 Journal of Cancer Therapy, 2013, 4, 13-27 Published Online November 2013 (http://www.scirp.org/journal/jct) http://dx.doi.org/10.4236/jct.2013.410A003 Open Access JCT 13 Recent Developments and Current Issues in the Treatment of Pancreatic Cancer Helmut Oettle Charité-Universitätsmedizin Berlin, Medizinische Klinik mit Schwerpunkt Hämatologie, Onkologie und Tumorimmunologie, Berlin, Germany. Email: helmut.oettle@charite.de Received July 18th, 2013; revised August 15th, 2013; accepted August 24th, 2013 Copyright © 2013 Helmut Oettle. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Presently, many questions exist about what the optimal regimen comprises for all stages and treatment settings for pan- creatic cancer. Since the CONKO-001 trial, adjuvant therapy following resection has become standard of care; however, outcomes are poor, with most patients experiencing disease recurrence, and new therapies have yet to be validated. Furthermore, the value of adjuvant radiotherapy has still not been clearly defined. Targeted treatment in combination with chemotherapy has been mostly disappointing so far in the adjuvant setting but immunotherapy holds potential for improving survival outcomes. Neoadjuvant treatment does not appear to provide much benefit in resectable patients but in the small subgroup of patients with borderline resectable/unresectable locally advanced disease it may increase the possibility of an R0 resection and, consequently, a substantial increase in survival duration. Use of capecitabine-based radiotherapy in patients with unresectable locally advanced disease appears to be more efficacious and better tolerated than gemcitabine-based chemoradiotherapy, with respect to survival outcomes. However, as with adjuvant treatment, the benefit of adding radiotherapy has not yet been definitively demonstrated. In patients with metastatic pancreatic cancer, targeting the stroma with nab-paclitaxel has shown promising results in a phase III trial setting when adminis- tered in combination with gemcitabine and, furthermore, this regimen is suitable for a broad range of patients due to its generally good tolerability profile. Because of its high toxicity, FOLFIRINOX is more suitable for younger patients with an excellent performance status who can withstand aggressive treatment and in patients with a worse performance status, gemcitabine monotherapy is considered to be a more appropriate treatment. Alternatively, gemcitabine in com- bination with erlotinib, the only targeted compound that has resulted in significant albeit small improvements in sur- vival in patients with advanced disease, could be selected. However, the benefit-risk profile of this regimen is only fa- vorable in a strictly defined, small patient subgroup who develop a treatment-related rash. Finally, with the elucidation of prognostic and predictive markers, treatment is expected to become ever more individualized, leading to improved efficacy outcomes and less unnecessary toxicity. Keywords: Pancreatic Cancer; Chemotherapy; Adjuvant Therapy; Neoadjuvant Therapy 1. Introduction With a median survival duration of approximately 6 months following diagnosis and a 5-year survival rate of less than 5%, pancreatic cancer is considered to have the poorest prognosis of any solid tumor [1]. Recent statistics show that pancreatic cancer is the fourth most common cause of death in the US and Europe and yet it accounts for only 3% of total cancer cases [2]. As well as display- ing a highly drug- and radio-therapy resistant phenotype, pancreatic cancer has high potential for local invasion and metastasis to distant sites compared with other solid tumors [3]. Indeed, for the few patients who have seem- ingly resectable disease at diagnosis, distant microme- tastases have usually already been established [2]. More- over, due to its initially highly asymptomatic nature, more than 80% of patients only present once disease is advanced [4]. However, although progress in the treat- ment of pancreatic cancer in the past few decades has been incremental, it is nevertheless steadily increasing. 2. Risk Factors Recently, a number of factors have been identified as  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 14 having a possible causative role in the development of pancreatic cancer, including non-O blood group, diabetes mellitus, low vegetable intake and a high-fat diet; al- though most of these have yet to be validated as defini- tive risk factors [5]. However, a causative role for to- bacco was determined several years ago, with smokers being found to have an elevated pancreatic cancer risk of 2.5 to 3.6 times that of a non-smoker [6]. Another well established risk factor is familial mutations, with ap- proximately 5% - 10% of patients having a family history of pancreatic cancer [7]. An individual from a family with at least four affected members is approximately 57 times more likely to develop pancreatic cancer when compared with an individual from a family containing no affected members [4]. 3. Genetics Our understanding of pancreatic cancer genetics has in- creased in the last decade with a number of germline and aquired somatic mutations being identified and mapped. The vast majority of patients with pancreatic cancer from any cause carry at least one of four known mutations, with 90% of tumors carrying a mutation in the KRAS oncogene and 95% of tumors with inactivation of the CDKN2A gene which codes for p16, a regulator of the G1-S transition of the cell cycle [8]. In approximately 50% of tumors, the tumor suppressor gene DPC4 is lost and in 50% - 75% of tumors, TP53, another tumor sup- pressor gene, is abnormal. Recently, whole genome se- quencing of 24 pancreatic cancers revealed on average 63 mutations per cancer, implying that pancreatic cancer is a very complex and heterogeneous disease and conse- quently that it might respond best to a multi-pronged and/or individualized treatment approach [9]. 