different leaf explants could not form roots on ME4. For root induction, adven- titious buds were cultured onto ME5, on which, nearly 100% of them formed roots to develop into complete plantlets (Figure 2(g)). 3.3. Regeneration on Medium Supplemented with BAP and NAA Over the past 30 years, many concentration combinations of BAP and NAA were tested to induce organogenesis of A. annua plants. Multiple combinations of different phy- tohormones such as BAP, NAA, IAA, KT and IBA have been optimized for different ecotypes [13-15,34,35, 38-43]. Based on these previous reports, we only chose one combination consisting of 1 mg/l BAP and 0.05 mg/l NAA to test regeneration capacity of leaves. Leaves #1 through #6 of seedlings were used for ex- plants to compare their responses to BAP and NAA. Re- sultant data showed differences in induction of both cal- lus and adventitious bud among explants (Figure 3(c); Figures 5(a)-(e)). After inoculation of 17 days, all ex- plants from leaves #4, 5 and 6 formed greenish compact calli. The average induction rate of calli from leaf #1 and 2 was approximately 63%, significantly lower than those values from other leaves (Figure 3(c)). As culture con- tinued, all explants from different leaves produced calli. Under microscope, calli induced by BAP and NAA were relatively compact and different from embryogenic calli induced by TDZ and IBA described above. In addition, of 6 positional leaves tested, explants from leaf #6 gave the highest induction rate of adventitious bud formation at day 17, the average percentage value of which was significantly higher than those values from leaves #1, 2, 3 and 4 (Figure 3(c)). Approximately 92% of explants from leaf #6 produced adventitious buds. Open Access AJPS  Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants Open Access AJPS 2211 Figure 3. Effec ts of media and le af positions on callus induction and adventitious bud for m ation. (a) Data show percentages of induction of embryogenic calli and formation of adventitious buds from le af #1 and 2 explants on ME1-ME4 (Table 1) after cultured 21 days; percentage values are mean values from 4 independent experiments, each of which was performed with 30 explants and technically repeated once; error bars are calculated from standard deviation. Columns labeled with different letters such as “A” and “B” indicate significant differences evaluated by Student’s T-test, P < 0.05, while labeled with the same letter such as “B” and “C” indicate insignificant differences. (b) Data show percentage values of embryogenic calli in- duction and formation of adventitious buds from explants of leaves #1 - 6 after inocul ation of 17 days on medium ME4; per- centage values are mean values of 4 independent experiments, each of which was performed with 15 explants and technically repeated once; error bars were calculated from standard deviation. Columns labeled with the same capitalized letters indi- cate insignificant differences (P values > 0.05), while columns labeled with different capitalized letters indicates significant differences (P < 0.05) evaluated by Student’s T-test. (c) Data show percentage values of induction of calli and formation of adventitious buds from explants of leaves #1 - 6 after cultur ed 17 days on MS medium supplemented with BAP (1 mg/l) and NAA (0.1 mg/l); percentage values are mean values of 4 independent experiments, each of which was performed with 15 ex- plants and technically repeated once; standard error bars were calculated from standard deviation; columns labeled with the same capitalized letter(s) indicate insignificant differences (P > 0.05), while columns labeled with different capitalized letters indicates significant differences (P < 0.05) evaluated by Student’s T-test. 