4. Tumor Microenvironment The importance of the microenvironment in tumor propagation has begun to be realized in recent years and is an area of great interest in the study of pancreatic can- cer. A prominent feature of the tumour microenviron- ment in pancreatic cancer is the formation of a dense complex stroma, known as a desmoplastic reaction, around the tumour which can comprise up to 90% of the tumor volume [10]. This compact fibrous yet dynamic tissue functions as more than just a mechanical barrier, playing host to a complex interplay between normal host epithet- lial cells, tumor cells, stromal fibroblasts, pancreatic stel- late cells, endothelial cells and adipocytes, immune and inflammatory cells as well as growth factors which acti- vate oncogenic signaling pathways [11]. There is now significant evidence that pancreatic stellate cells play a key role in stromal formation and turnover. Following activation by growth factors, pancreatic stellate cells se- crete collagen as well as other extracellular matrix (ECM) proteins [12,13]. Furthermore, pancreatic stellate cells contribute to the hypoxia/fibrosis cycle in the peritu- moural stroma via abnormal ECM protein secretion and amplification of endostatin production by tumor cells [14]. They also regulate stromal turnover and reabsorp- tion, mostly through generation of matrix metallopro- teinases. Proteins produced by stromal cells and associ- ated with poor prognosis to treatment include stromal cell-derived factor, chemokines, cyclooxygenase-2, PDGF receptor, hedgehog pathway elements, vascular endothe- lial growth factor, integrins, and SPARC (Secreted Pro- tein Acidic And Rich in Cysteine) [4]. As a consequence of our increased understanding of the tumour microenvi- ronment, new therapeutic opportunities have arisen. For example, the nanoparticle albumin-bound (nab)- paclitaxel is able to bind with SPARC, which is found in abundant levels in the stroma, resulting in increased de- livery of paclitaxel to tumor cells [15]. Another emerging area of research is of the small subset of cells within a tumor termed stem cells. Pancreatic stem cells make up approximately 0.5% - 1% of all tumour cells and express the protein markers CD44, CD24 and epithelial-specific antigen [16]. Responsible for tumor initiation and propa- gation, they are also hypothesized to contribute to me- tastasis although, thus far, solid evidence is lacking in this area. However, stronger evidence has come to light for a role of stem cells in the typically high resistance displayed by pancreatic cancer towards chemotherapy and radiotherapy. In an in-vitro study [17], exposure to gemcitabine resulted in an enriched population of CD133+ cells in the 13.6 p pancreatic cancer cell line and in another in-vitro study [16], exposure to gemcit- abine and ionising radiation resulted in an enriched population of CD44, CD24, and ESA in xenografts of human pancreatic cancer. However, although our under- standing of the molecular processes that underpin the development and propagation of pancreatic cancer has deepened in recent years, translation of this knowledge into effective therapies has yet to be realized. 5. Adjuvant Therapy 5.1. Chemotherapy/Chemoradiotherapy Currently, surgical resection is the only curative treat- ment for stage I/II pancreatic cancer (Figure 1). Never- theless, following surgery, rates of locoregional and dis- tant recurrence are high, occurring in 50% - 80% and more than 70% of patients, respectively. As a result, 5- year survival rates following surgery with a curative in- tent are low, ranging from 10% - 15% [18] and, conse- quently, adjuvant therapy is considered an important facet of treatment for early-stage pancreatic cancer. Al- though accepted now as standard of care, for a long time Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer Open Access JCT 15 Figure 1. Current issues in pancreatic cancer treatment. the benefits of administering adjuvant chemotherapy with or without radiotherapy following resection were unclear. In trials conducted in the late 1960s and early 1970s, patients with advanced unresectable pancreatic cancer received external beam radiotherapy with or without systemic chemotherapy [19,20]. As a result of the antitumour activity observed in these studies, the first randomized trial of adjuvant therapy was initiated by the Gastrointestinal Tumour Study Group (GITSG) in which patients undergoing resection with curative intent re- ceived 5-FU + folinic acid administered with radiother- apy or underwent observation alone [21]. Significantly (p = 0.03) longer median survival durations were observed in patients who had received adjuvant therapy, compared with those who had not (20 vs. 11 months) [21]. Unfor- tunately, this trial was found to have multiple shortcom- ings and reliable conclusions could not be drawn from it [2]. In response to these criticisms, the GITSG conducted a single-arm study (n = 30) comprising the same adjuvant treatment protocol which demonstrated similar results [22]. Adjuvant therapy therefore appeared to be benefi- cial although it still remained unclear as to whether che- motherapy alone or chemoradiotherapy was responsible for these improvements in survival. Following the GITSG trial, two pivotal trials, (EORTC-40891 and ESPAC-1) were conducted which showed contradictory outcomes. In the EORTC-40891 trial, 5-FU + folinic acid-based chemoradiotherapy did not significantly improve overall survival at 5 years when compared with observation alone in the overall patient population which included patients with periampullary tumors (median of 24.5 vs. 19.0 months) yet a trend (p = 0.099) towards a survival benefit was observed in patients with pancreatic head cancer (median survival duration of 17.1 vs. 12.6 months) [23]. Moreover, a long-term survival analysis also showed no significant benefit of adjuvant treatment in the overall patient population, with median survival du- rations of 1.8 and 1.6 years in the chemoradiotherapy and observation alone arms, respectively [24]. However, due to inadequate statistical power, one of the numerous shortcomings of the EORTC-40891 trial, a possible sur- vival advantage of adjuvant chemoradiation could not be definitively ruled out. In the ESPAC-1 trial, which util- ized a 2 × 2 factorial design, 5-FU + folinic acid-based chemoradiotherapy actually had a deleterious effect on survival, resulting in a significantly (p = 0.05) lower sur- vival rate at 5 years, compared with no chemoradiother- apy (10% vs. 20% of patients). However, adjuvant 5FU + folinic acid was found to be significantly (p = 0.009) more efficacious than no adjuvant 5-FU + folinic acid, with respective survival rates at 5 years of 21% and 8% [25]. Due to these conflicting outcomes, the benefit of ad- juvant therapy remained debatable, especially with re- spect to addition of radiotherapy to chemotherapy. How- ever, subsequent publication of several larger and more well-designed landmark clinical trials has shed more light on this issue. Due to its proven efficacy in the palliative setting, gemcitabine was investigated as a possible adjuvant ther-  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 16 apy in several phase III studies. In the CONKO-001 study, we randomly assigned patients to receive adjuvant gemcitabine or to undergo observation alone [26]. A sig- nificant (p < 0.001) and clinically relevant benefit was seen in the patients receiving adjuvant therapy, with a median disease-free survival interval of 13.4 months be- ing achieved, compared with 6.9 months in the treatment group undergoing observation alone. Upon subgroup analysis, significant benefit was seen across all sub- groups, including in patients with an R1 resection (15.4 vs. 5.5 months; p < 0.001), who were node-negative (22.4 vs. 10.4 months; p = 0.006) or with tumor stage T1-2 (27.5 vs. 10.0 months; p < 0.05). Interestingly, gemcitabine recipients with an R1 resection survived for longer disease-free than gemcitabine recipients who had achieved an R0 resection (median disease-free survival interval of 15.4 vs. 13.1 months). As well as prolonging disease-free survival, adjuvant gemcitabine also signifi- cantly (p < 0.01), albeit to a lesser degree, improved the hard endpoint of overall survival, with final results show- ing gemcitabine recipients surviving for a median of 22.8 months, compared with 20.2 months in the observation alone arm [26]. Again, significant benefit was observed in gemcitabine recipients who had undergone an R1 re- section, with overall survival in this group being almost as long as gemcitabine recipients who had undergone an R0 resection (median of 22.1 vs. 22.8 months). This re- duction in the magnitude of benefit from gemcitabine treatment in the overall patient population may have been due to the majority of patients under observation alone subsequently crossing over to the gemcitabine arm upon progression. With respect to the resection margin, sig- nificant (p < 0.05) benefit in overall survival remained in patients who had undergone an R0 resection (median of 22.8 vs. 20.3 months) whereas only a trend towards im- proved overall survival was observed in patients who had undergone an R1 resection (median of 22.1 vs. 14.1 months) [26]. The large ESPAC-3 version 2 trial (n = 1088) [27] was subsequently designed to compare the efficacy of adju- vant 5-FU + folinic acid, already established as standard of care for advanced pancreatic cancer, with gemcitabine which had earlier been validated as adjuvant therapy in the CONKO-001 trial. There had initially been an arm undergoing observation alone but this was discontinued prematurely due to the final results of ESPAC-1 demon- strating a benefit for adjuvant therapy. The main aim was to determine whether gemcitabine would result in an improvement in overall survival. However, no significant difference between the gemcitabine and 5-FU + folinic arms was observed in median overall survival duration, median progression-free survival duration or quality of life. Gemcitabine appeared to be better tolerated than 5-FU + folinic acid which had resulted in significantly (p < 0.001) increased rates of grade 3 or 4 stomatitis, grade 3 and 4 diarrhea and serious adverse events [27]. These results were in contrast to an earlier smaller study [28] in which gemcitabine resulted in significantly (p < 0.01) greater antitumour activity as well as a sig- nificantly (p < 0.01) higher overall survival rate at 2 years when compared with fluorouracil. However, the dose of 5-FU used in this earlier study was lower than the fluorouracil dose used in ESPAC-3 version 2. The benefit of using gemcitabine as part of adjuvant chemoradiotherapy was again left unanswered in a trial conducted by the Radiation Therapy Oncology Group (RTOG-9704) [29]. In this trial, patients were randomly assigned to receive either adjuvant gemcitabine or 5-FU (control), both administered before and after 5-FU-based chemoradiotherapy. No significant difference in the pri- mary endpoint of overall survival was reported between the treatment arms. However, in patients with cancer of the pancreas head, gemcitabine resulted in a trend (p = 0.09) towards a longer overall survival duration (median of 20.5 vs. 16.9 months), upon multivariate analysis. Use of S-1 (gimeracil/oteracil/tegafur) as adjuvant therapy was explored by the Japan Adjuvant Study Group of Pancreatic Cancer in the JASPAC 01 trial [30]. In this noninferiority study, Japanese patients were ran- domised to S-1 or gemcitabine with the aim of determin- ing whether S-1 would be any less efficacious than gem- citabine as adjuvant therapy. S-1 was found to be not only significantly (p < 0.001) noninferior to gemcitabine, but was also found to be significantly (p < 0.001) supe- rior, with respect to overall survival at 2 years (70% vs. 53% in the full analysis set; HR 0.56; 95% CI 0.36 - 0.87). Due to demonstrating efficacy in patients with ad- vanced pancreatic cancer, capecitabine is being explored as a possible option in adjuvant therapy. In the currently recruiting ESPAC-4 trial (EudraCT2007-004299-38), patients with resectable pancreatic cancer or periampul- lary cancer are being randomised to gemcitabine + cape- citabine or gemcitabine monotherapy. Completion is scheduled for November 2014. Addition of radiotherapy to adjuvant chemotherapy remains controversial, with some studies reporting a clinical benefit but others, such as the ESPAC-1 trial, showing a deleterious effect [25]. Results from RTOG- 9704 and CONKO-001 seem to suggest that adjuvant chemoradiotherapy is no more efficacious than adjuvant chemotherapy [26,29]. However, this should be inter- preted cautiously as the patient populations in these trials differed, with CONKO-001 having fewer patients with margin- or node-positive disease as well as an inclusion criterion of patients with CA19-9 concentrations of 90 U/mL or less. In an exploratory subgroup analysis from a meta-analysis conducted by Stocken et al., chemoradio- Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 17 therapy was found to be significantly more effective than chemotherapy in patients with positive (R1) resection margins [31]. For most oncology practices in Europe adjuvant chemotherapy alone is considered standard of care and adjuvant chemoradiotherapy is generally not administered whereas in the US adjuvant chemoradio- therapy and subsequent chemotherapy is the standard of care [32]. 5.2. Targeted Therapies After showing a small but significant benefit in overall survival in the phase III PA.3 trial [33] in advanced pan- creatic cancer patients, erlotinib is being assessed as part of gemcitabine-based adjuvant therapy in the CONKO- 005 trial (EudraCT2007-003813-15) in patients who have undergone an R0 resection. In another study cur- rently being conducted by the Charité Oncology Group (CONKO-006; EudraCT2007-000718-35), the multi- kinase inhibitor sorafenib is being explored as part of combination adjuvant therapy with gemcitabine, com- pared with gemcitabine alone, in patients with an R1 resection of pancreatic cancer. The benefit of adjuvant gemcitabine in patients with an R1 resection had already been demonstrated in subgroup analysis of CONKO-001, in which gemcitabine recipients with an R1 resection survived for almost 10 months longer than patients with an R1 resection who were under observation alone. Therefore, the aim of this study is to determine whether addition of sorafenib could increase disease-free survival (primary endpoint) and subsequently overall survival (secondary endpoint) in this patient subgroup. Duration of therapy will also be assessed, with patients being treated for one year in the CONKO-006 study, compared with only six months in the CONKO-001 and CONKO- 005 studies. 