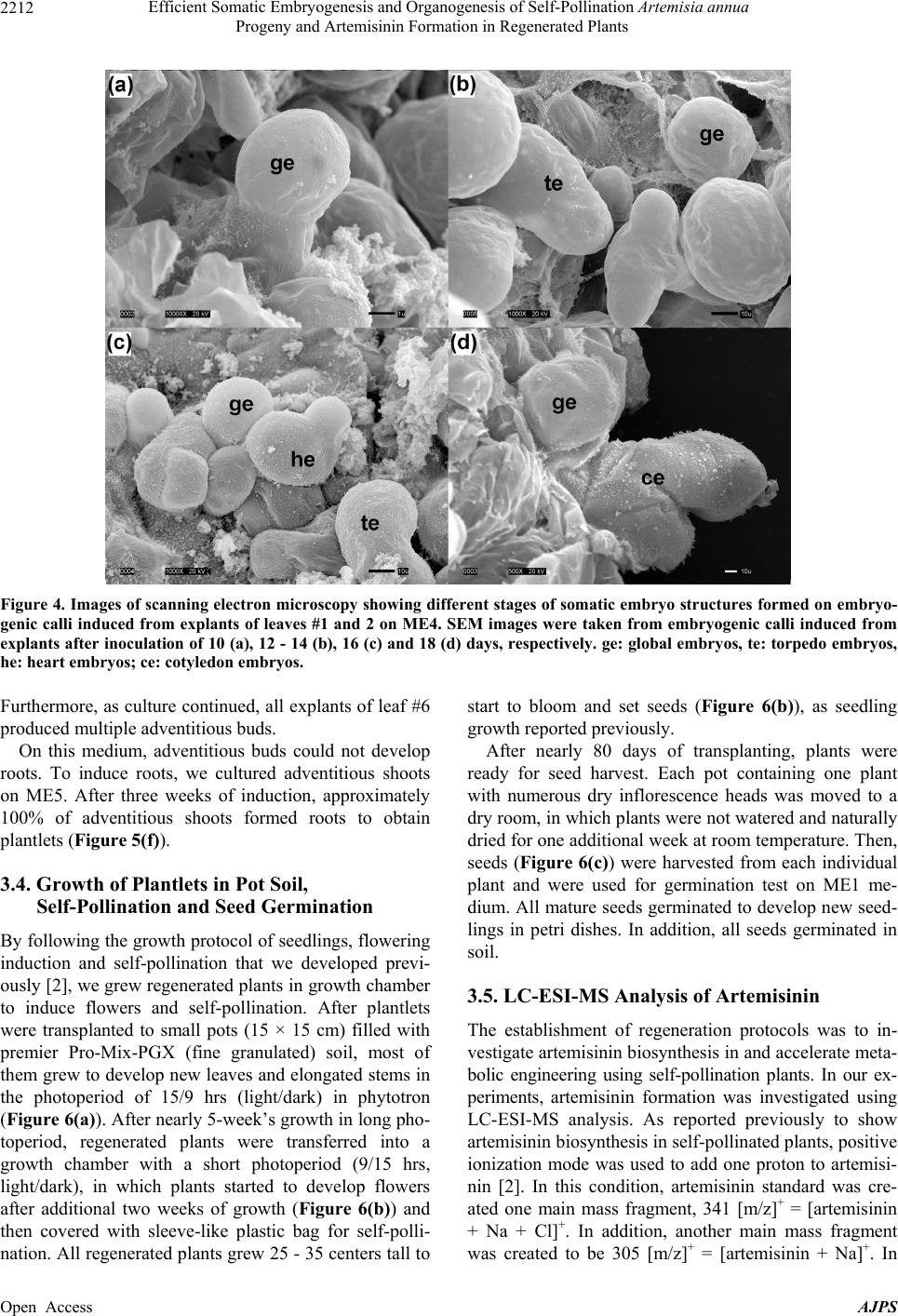 Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants 2212 Figure 4. Images of scanning electron microscopy showing different stages of somatic embryo structures formed on embryo- genic calli induced from explants of leaves #1 and 2 on ME4. SEM images were taken from embryogenic calli induced from explants after inoculation of 10 (a), 12 - 14 (b), 16 (c) and 18 (d) days, respectively. ge: global embryos, te: torpedo embryos, he: heart embryos; ce: coty ledon embryos. Furthermore, as culture continued, all explants of leaf #6 produced multiple adventitious buds. On this medium, adventitious buds could not develop roots. To induce roots, we cultured adventitious shoots on ME5. After three weeks of induction, approximately 100% of adventitious shoots formed roots to obtain plantlets (Figure 5(f)). 3.4. Growth of Plantlets in Pot Soil, Self-Pollination and Seed Germination By following the growth protocol of seedlings, flowering induction and self-pollination that we developed previ- ously [2], we grew regenerated plants in growth chamber to induce flowers and self-pollination. After plantlets were transplanted to small pots (15 × 15 cm) filled with premier Pro-Mix-PGX (fine granulated) soil, most of them grew to develop new leaves and elongated stems in the photoperiod of 15/9 hrs (light/dark) in phytotron (Figure 6(a)). After nearly 5-week’s growth in long pho- toperiod, regenerated plants were transferred into a growth chamber with a short photoperiod (9/15 hrs, light/dark), in which plants started to develop flowers after additional two weeks of growth (Figure 6(b)) and then covered with sleeve-like plastic bag for self-polli- nation. All regenerated plants grew 25 - 35 centers tall to start to bloom and set seeds (Figure 6(b)), as seedling growth reported previously. After nearly 80 days of transplanting, plants were ready for seed harvest. Each pot containing one plant with numerous dry inflorescence heads was moved to a dry room, in which plants were not watered and naturally dried for one additional week at room temperature. Then, seeds (Figure 6(c)) were harvested from each individual plant and were used for germination test on ME1 me- dium. All mature seeds germinated to develop new seed- lings in petri dishes. In addition, all seeds germinated in soil. 3.5. LC-ESI-MS Analysis of Artemisinin The establishment of regeneration protocols was to in- vestigate artemisinin biosynthesis in and accelerate meta- bolic engineering using self-pollination plants. In our ex- periments, artemisinin formation was investigated using LC-ESI-MS analysis. As reported previously to show artemisinin biosynthesis in self-pollinated plants, positive ionization mode was used to add one proton to artemisi- nin [2]. In this condition, artemisinin standard was cre- ated one main mass fragment, 341 [m/z]+ = [artemisinin + Na + Cl]+. In addition, another main mass fragment was created to be 305 [m/z]+ = [artemisinin + Na]+. In Open Access AJPS 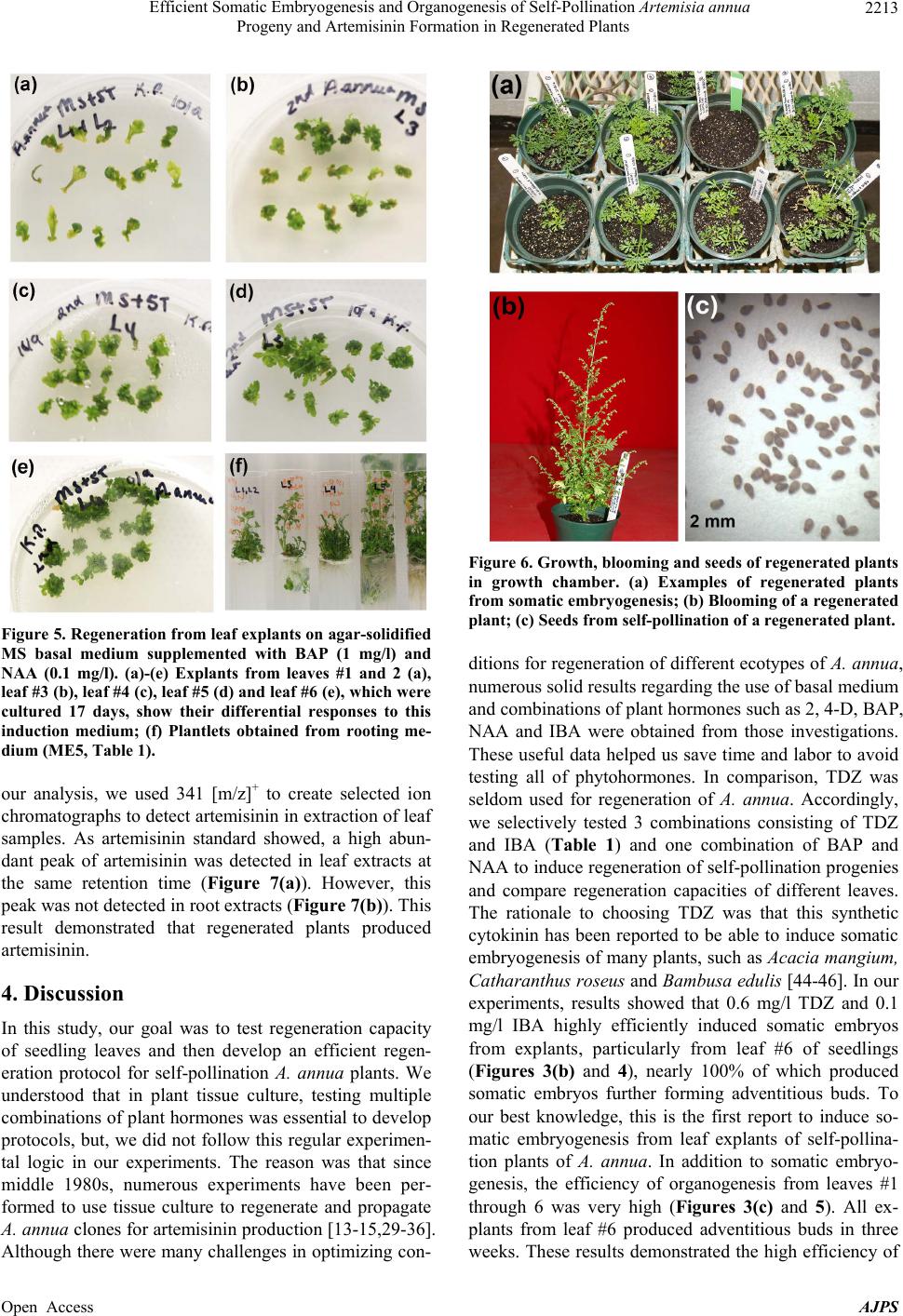 Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants 2213 Figure 5. Regeneration from leaf explants on agar-solidified MS basal medium supplemented with BAP (1 mg/l) and NAA (0.1 mg/l). (a)-(e) Explants from leaves #1 and 2 (a), leaf #3 (b), leaf #4 (c), leaf #5 (d) and leaf #6 (e), which were cultured 17 days, show their differential responses to this induction medium; (f) Plantlets obtained from rooting me- dium (ME5, Table 1). our analysis, we used 341 [m/z]+ to create selected ion chromatographs to detect artemisinin in extraction of leaf samples. As artemisinin standard showed, a high abun- dant peak of artemisinin was detected in leaf extracts at the same retention time (Figure 7(a)). However, this peak was not detected in root extracts (Figure 7(b)). This result demonstrated that regenerated plants produced artemisinin. 4. Discussion In this study, our goal was to test regeneration capacity of seedling leaves and then develop an efficient regen- eration protocol for self-pollination A. annua plants. We understood that in plant tissue culture, testing multiple combinations of plant hormones was essential to develop protocols, but, we did not follow this regular experimen- tal logic in our experiments. The reason was that since middle 1980s, numerous experiments have been per- formed to use tissue culture to regenerate and propagate A. annua clones for artemisinin production [13-15,29-36]. Although there were many challenges in optimizing con- Figure 6. Growth, blooming and seeds of regenerated plants in growth chamber. (a) Examples of regenerated plants from somatic embryogenesis; (b) Blooming of a regenerated plant; (c) Seeds from self-pollination of a regenerated plant. ditions for regeneration of different ecotypes of A. annua, numerous solid results regarding the use of basal medium and combinations of plant hormones such as 2, 4-D, BAP, NAA and IBA were obtained from those investigations. These useful data helped us save time and labor to avoid testing all of phytohormones. In comparison, TDZ was seldom used for regeneration of A. annua. Accordingly, we selectively tested 3 combinations consisting of TDZ and IBA (Table 1) and one combination of BAP and NAA to induce regeneration of self-pollination progenies and compare regeneration capacities of different leaves. The rationale to choosing TDZ was that this synthetic cytokinin has been reported to be able to induce somatic embryogenesis of many plants, such as Acacia mangium, Catharanthus roseus and Bambusa edulis [44-46]. In our experiments, results showed that 0.6 mg/l TDZ and 0.1 mg/l IBA highly efficiently induced somatic embryos from explants, particularly from leaf #6 of seedlings (Figures 3(b) and 4), nearly 100% of which produced somatic embryos further forming adventitious buds. To our best knowledge, this is the first report to induce so- matic embryogenesis from leaf explants of self-pollina- tion plants of A. annua. In addition to somatic embryo- genesis, the efficiency of organogenesis from leaves #1 through 6 was very high (Figures 3(c) and 5). All ex- plants from leaf #6 produced adventitious buds in three weeks. These results demonstrated the high efficiency of Open Access AJPS  Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants Open Access AJPS 2214 Figure 7. Selected ion chromatographs showing formation of artemisinin in leaves of regenerated plants. (a) A peak showing artemisinin in crude leaf extraction characterized by a mass-to-charge of 341 [m/z]+; (b) No peaks at 341 [m/z]+ detected from crude root extraction; (c) An authentic standard of artemisinin characterized by a mass-to-charge of 341 [m/z]+. regeneration capacity of leaves of self-pollination plants. Another goal of this investigation was to compare re- generation capacity of different positional leaves of seed- lings and to select explant resources for future genetic transformation to enhance metabolic engineering of ar- temisinin. We understand that testing all positional leaves can provide a comprehensive result showing re- generation capacities of all leaves. In consideration of reducing time, labor and spaces, we chose 3-week to 5-week old seedlings grown on agar medium in our in- vestigation. This time frame allowed us testing regenera- tion of explants in a relatively