5.3. Immunotherapy Immunotherapy is a therapeutic field that may hold great potential in the adjuvant setting, with the main aims be- ing to inhibit regulatory T-cells which suppress the im- mune response to pancreatic cancer or to prime the im- mune system to recognize cancer cells via immunosti- mulatory pancreatic cancer antigens or use of geneti- cally-modified irradiated pancreatic cancer cells [34]. Algenpantucel-L, which is the most clinically advanced pancreatic cancer vaccine currently in development, comprises an irradiated live combination of two human pancreatic cancer cell lines which present a non-human surface epitope (alpha-galactosyl), thus stimulating an immune response. A multi-institutional single-arm phase II trial assessed the efficacy of algenpantucel-L, when added to standard of care (adjuvant chemotherapy or chemoradiotherapy with gemcitabine or 5-FU) [35]. In this trial, the one-year survival rate was higher than that observed in the RTOG-9704 trial (86% vs. 69% of pa- tients), even though lymph node involvement was higher at baseline (81% vs. 68% positive nodes). Furthermore, algenpantucel-L was well tolerated [35]. Based on these encouraging data, the randomised IMPRESS trial by Fisher et al. (NCT01072981) was initiated in April 2010 and is scheduled to be finished by January 2014. In this study, 922 patients will receive standard of care with or without algenpantucel-L [34]. In a phase II single-centre trial, a granulocyte-macrophage colony stimulating fac- tor-transduced allogeneic whole cell vaccine was admin- istered following adjuvant fluorouracil-based chemora- diotherapy. Disease-free survival and overall survival rates at one year were 67% and 85%, respectively. Tol- erability was good, with the most frequently reported adverse events being transient injection-site reactions [36]. Other vaccines containing ras-peptide, telomerase peptide, mucin + carcinoembryonic antigen and survivin are also in early-phase development as are vaccines comprising antigen-pulsed dendritic cells presenting ei- ther mucin 1 or carcinoembryonic antigen [34]. 6. Neoadjuvant Therapy As a result of waiting list times for surgery and the sub- sequent time needed for postoperative recovery, the time between diagnosis and receipt of chemotherapy can be 2 months or more. Due to micro-metastases in lymph nodes, lung, peritoneum and liver being present at diag- nosis in the majority of patients, a rationale exists for the use of neoadjuvant therapy. Furthermore, as patients are at an earlier disease stage and therefore have an im- proved performance status, it is more likely that they will be eligible for treatment, compared with patients waiting to undergo adjuvant therapy. Neoadjuvant treatment may also reduce the risk of peritoneal tumor cell implantation during surgery due to decreasing intraoperative tumor spillage and may also result in more definitive surgical resections [37]. Unfortunately, clinical outcomes for neoadjuvant ther- apy have mostly been disappointing although positive results were shown in a small phase II study [38] in which 28 patients with resectable adenocarcinoma of the pancreatic head received 4 courses of neoadjuvant gem- citabine + cisplatin. Resection rate was 93%, above that of the predetermined primary endpoint of at least 70% and improvements in nutritional status and quality of life were observed. Another phase II study [39] compared gemcitabine monotherapy with gemcitabine + cisplatin. Resection rates were 38% and 70%, respectively, but survival outcomes were poor, with patients only surviv- ing a median of 9.9 and 15.6 months, respectively. Ad- ministration of subsequent chemoradiotherapy following Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 18 initial neoadjuvant chemotherapy has also been assessed in the phase II setting. One phase II trial [40] assessed gemcitabine then gemcitabine-based radiotherapy in 20 patients and another phase II trial [41] assessed cisplatin + gemcitabine then gemcitabine + radiotherapy in 90 patients. Disappointingly, survival outcomes were gener- ally no better than those observed with surgery alone. Furthermore, in a meta-analysis [42] comprising 4394 patients, resection rates in patients with initially re- sectable tumours were found to be the same, regardless of whether or not patients received neoadjuvant treatment. Therefore, although there is a hypothetical rationale for use of neoadjuvant therapy in patients with resectable pancreatic cancer, stronger clinical data are needed to support its use in standard practice. Currently, a neoadjuvant regimen comprising nab-pa- clitaxel and gemcitabine is being explored in a phase II pilot trial [43]. Preliminary results were presented at ASCO 2013 in which this regimen was determined as being feasible and warranting study in a larger trial. 7. Borderline Resectable/Unresectable Pancreatic Cancer—A New Indication? Patients with borderline resectable/unresectable locally advanced pancreatic cancer constitute a unique patient population. In patients who are borderline resectable, neoadjuvant treatment offers an increased chance of an R0 resection and in those patients who are borderline unresectable, neoadjuvant treatment offers an increased chance of resection, including the possibility of an R0 resection and, consequently, for both patient populations, a much extended survival duration. In the previously mentioned meta-analysis by Gillen et al. [42], although benefit from neoadjuvant therapy was not demonstrated in patients with resectable tumours, approximately one third of patients who had initially unresectable tumours at baseline were able to undergo resection following neoadjuvant therapy, especially if combination chemo- therapy was administered. Survival in this group was similar to that observed in patients with initially resectable tumours. A large retrospective series at a single institu- tion subsequently confirmed these data [44]. Patients with locally-advanced unresectable pancreatic cancer re- ceived neoadjuvant gemcitabine-based chemoradiother- apy. Of the 215 patients studied, 26% were able to un- dergo secondary resection, with the median overall sur- vival duration being substantially longer in the 36% of resected patients who underwent an R0 resection, com- pared with those patients who did not undergo any resec- tion at all (22.1 vs. 11.9 months). Moreover, in an analy- sis of patients undergoing curative pancreatoduodenec- tomy or total pancreatectomy [45], patients requiring a portal/superior mesenteric vein resection had a signifi- cantly (p < 0.05) shorter mean survival time, compared with patients who did not require this resection. However, adjuvant gemcitabine improved the prognosis of these patients such that the mean survival time was similar between these 2 groups of patients. 