short period. Our data showed that although explants from the 1st to the 6th leaves of seedlings could efficiently produce embryo- genic calli and adventitious buds on ME4, the average values of the 6th leaf were higher than those of other leaves in nearly 3-week period after inoculation (Figure 3(b)). Actually, after continuous culture to 4 weeks, all explants of the 6th leaf produced adventitious buds. In addition, on the medium containing BAP and NAA, ex- plants from the 6th leaf showed the highest percentage of adventitious bud induction in approximately 3 weeks after inoculation (Figure 3(c)). These results indicate that the 6th leaf tested in our experiments has the highest regeneration capacity. The possible reason was that on 5-week old seedlings, the 6th leaf was less mature than others, thus gave the highest efficiency. The other possi- ble reason was that the 6th leaf itself had higher regenera- tion capacity than others. This is because the spatial posi- tions of tissues have been reported to dramatically affect regeneration of plants, the examples of which are Popu- lus trichocarpa [47], A. mangium [48] and Cornus cana- densis [49]. This investigation is to develop self-pollination A. an- nua plants as a platform to understand genetics of ar- temisinin biosynthesis and to enhance metabolic engi- neering for high yield. As we know, the final elucidation of biosynthetic pathways of natural products essentially needs genetic evidence. For example, genetic evidence from Arabidopsis thaliana and other model plants has helped the intensive understanding of anthocyanin and proanthocyanidin pathways in the plant kingdom [50-54]. To date, biochemical, molecular and synthetic evidence has demonstrated the enzymatic steps from amorphor-4, 11-diene to artemisinic acid and dihydroartemisinic acid [22-26,55] and mapping of F1 hybrid of A. annua has helped identify loci associated with artemisinin forma- tion [5], however, genetic evidence, such as knockout of genes and their impact on artemisinin productions, re- mains largely lacking. One of crucial reasons has been the challenge of the heterogeneous progeny resulting from the cross-hybridization preference of A. annua [2,9,27,28]. This heterogeneity of progeny increases dif- ficulty to select mutant plants to identify pathway and regulatory genes involved in artemisinin biosynthesis. In addition, genetic transformation of A. annua has been a challenging hurdle in metabolic engineering of artemisi- nin most likely due to heterogeneity of progeny [43]. We have developed self-pollination plants [2]. Progenies of F5 and F6 generations have not shown any segregation in  Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants 2215 plant growth and morphology as well as artemisinin for- mation, indicating that they are mostly likely inbred ho- mozygous plants. We believe that the protocol of effi- cient regeneration developed in present study will help accelerate the use of self-pollination plants to develop genetic approaches such as mutagenesis to elucidate biosynthetic steps and regulatory mechanism of artemisi- nin formation. 5. Conclusion The high regeneration variation of different ecotypes of A. annua plants has been reported to be a severe hurdle for the success of genetic transformation. The main reason likely is the segregation of progeny plants resulting from natural cross-hybridization. Our experiments demonstrate a high and reproducible regeneration efficiency of self- pollinated A. annua progeny through both somatic em- bryogenesis and organogenesis. Positional effects of leaves from juvenile seedlings on callus induction and regeneration are observed in our experiments. In the se- lected first six leaves of seedlings, the sixth leaf shows the rapidest response to induction of embryogenic callus and organogenesis as well as regeneration. Regenerated plants from both somatic embryogenesis and organo- genesis produced a valuable level of artemisinin. Our data show that self-pollinated A. annua plants form an appropriate platform to genetically understand artemisi- nin biosynthesis and enhance metabolic engineering. 6. Acknowledgements This research was funded by Multidisciplinary Research Grant (MRG) Program at North Carolina Biotechnology Center. We are grateful to Mrs. Valerie Knowlton at Center for Electron Microscopy for her assistance in SEM. REFERENCES [1] WHO, “Artemisinin and Its Derivatives as Anti-Malarial Drugs,” WHO, Geneva, 1998. [2] F. Alejos-Gonzalez, G. S. Qu, L. L. Zhou, C. H. Saravitz, J. L. Shurtleff and D.-Y. Xie, “Characterization of De- velopment and Artemisinin Biosynthesis in Self-Pollinated Artemisia annua Plants,” Planta, Vol. 234, No. 4, 2011, pp. 685-697. [3] WHO, “Meeting on the Production of Artemisinin and Artemisinin-Based Combination Therapies,” WHO, Aru- sha, 2006. [4] WHO, “Good Procurement Practices for Artemisinin- Based Antimalarial Medicines,” WHO Global Malaria Programme, WHO, Geneva, 2010. [5] I. A. Graham, K. Bessr, S. Blumer, C. A. Branigan, T. Czechowski, L. Elias, I. Guterman, D. Harvey, P. G. Isaac, A. M. Khan, T. R. Larson, Y. Li, T. Pawson, T. Penfield, M. F. Smallwood, V. Segura, T. Townsend, D. Vyas, T. Winzer and D. Bowles, “The Genetic Map of Artemisia annua L. Identifies Loci Affecting Yield of the Antima- larial Drug Artemisinin,” Science, Vol. 327, No. 5963, 2010, pp. 328-331. http://dx.doi.org/10.1126/science.1182612 [6] J.-M. Liu, M.-Y. Ni, J-F. Fan, Y.-Y. Tu, Z.-H. Wu and Y.-L. Wu, “Structure and Reaction of Arteannuin,” Acta Chimica Sinica, Vol. 37, No. 2, 1979, pp. 129-143. [7] Zhong-Yi-Yan-Jiu-Yue-Zhong-Yao-Yan-Jiu-Suo, “Anti- malarial Studies of Artemisia annua L:1971-1978,” Zhong Yi Yan Jiu Yue Zhong Yao Yan Jiu Suo, Beijing, 1978. [8] WHO, “The Role of Artemisinin and Its Derivatives in the Current Treatment of Malaria (1994-1995),” Unpub- lished Document, WHO/MAL/94.1067, World Health Organization, Geneva, 1994. [9] J. F. S. Ferreira, “Cultivation and Genetics of Artemisia annua L. for Increased Production of the Antimalarial Artemisinin,” Plant Genetic Resources: Characterization and Utilization, Vol. 3, No. 2, 2005, pp. 206-229. http://dx.doi.org/10.1079/PGR200585 [10] E. V. Geldre, A. Vergauwe and E. V. den Eeckhout, “State of the Art of the Production of the Antimalarial Compound Artemisinin in Plants,” Plant Molecular Bi- ology, Vol. 33, No. 2, 1997, pp. 199-209. http://dx.doi.org/10.1023/A:1005716600612 [11] J. Cockram, C. Hill, C. Burns, R. R. J. Arroo, J. G. Wool- ley, I. Flockart, T. Robison, C. J. Atkinson, M. J. Davies, N. Dungey, A. J. Greenland, L. Smith and S. Bentley, “Screening a Diverse Collection of Artemisia annua Germplasm Accessions for the Antimalarial Compound, Artemisinin,” Plant Genetic Resources, Vol. 10, No. 2, 2012, pp. 152-154. http://dx.doi.org/10.1017/S1479262112000159 [12] T. E. Wallaart, N. Pras and W. J. Quax, “Seasonal Varia- tions of Artemisinin and Its Biosynthetic Precursors in Tetraploid Artemisia annua Plants Compared with the Diploid Wild-Type,” Planta Medica, Vol. 65, No. 8, 1999, pp. 723-728. http://dx.doi.org/10.1055/s-1999-14094 [13] M. S. R. Nair, N. Acton, D. L. Klayman, K. Kendrick, D. V. Basile and S. Mante, “Production of Artemisinin in Tissue Cultures of Artemisia annua,” Journal of Natural Products, Vol. 49, No. 3, 1986, pp. 504-507. [14] D. V. Basile, N. Akhtari, Y. Durand and M. S. R. Nair, “Toward the