8. Unresectable Locally Advanced Pancreatic Cancer 8.1. Chemoradiotherapy For patients with locally advanced unresectable disease, treatment options, including whether or not to administer radiotherapy, are not yet clearly defined. In a phase III study [46], chemoradiotherapy comprising fluorouracil + cisplatin and a total radiotherapy dose of 60Gy resulted in a significantly (p = 0.03) shorter median overall sur- vival duration, compared with gemcitabine alone (me- dian of 8.6 vs. 13.0 months), as well as increased moder- ate-to-severe toxicity. Yet in a subsequent phase III trial conducted by the Eastern Cooperative Oncology Group [47], chemoradiotherapy comprising gemcitabine (600 mg/ m2/week) and radiotherapy administered to a total dose of 50.4 Gy was found to significantly (p = 0.017) improve overall survival over gemcitabine alone (median of 11.1 vs. 9.2 months). Unfortunately, due to limited statistical power resulting from poor patient accrual, this study was terminated prematurely and was not able to provide definitive evidence that could impact standard of care. The SCALOP trial [48] was the first randomized mul- ticenter trial to compare capecitabine-based chemoradio- therapy with gemcitabine-based chemoradiotherapy, both administered following induction treatment with gemcit- abine + capecitabine, in patients with locally advanced disease. Radiotherapy was 3D conformal or intensity- modulated and was administered as 5.5 fractions per week to a total dose of 50.4Gy, although only 68% - 69% of patients received the full protocol dose. Progression- free survival rates at 9 months (primary endpoint) were 63% and 51%, respectively, which met prespecified cri- teria according to a Fleming’s design. However, capecit- abine resulted in a non-significantly (p = 0.111) longer median progression-free survival interval, compared with gemcitabine (12.0 vs. 10.4 months; HR 0.60; 95% CI 0.32 - 1.12), including a longer median local progres- sion-free survival interval (14.6 vs. 12.0 months) as well as a longer distant progression-free survival interval (14.3 vs. 11.9 months). More importantly, capecitabine was found to significantly (p = 0.012) extend overall survival, compared with gemcitabine (median of 15.2 vs. 13.4 months; HR 0.39; 95% CI 0.18 - 0.81). With respect to secondary endpoints, 2 patients (6%) in the capecit- abine arm alone experienced a complete response but, in general, response rates were similar between treatment Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 19 arms. Of the 58% of patients with progression in each treatment arm, 33% and 32%, respectively, experienced local relapse, 52% and 46%, respectively, experienced metastatic relapse and 14% and 23%, respectively, ex- perienced both types of relapse. Following radiotherapy, similar numbers of patients were eligible for resection (6% vs. 8% of patients, respectively). Furthermore, cape- citabine resulted in fewer grade 3 and 4 adverse events, compared with gemcitabine (11% vs. 37% of patients), including significantly (p = 0.007) fewer grade 3 and 4 hematological adverse events (0% vs. 18% of patients) and a trend (p = 0.095) towards fewer grade 3 and 4 non-hematological adverse events (11% vs. 26% of pa- tients) [48]. The 2 × 2 factorial LAP07 study [49] aimed to com- pare switching to capecitabine-based chemoradiotherapy with continuing either erlotinib + gemcitabine or gem- citabine monotherapy. Patients who had received er- lotinib during the initial induction treatment could con- tinue to receive erlotinib as maintenance therapy, regard- less of whether or not they were randomized to continue treatment. Disappointingly, no significant difference in the primary endpoint of overall survival was observed between the chemoradiotherapy and the chemotherapy arms (15.3 vs. 16.5 months; HR 1.03; 95% CI 0.79 - 1.03) and nor was there any significant difference in progres- sion-free survival (HR 0.9; 95% CI 0.7 - 1.1). Comparison of chemoradiotherapy versus chemother- apy alone following induction with either gemcitabine, gemcitabine + nab-paclitaxel or 5-FU + folinic acid + irinotecan + oxaliplatin (FOLFIRINOX) is being inves- tigated in the CONKO-007 trial by Fietkau and Oettle (NCT01827553). Following completion of radiotherapy, patients will continue to receive the same chemotherapy regimen as they received for induction therapy until dis- ease progression. Overall survival is the primary end- point and resectability status is being assessed as a sec- ondary endpoint. 8.2. Investigational Agents in Locally Advanced Pancreatic Cancer Gene therapy was recently assessed in a phase III trial in which golnerminogene pradenovec, a genetically-modi- fied adenovirus 5 vector encoding tumour necrosis fac- tor-alpha, was administered with 5-FU + radiotherapy and compared with 5-FU + radiotherapy alone. Nonsig- nificant improvement in the primary endpoint of overall survival was observed in the investigational treatment arm [50]. 9. Metastatic Pancreatic Cancer 9.1. First-line Therapy Gemcitabine has comprised the first-line standard of care for metastatic pancreatic cancer since 1997 when a piv- otal study by Burris et al. demonstrated superiority of gemcitabine over 5-FU [28]. Subsequently many differ- ent combinations using gemcitabine as a backbone have been evaluated including 5-FU [51], capecitabine [52], oxaliplatin [53], cisplatin [54], irinotecan [55] and pe- metrexed [56]. Overall, these combination regimes failed to demonstrate significant prolonged survival as com- pared to gemcitabine alone although a significant but modest benefit in survival of gemcitabine-based combi- nation regimens in patients with good performance status was demonstrated in 2 meta-analyses [57,58]. 