Production of Artemisinin through Tissue Culture: Nutrient-Hormone Combinations Suitable for Cell Suspension Cultures,” In Vitro Cellular and Devel- opmental Biology: Plant, Vol. 29, No. 3, 1993, pp. 143- 147. http://dx.doi.org/10.1007/BF02632286 [15] J. F. S. Ferreira, J. E. Simon and J. Janick, “Relationship of Artemisinin Content of Tissue-Cultured, Greenhouse, and Field-Grown Plants of Artemisia annua,” Planta Medica, Vol. 61, No. 4, 1995, pp. 351-355. http://dx.doi.org/10.1055/s-2006-958098 [16] G. Cai, G. Li, H. Ye and G. F. Li, “Hairy Root Culture of Artemisia annua L. by Ri Plasmid Transformation and Open Access AJPS  Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants 2216 Biosynthesis of Artemisinin,” Chinese Journal of Bio- technology, Vol. 11, No. 4, 1995, pp. 227-235. [17] D.-Y. Xie, H. Ye, G. Li and Z. Guo, “Artemisia annua L. Transformation with Different Agrobacterium rhizogene- sis and Large Scale Culture of Hairy Roots for Artemisi- nin (Qinghaosu) Production,” In: Agricultural Biotech- nology: Laboratory, Field and Market, CPN Publications, Darwin, 1998, pp. 134-136. [18] S. Banerjee, M. Zehra and M. M. Kumar, “Agrobacterium rhizogenes-Mediated Transformation of Artemisia annua: Production of Transgenic Plants,” Planta Medica, Vol. 63, No. 5, 1997, pp. 467-469. http://dx.doi.org/10.1055/s-2006-957737 [19] B. Ghost, S. Mukherjee and S. Jha, “Genetic Transforma- tion of Artemisia annua by Agrobacterium tumefaciens and Artemisinin Synthesis in Transformed Cultures,” Plant Science, Vol. 122, No. 2, 1997, pp. 193-199. http://dx.doi.org/10.1016/S0168-9452(96)04558-X [20] K. H. Teoh, D. R. Polichak, D. W. Reed, G. Nowak and P. S. Covello, “Artemisia annua L. (Asteraceae) Trichome- Specific cDNAs Reveal CYP71AV1, a Cytochrome P450 with a Key Role in the Biosynthesis of the Antimalarial Sesquiterpene Lactone Artemisinin,” FEBS Letters, Vol. 580, No. 5, 2006, pp. 1411-1416. http://dx.doi.org/10.1016/j.febslet.2006.01.065 [21] L. Zhang, F. Y. Jing, F. P. Li, M. Y. Li, Y. L. Wang, G. F. Wang, X. F. Sun and K. X. Tang, “Development of trans- genic Artemisia annua (Chinese Wormwood) Plants with an Enhanced Content of Artemisinin, an Effective Anti- Malarial Drug, by Hairpin-RNA-Mediated Gene Silenc- ing,” Biotechnology Applied Biochemistry, Vol. 52, No. 3, 2009, pp. 199-207. http://dx.doi.org/10.1042/BA20080068 [22] H. J. Bouwmeester, T. E. Wallaart, M. H. A. Jansen, B. van Loo, B. J. M. Jansen, M. A. Posthumus, C. O. Schmidt, J.-W. Kraker, W. A. Konig and M. C. R. Franssen, “Amorpha-4,11-Diene Synthase Catalyses the First Probable Step in Artemisinin Biosynthesis,” Phyto- chemistry, Vol. 52, No. 5, 1999, pp. 843-854. http://dx.doi.org/10.1016/S0031-9422(99)00206-X [23] P. S. Covello, K. H. Teoh, D. R. Polichuk, D. W. Reed and G. Nowak, “Functional Genomics and the Biosynthe- sis of Artemisinin,” Phytochemistry, Vol. 68, No. 14, 2007, pp. 1864-1971. http://dx.doi.org/10.1016/j.phytochem.2007.02.016 [24] D.-K. Ro, E. M. Paradise, M. Oueller, K. J. Fisher, K. L. Newman, J. M. Ndungu, K. A. Ho, R. A. Eachus, T. S. Ham, J. Kirby, M. C. Y. Chang, S. T. Withers, Y. Shiba, R. Sarpong and J. D. Keasling, “Production of the Anti- malarial Drug Precursor Artemisinic Acid in Engineered Yeast,” Nature, Vol. 440, No. 7086, 2006, pp. 940-943. http://dx.doi.org/10.1038/nature04640 [25] Y. Zhang, K. H. Teoh, D. W. Reed and P. S. Covello, “Molecular Cloning and Characterization of Dbr1, a 2- Alkenal Reductase from Artemisia annua,” Botany, Vol. 87, No. 6, 2009, pp. 643-649. http://dx.doi.org/10.1139/B09-033 [26] Y. Zhang, K. H. Teoh, D. W. Reed, L. Maes, A. Goossens, D. J. H. Olson, A. R. S. Ross and P. S. Covello, “The molecular Cloning of Artemisinic