9.2. Targeted Therapies Although our understanding of the molecular and genetic changes associated with the development and propaga- tion of pancreatic cancer has increased over recent years, there is still a lack of suitably targeted drugs for this dis- ease. Despite demonstrating efficacy in other solid tu- mours, targeted therapies have so far resulted in disap- pointing outcomes in advanced pancreatic cancer. The only targeted compound which has demonstrated pro- longed survival in a phase III clinical trial setting was erlotinib when administered in combination with gem- citabine [33]. In the phase III PA.3 trial, erlotinib in combination with gemcitabine resulted in a small but significantly (p < 0.05) greater extension in overall sur- vival, compared with gemcitabine alone (HR 0.82; me- dian of 6.24 vs. 5.91 months). However, as this increase was not considered clinically relevant by most oncolo- gists erlotinib has not been broadly adopted as part of standard of care. Other targeted therapies in combination with gemcitabine that have been investigated, including monoclonal antibodies such as bevacizumab and cetuxi- mab or antiangiogenic multikinase inhibitors such as axitinib and sorafenib, have failed to show survival bene- fit in advanced pancreatic cancer [59-63]. Development of the once-promising hedgehog signaling pathway in- hibitor vismodegib (GDC 0449) for the treatment of ad- vanced pancreatic cancer was discontinued recently due to unfavorable results. Many other targeted therapies are currently being in- vestigated in early-phase clinical trials, including the hypoxia-targeted drug TH-302 [NCT01746979], the anti- IGF-R1 antibody MK 0646 [NCT00769483], and the PARP inhibitor ABT-888 [NCT01489865], although phase III confirmatory studies will be needed to defini- tively prove the clinical benefit of these and other agents. 9.3. Polychemotherapy with FOLFIRINOX Substantial progress in the treatment of advanced pan- creatic cancer has been made by the introduction of the FOLFIRINOX regimen (oxaliplatin, irinotecan fluorouracil, and leucovorin) [64]. In this phase III clinical trial, which Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 20 compared FOLFIRINOX with the current treatment standard gemcitabine, patients receiving FOLFIRINOX benefited significantly in median progression-free sur- vival (6.4 months vs. 3.3 months, p < 0.001) and also in the primary endpoint of overall survival (11.1 months vs. 6.8 months, p < 0.001). However, it should be noted that FOLFIRINOX resulted in higher toxicity as compared to gemcitabine. Specifically, patients presented with more grade 3 or 4 neutropenia (45.7% vs. 21.0%, p < 0.001) and febrile neutropenia (5.4% vs. 1.2%), which required use of granulocyte-colony stimulating factor. The toler- ability profile of FOLFIRINOX was also less favorable in terms of grade 3 or 4 thrombocytopenia (9.1% vs. 3.6%, p = 0.04), grade 3 or 4 diarrhea (12.7% vs. 1.8%, p < 0.001) and grade 3 or 4 sensory neuropathy (9.0% vs. 0%, p < 0.001). Interestingly, despite the increased toxic- ity of FOLFIRINOX regimen, time to deterioration in quality of life was similar to that observed with gemcit- abine. However, since inclusion critera were strict (pa- tients had to be younger than 76 years with a high per- formance status [ECOG 0 or 1] with no cardiac ischemia and good hepatobiliary function) this does not represent a real life setting and subsequently the clinical impact of this study is thought to be modest. Moreover, FOLFIR- INOX has not been readily adopted by oncologists prac- tice due to general safety concerns about the risk of cholangitis in stented patients [65]. Currently, a modified FOLFIRINOX regimen with expected reduced toxicity due to omission of the 5-FU bolus (FOLFOXIRI) is be- ing investigated [66]. 9.4. Targeting the Stroma with Nab-Paclitaxel A unique feature of pancreatic cancer is an abundant stroma which impairs drug delivery by reducing drug diffusion into the primary tumour. This is thought to be a major factor in the notable resistance of pancreatic can- cer to chemotherapy [67] and, consequently, many re- searchers have tried to improve drug delivery by target- ing the stroma of pancreatic cancer cells. A recent break- through in this area is nanoparticle albumin-bound (nab)- paclitaxel that was initially developed to reduce treat- ment toxicity by allowing omission of the solvent needed to dissolve oily drug formulations of paclitaxel. Pancre- atic peritumoral fibroblasts cancers overexpress SPARC [68], which serves, via mediation of the albumin-binding protein gp60, as a strong binding protein for nab-pacli- taxel [69] (Figure 2). Von Hoff et al. demonstrated con- vincing evidence of this principle in a phase I/II trial, with a response rate to nab-paclitaxel of 48%, a median overall survival (OS) of 12.2 months and 1-year survival rate of 48% [70]. They also reported interesting preclini- cal data; most importantly stroma depletion resulted in a 2.8-fold higher intratumoral gemcitabine concentration in mice receiving nab-paclitaxel plus gemcitabine when compared to gemcitabine alone. At this years ASCO gas- trointestinal cancers symposium, Von Hoff et al. pre- sented the results of a phase III trial in 861 patients (MPACT), which confirmed the phase II data [71]. Nab- paclitaxel plus gemcitabine was superior to gemcitabine for all efficacy endpoints with a median overall survival of 8.5 vs. 6.7 months (HR 0.72; p = 0.000015), a median progression free survival of 5.5 vs. 3.7 months (HR 0.69; p = 0.000024), an investigator-assessed overall response rate of 29% vs. 8% (p = 3.3 × 10−16), and an improved one-year overall survival of 35% vs. 22% (p = 0.0002). These improvements in efficacy for nab-paclitaxel plus gemcitabine were accompanied by good tolerability data. With comparable dose intensities being administered in both treatment arms, grade 3 or higher adverse events with nab-paclitaxel comprised neutropenia (38% vs. 27%), febrile neutropenia (3% vs. 1%), thrombocyto- penia (13% vs. 9%), anemia (13% vs. 12%) fatigue (17% vs. 7%), and diarrhea (6% vs. 1%). Grade 3 or higher pe- ripheral neuropathy occurred more often with nab-pacli- taxel plus gemcitabine (17% vs. 1%) but improved to no more than grade 1 over a median period of 29 days [71]. 9.5. Is Nab-Paclitaxel a New Treatment Standard? Despite the inherent limitations of inter-study compari- son, the tabular overview (Tables 1-3) shows that in comparison to FOLFIRINOX, the nab-paclitaxel regime provides similar efficacy to FOLFIRINOX but with bet- ter tolerability. In contrast to FOLFIRINOX, nab-pacli- taxel + gemcitabine was better tolerated even though the patient population included less fit patients. Notably, nab-paclitaxel + gemcitabine resulted in a lower rate of grade 3 and grade 4 neutropenia, compared with FOLFIR- INOX (45.7% vs. 38%). Grade 3 and 4 neuropathy was found to be higher with nab-paclitaxel (17% vs. 9%), but improved quickly within 29 days to no more than grade 1 [64,71]. Looking at different subgroups patients of the MPACT trial upon multivariate analyses it was revealed that patients with a Karnofsky performance status of 70 - 80 (HR 0.61), one metastatic site (HR 0.41), more than 3 metastatic sites (HR 0.5) and increased (≥59 × ULN) CA-19-9 (HR 0.61) particularly benefited from nab-pa- clitaxel + gemcitabine [72]. In comparison with gemcit- abine alone [28] and gemcitabine + erlotinib [33], nab- paclitaxel + gemcitabine resulted in a substantial benefit in overall survival (8.5 month vs. 6.24 month vs. 5.65 month), mitigating the increased toxicity of nab-pacli- taxel. In conclusion, nab-paclitaxel + gemcitabine has a favorable toxicity profile and is a potentially new stan- dard therapy for the treatment of metastatic pancreatic cancer in a broad range of patients. FOLFIRINOX should be restricted to younger patients who have an ex- cellent performance status and are willing to undergo Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer Open Access JCT 21 Figure 2. Mechanism of nab-paclitaxel drug-delivery. Table 1. Patient demographics in selected pivotal phase III trials in patients with advanced disease. Study GEM [28] GEM/ERL [33] FOLFIRINOX [64] NABPAC [71] Age (range) 62 (37 - 79) 63.7 (37 - 84) 61 (25 - 76) 62 (27 - 88) Performance status 30% KPS 80 - 90 70% KPS 50 - 70 29.8% ECOG 0 50.9% ECOG 1 18.9% ECOG 2 37.4% ECOG 0 61.9% ECOG 1 0.6% ECOG 2 58% KPS 90 - 100 42% KPS 70 - 80 Liver metastases NR NR 87.6 % 85% Head of pancreas NR NR 39.2 % 44% CA19.9 ≥ 59 ULN NR NR 41.5 % 46% GEM, gemcitabine; ERL, erlotinib; NABPAC, nab-paclitaxel; KPS, Karnofsky performance status; ECOG, Eastern Cooperative Oncology Group performance status; ULN, upper limit of normal; NR, not reported. validated the value of fluoropyrimidine-based regimes in second-line treatment. Oxaliplatin plus capecitabine (XELOX) showed comparable efficacy in a phase II trial and offers the possibility of oral fluoropyrimidine treat- ment [75]. A recently presented randomized phase II trial [76] compared the most readily available fluoropyri- midine (5-FU, UFT or S-1) with continuation of gemcit- abine. Fluoropyrimidine-treated patients benefited from a non-significantly prolonged progression-free survival of 113 days vs. 50 days (p = 0.1050) and a significantly improved overall survival of 226 days vs. 161 days (p = 0.0384). The results of another randomized phase II trial suggests that the combination of a fluoropyrimidine with irinotecan could also be of value as second-line treatment [77]. Summing up the currently available evidence, pa- tients with a good performance status who have pro- gressed on gemcitabine-based therapy are recommended to receive second-line therapy consisting of oxaliplatin and an (oral) fluoropyrimidine. more aggressive treatment. In patients with a Karnofsky performance status of <70%, gemcitabine monotherapy can be administered, if applicable with erlotinib being added but then subsequently discontinued if no rash has developed within the first 10 - 15 days. 9.6. Second-Line Therapy There is no generally accepted consensus with regard to second-line therapy in pancreatic cancer. Nevertheless, a growing number of studies suggest that patients can benefit from second-line therapy. In the CONKO-003 trial [73,74], we randomly assigned patients to treatment with oxaliplatin, folinic acid and 5-FU (OFF) or best sup- portive care. Median survival in patients receiving sec- ond-line treatment with OFF was 4.82 months versus 2.30 months with best supportive care alone (HR 0.45, p = 0.008). Meanwhile, subsequent phase II trials have further 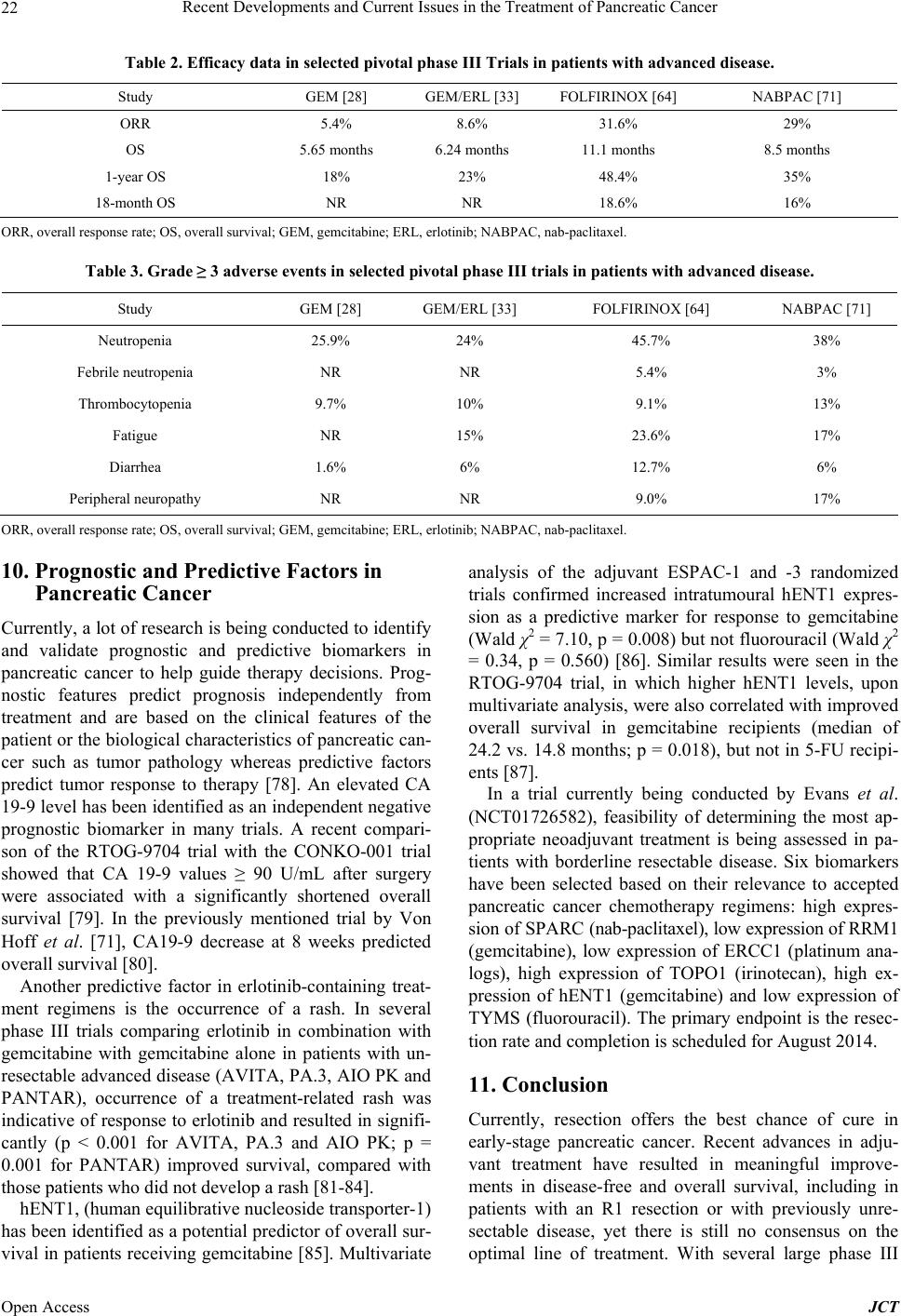 Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 22 Table 2. Efficacy data in selected pivotal phase III Trials in patients with advanced disease. Study GEM [28] GEM/ERL [33] FOLFIRINOX [64] NABPAC [71] ORR 5.4% 8.6% 31.6% 29% OS 5.65 months 6.24 months 11.1 months 8.5 months 1-year OS 18% 23% 48.4% 35% 18-month OS NR NR 18.6% 16% ORR, overall response rate; OS, overall survival; GEM, gemcitabine; ERL, erlotinib; NABPAC, nab-paclitaxel. Table 3. Grade ≥ 3 adverse events in selected pivotal phase III trials in patients with advanced disease. Study GEM [28] GEM/ERL [33] FOLFIRINOX [64] NABPAC [71] Neutropenia 25.9% 24% 45.7% 38% Febrile neutropenia NR NR 5.4% 3% Thrombocytopenia 9.7% 10% 9.1% 13% Fatigue NR 15% 23.6% 17% Diarrhea 1.6% 6% 12.7% 6% Peripheral neuropathy NR NR 9.0% 17% ORR, overall response rate; OS, overall survival; GEM, gemcitabine; ERL, erlotinib; NABPAC, nab-paclitaxel. 10. Prognostic and Predictive Factors in Pancreatic Cancer Currently, a lot of research is being conducted to identify and validate prognostic and predictive biomarkers in pancreatic cancer to help guide therapy decisions. Prog- nostic features predict prognosis independently from treatment and are based on the clinical features of the patient or the biological characteristics of pancreatic can- cer such as tumor pathology whereas predictive factors predict tumor response to therapy [78]. An elevated CA 19-9 level has been identified as an independent negative prognostic biomarker in many trials. A recent compari- son of the RTOG-9704 trial with the CONKO-001 trial showed that CA 19-9 values ≥ 90 U/mL after surgery were associated with a significantly shortened overall survival [79]. In the previously mentioned trial by Von Hoff et al. [71], CA19-9 decrease at 8 weeks predicted overall survival [80]. Another predictive factor in erlotinib-containing treat- ment regimens is the occurrence of a rash. In several phase III trials comparing erlotinib in combination with gemcitabine with gemcitabine alone in patients with un- resectable advanced disease (AVITA, PA.3, AIO PK and PANTAR), occurrence of a treatment-related rash was indicative of response to erlotinib and resulted in signifi- cantly (p < 0.001 for AVITA, PA.3 and AIO PK; p = 0.001 for PANTAR) improved survival, compared with those patients who did not develop a rash [81-84]. hENT1, (human equilibrative nucleoside transporter-1) has been identified as a potential predictor of overall sur- vival in patients receiving gemcitabine [85]. Multivariate analysis of the adjuvant ESPAC-1 and -3 randomized trials confirmed increased intratumoural hENT1 expres- sion as a predictive marker for response to gemcitabine (Wald χ2 = 7.10, p = 0.008) but not fluorouracil (Wald χ2 = 0.34, p = 0.560) [86]. Similar results were seen in the RTOG-9704 trial, in which higher hENT1 levels, upon multivariate analysis, were also correlated with improved overall survival in gemcitabine recipients (median of 24.2 vs. 14.8 months; p = 0.018), but not in 5-FU recipi- ents [87]. In a trial currently being conducted by Evans et al. (NCT01726582), feasibility of determining the most ap- propriate neoadjuvant treatment is being assessed in pa- tients with borderline resectable disease. Six biomarkers have been selected based on their relevance to accepted pancreatic cancer chemotherapy regimens: high expres- sion of SPARC (nab-paclitaxel), low expression of RRM1 (gemcitabine), low expression of ERCC1 (platinum ana- logs), high expression of TOPO1 (irinotecan), high ex- pression of hENT1 (gemcitabine) and low expression of TYMS (fluorouracil). The primary endpoint is the resec- tion rate and completion is scheduled for August 2014. 