Aldehyde Δ-11(13) Reductase and Its Role in Glandular Trichome-Dependent Biosynthesis of Artemisinin in Artemisia annua,” Journal Biological Chemistry, Vol. 283, No. 31, 2008, pp. 21501- 21508. http://dx.doi.org/10.1074/jbc.M803090200 [27] N. Delabays, X. Simonnet and M. Gaudin, “The Genetics of Artemisinin Content in Artemisia annua L. and the Breeding of High Yielding Cultivars,” Current Medicinal Chemistry, 2001. Vol. 8, No. 15, 2001, pp. 1795-1801. [28] N. Delabays, A. Benakis and G. Collet, “Selection and Breeding for High Artemisinin (Qinghaosu) Yielding Strains of Artemisia annua,” Acta Horticulture, Vol. 330, No. 1, 1993, pp. 203-207. [29] D.-Y. Xie, H. Ye and G. Li, “The Progress of Artemisia annua Research—The Application of Biotechnology and Prospects,” Chinese Bulletin Botany, Vol. 12, No. 4, 1995, pp. 28-31. (in Chinese) [30] H. J. Woerdenbag, N. Pras and A. W. Alfermann, “Pro- duction of Artemisinin in Shoot Cultures of Artemisia annua,” Planta M e d ica, Vol. 57, 1991, pp. A91-A92. http://dx.doi.org/10.1055/s-2006-960369 [31] H. M. Elhag, M. M. El-Domiaty, F. S. El-Olemy, J. S. Mossa and M. M. El-Olemy, “Selection and Micropropa- gation of High Artemisinin Producing Clones of Artemisia annua L.,” Phytotherapy Research, Vol. 6, No. 1, 1992, pp. 20-24. http://dx.doi.org/10.1002/ptr.2650060106 [32] H. J. Woerdenbag, J. F. J. Lüers, Wv. Uden, N. Pras, T. M. Malingré and A. W. Alfermann, “Production of the New Antimalarial Drug Artemisinin in Shoot Cultures of Artemisia annua,” Planta Medica, Vol. 58, No. S1, 1992, pp. 620-621. http://dx.doi.org/10.1055/s-2006-961621 [33] H. J. Woerdenbag, J. F. J. Luers, W. Vanuden, N. Pras, T. M. Malingre and A. W. Alfermann, “Production of the New Antimalarial Drug Artemisinin in Shoot Cultures of Artemisia annua L,” Plant Cell, Tissue, and Organ Cul- ture, Vol. 32, No. 2, 1993, pp. 247-257. http://dx.doi.org/10.1007/BF00029850 [34] N. B. Paniego and A. M. Giulietti, “Artemisia annua L.: Dedifferentiated and differentiated cultures,” Plant Cell, Tissue, and Organ Culture, Vol. 36, No. 2, 1994, pp. 163- 168. http://dx.doi.org/10.1007/BF00037715 [35] A. Gulati, S. Bharel, S. K. Jain, M. Z. Abdin and P. S. Srivastava, “In Vitro Micropropagation and Flowering in Artemisia annua,” Journal of Plant Biochemistry and Biotechnology, Vol. 5, No. 1, 1996, pp. 31-35. http://dx.doi.org/10.1007/BF03262976 [36] W. Lualon, W. De-Eknamkul, H. Tanaka, Y. Shoyama and W. Putalun, “Artemisinin Production by Shoot Re- generation of Artemisia annua L. Using Thidiazuron,” Zeitschrift für Naturforschung C, Vol. 63, No. 1/2, 2008, pp. 96-100. [37] T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cul- ture,” Physiologia Plantarum, Vol. 15, No. 3, 1962, pp. 473-497. http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x Open Access AJPS  Efficient Somatic Embryogenesis and Organogenesis of Self-Pollination Artemisia annua Progeny and Artemisinin Formation in Regenerated Plants Open Access AJPS 2217 [38] K. L. Chan, C. K. H. Teo, S. Jinadasa and K. H. Yuen, “Selection of High Artemisinin Yielding Artemisia an- nua,” Planta Medica, Vol. 61, No. 3, 1995, pp. 285-287. http://dx.doi.org/10.1055/s-2006-958078 [39] D. C. Jain, A. K. Mathur, M. M. Gupta, A. K. Singh, R. K. Verma, A. P. Gupta and S. Kumar, “Isolation of High Artemisinin-Yielding Clones of Artemisia annua,” Phy- tochemistry, Vol. 43, No. 5, 1996, pp. 993-1001. http://dx.doi.org/10.1016/S0031-9422(96)00369-X [40] A. Vergauwe, R. Cammaert, D. Vandenberghe, C. Gene- tello, D. Inzé, M. Montagu and E. Eeckhout, “Agrobacte- rium tumefaciens-Mediated Transformation of Artemisia annua L. and Regeneration of Transgenic Plants,” Plant Cell Reports, Vol. 15, No. 12, 1996, pp. 929-933. http://dx.doi.org/10.1007/BF00231590 [41] A. Vergauwe, E. Van Geldre, D. Inzé, M. Van Montagu and E. Van den Eeckhout, “Factors Influencing Agrobac- terium tumefaciens-Mediated Transformation of Artemisia annua L.,” Plant Cell Reports, Vol. 18, No. 1, 1998, pp. 105-110. http://dx.doi.org/10.1007/s002990050540 [42] J.-L. Han, H. Wang, H.-C. Ye, Y. Liu, Z.-Q. Li, Y. Zhang, Y.-S. Zhang, F. Yan and G.-F. Li, “High Efficiency of Genetic Transformation and Regeneration of Artemisia annua L. via Agrobacterium tumefaciens-Mediated Pro- cedure,” Plant Science, Vol. 168, No. 1, 2005, pp. 73-80. http://dx.doi.org/10.1016/j.plantsci.2004.07.020 [43] B. Y. Liu, H. Wang, Z. G. Du, G. F. Li and H. C. Ye, “Metabolic Engineering of Artemisinin Biosynthesis in Artemisia annua L.,” Plant Cell Reports, Vol. 30, No. 5, 2011, pp. 689-694. http://dx.doi.org/10.1007/s00299-010-0967-9 [44] D.-Y. Xie and Y. Hong, “Regeneration of Acacia man- gium through Somatic Embryogenesis,” Plant Cell Re- ports, Vol. 20, No. 1, 2001, pp. 34-40. http://dx.doi.org/10.1007/s002990000288 [45] C.-C. Lin, C.-S. Lin and W.-C. Chang, “In Vitro Flower- ing of Bambusa edulis and Subsequent Plantlet Survival,” Plant Cell, Tissue and Organ Culture, Vol. 2, No. 1, 2003, pp. 71-78. http://dx.doi.org/10.1023/A:1021281217589 [46] M. Dhandapani, D. Kim and S.-B. Hong, “Efficient Plant Regeneration via Somatic Embryogenesis and Organo- genesis from the Explants of Catharanthus roseus,” In Vitro Cellular & Developmental Biology: Plant, Vol. 44, No. 1, 2008, pp. 18-25. http://dx.doi.org/10.1007/s11627-007-9094-x [47] J. Y. Song, S. F. Lu, Z. Z. Chen, R. Lourenco and V. L. Chian, “Genetic Transformation of Populus trichocarpa Genotype Nisqually-1: A Functional Genomic Tool for Woody Plants,” Plant Cell Physiology, Vol. 47, No. 11, 2006, pp. 1582-1589. [48] D.-Y. Xie and Y. Hong, “Agrobacterium-Mediated Ge- netic Transformation of Acacia mangium,” Plant Cell Reports, Vol. 20, No. 10, 2002, pp. 917-922. http://dx.doi.org/10.1007/s00299-001-0397-9 [49] C.-M. Feng, R. Qu, L.-L. Zhou and D.-X. Xie, “Shoot regeneration of dwarf dogwood (Cornus canadensis L.) and Morphological Characterization of the Regenerated Plants,” Plant Cell, Tissue and Organ Culture, Vol. 97, No. 1, 2009, pp. 27-37. http://dx.doi.org/10.1007/s11240-009-9495-0 [50] D.-Y. Xie and R. A. Dixon, “Proanthocyanidin Biosyn- thesis—Still More Questions than Answers?” Phyto- chemistry, Vol. 66, No. 18, 2005, pp. 2127-2144. http://dx.doi.org/10.1016/j.phytochem.2005.01.008 [51] R. A. Dixon, D.-Y. Xie and S. B. Sharma, “Proanthocya- nidins—A Final Frontier in Flavonoid Research?” New Phytologist, Vol. 165, No. 1, 2005, pp. 9-28. http://dx.doi.org/10.1111/j.1469-8137.2004.01217.x [52] D.-Y. Xie, S. B. Sharma, N. L. Paiva, D. Ferreira and R. A. Dixon, “Role of Anthocyanidin Reductase, Encoded by BANYULS in Plant Flavonoid Biosynthesis,” Science, Vol. 299, No. 5605, 2003, pp. 396-399. http://dx.doi.org/10.1126/science.1078540 [53] Kakegawa, K. Kakegawa, J. Suda, M. Sugiyama and A. Komamine, “Regulation of Anthocyanin Biosynthesis in Cell Suspension Cultures of Vitis in Relation to Cell Di- vision,” Physiologia Plantarum, Vol. 94, No. 4, 1995, pp. 661-666. http://dx.doi.org/10.1111/j.1399-3054.1995.tb00981.x [54] B. Winkel-Shirley, “Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Bio- technology,” Plant Physiology, Vol. 126, No. 2, 2001, pp. 485-493. http://dx.doi.org/10.1104/pp.126.2.485 [55] K. H. Teoh, D. R. Polichuk, D. W. Reed and P. S. Covello, “Molecular Cloning of an Aldehyde Dehydrogenase Im- plicated in Artemisinin Biosynthesis in Artemisia annua,” Botany, Vol. 87, No. 6, 2009, pp. 635-642. http://dx.doi.org/10.1139/B09-032
|