11. Conclusion Currently, resection offers the best chance of cure in early-stage pancreatic cancer. Recent advances in adju- vant treatment have resulted in meaningful improve- ments in disease-free and overall survival, including in patients with an R1 resection or with previously unre- sectable disease, yet there is still no consensus on the optimal line of treatment. With several large phase III Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 23 trials scheduled to be completed in the next years, in- cluding at least one investigating the emerging area of immunotherapy, hopefully the way forward will become better elucidated. Although progress has been made in the treatment of metastatic pancreatic cancer in the last few decades, it is clear that new strategies are needed if patients’ lives are to be substantially extended and the realization is starting to emerge that targeting the primary tumor alone is not enough in the most resilient malignan- cies. The relatively recent recognition of the tumor mi- croenvironment as a key player in tumor development and immune evasion and, moreover, that the stroma is a major factor in the notable drug resistance of pancreatic cancer, have marked the beginning of somewhat of a paradigm shift in the way pancreatic cancer and its treatments are viewed. New therapies, in which a multi- pronged approach is employed, targeting not only the primary tumor, but also the surrounding structures such as the tumor stroma, are starting to be explored with the hope of increasing response rates and subsequently im- proving survival outcomes. Nab-paclitaxel heralds the beginning of this new era, demonstrating significantly greater efficacy in advanced disease when administered with gemcitabine in the phase III setting than the current standard of care, gemcitabine monotherapy, and with less toxicity than FOLFIRINOX. It will be interesting to see if this proven efficacy in the palliative setting can be ex- trapolated to the adjuvant and neoadjuvant setting as well as in patients with locally advanced unresectable disease. Nab-paclitaxel should be considered as an eminent thera- peutic option in combination with gemcitabine in the treatment of patients with pancreatic cancer. 12. Acknowledgements The authors wish to express their gratitude to all of the participants who have taken part in clinical trials as well as to the study investigators, study nurses and data col- lectors and, finally, to the study groups and donors, all of whom have helped contribute to developing more effec- tive therapies for pancreatic cancer. Researchers continue to search for new explanations, resulting in an increased understanding of pancreatic cancer biology and, ulti- mately, translation of this knowledge into novel ap- proaches in the treatment of this challenging disease. Medical writing assistance was provided by Dr. Marc Esser and Melody Watson at co.faktor (Berlin, Ger- many), funded by Celgene Corporation. The author is fully responsible for all content and edi- torial decisions for this manuscript. REFERENCES [1] C. L. Wolfgang, J. M. Herman, D. A. Laheru, A. P. Klein, M. A. Erdek, et al., “Recent Progress in Pancreatic Can- cer,” CA: A Cancer Journal for Clinicans, Vol. 63, No. 5, 2013, pp. 318-348. http://dx.doi.org/10.1002/caac.21190 [2] L. Lombardi, T. Troiano, N. Silvestris, L. Nanni, T. P. Latiano, et al., “Combined Modality Treatments in Pan- creatic Cancer,” Expert Opinion on Therapeutic Targets, Vol. 16, Suppl. 2, 2012, pp. S71-81. http://dx.doi.org/10.1517/14728222.2012.662959 [3] R. A. Stathis and M. J. Moore, “Advanced Pancreatic Carcinoma: Current Treatment and Future Challenges,” Nature Reviews. Clinical Oncology, Vol. 7, No. 3, 2010, pp. 163-172. http://dx.doi.org/10.1038/nrclinonc.2009.236 [4] M. Hidalgo, “Pancreatic Cancer,” The New England Journal of Medicine, Vol. 362, No. 17, 2010, pp. 1605- 1617. http://dx.doi.org/10.1056/NEJMra0901557 [5] A. Vincent, J. Herman, R. Schulick, R. H. Hruban and M. Goggins, “Pancreatic Cancer,” Lancet, Vol. 378, No. 9791, 2001, pp. 607-620. http://dx.doi.org/10.1016/S0140-6736(10)62307-0 [6] M. M. Hassan, M. L. Bondy, R. A. Wolff, J. L. Ab- bruzzese, J. N. Vauthey, et al., “Risk Factors for Pancre- atic Cancer: Case-Control Study,” The American Journal of Gastroenterology, Vol. 102, No. 12, 2007, pp. 2696- 2707. http://dx.doi.org/10.1111/j.1572-0241.2007.01510.x [7] C. Shi, H. R. Hruban and A. P. Klein, “Familial Pancre- atic Cancer,” Archives of Pathology & Laboratory Medi- cine, Vol. 133, No. 3, 2009, pp. 365-374. [8] A. Maitra and H. R. Hruban, “Pancreatic Cancer,” Annual Review of Pathology: Mechanisms of Disease, Vol. 3, 2008, pp. 157-188. http://dx.doi.org/10.1146/annurev.pathmechdis.3.121806. 154305 [9] S. Jones, X. Zhang, D. W. Parsons, J. C. Lin, R. J. Leary, et al., “Core Signaling Pathways in Human Pancreatic Cancers Revealed by Global Genomic Analyses,” Science, Vol. 321, No. 5897, 2008, pp. 1801-1806. http://dx.doi.org/10.1126/science.1164368 [10] G. Luo, J. Long, B. Zhang, C. Liu, J. Xu, et al., “Stroma and Pancreatic Ductal Adenocarcinoma: An Interaction Loop,” Biochimica et Biophysica Acta, Vol. 1826, No. 1, 2012, pp. 170-178. http://dx.doi.org/10.1016/j.bbcan.2012.04.002 [11] D. Mahadevan, D. D. Von Hoff, “Tumor-Stroma Inter- actions in Pancreatic Ductal Adenocarcinoma,” Mole- cular Cancer Therapeutics, Vol. 6, No. 4, 2007, pp. 1186- 1197. http://dx.doi.org/10.1158/1535-7163.MCT-06-0686 [12] M. V. Apte, S. Park, P. A. Phillips, N. Santucci, D. Gold- stein, et al., “Desmoplastic Reaction in Pancreatic Cancer: Role of Pancreatic Stellate Cells,” Pancreas, Vol. 29, No. 3, 2004, pp. 179-187. http://dx.doi.org/10.3389/fphys.2012.00344 [13] A. Masamune and T. Shimosegawa, “Signal Transduction in Pancreatic Stellate Cells,” Journal of Gastroenterology, Vol. 44, No. 4, 2009, pp. 249-260. http://dx.doi.org/10.1007/s00535-009-0013-2 [14] M. Erkan, C. Reiser-Erkan, C. W. Michalski, S. Deucker, D. Sauliunaite, et al., “Cancer-Stellate Cell Interactions Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 24 Perpetuate the Hypoxia-fibrosis Cycle in Pancreatic Duc- tal Adenocarcinoma,” Neoplasia, Vol. 11, No. 5, 2009, pp. 497-508. http://dx.doi.org/10.1593/neo.81618 [15] N. P. Desai, V. Trieu, L. Y. Hwang, R. Wu, P. Soon- Shiong, et al., “Improved Effectiveness of Nanoparticle Albumin-bound (nab) Paclitaxel versus Polysorbate-Based Docetaxel in Multiple Xenografts as a Function of HER2 and SPARC Status,” Anti-Cancer Drugs, Vol. 19, No. 9, 2008, pp. 899-909. http://dx.doi.org/10.1097/CAD.0b013e32830f9046 [16] C. J. Lee, J. Dosch and D. M. Simeone, “Pancreatic Can- cer Stem Cells,” Journal of Clinical Oncology, Vol. 26, No. 17, 2008, pp. 2806-2812. http://dx.doi.org/10.1200/JCO.2008.16.6702 [17] P. C. Hermann, S. L. Huber, T. Herrler, A. Aicher, J. W. Ellwart, et al., “Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer,” Cell Stem Cell, Vol. 1, No. 3, 2007, pp. 313-323. http://dx.doi.org/10.1016/j.stem.2007.06.002 [18] G. Bond-Smith, N. Banga, T. M. Hammond and C. J. Imber, “Pancreatic Adenocarcinoma,” BMJ, Vol. 344, 2012, Article ID: e2476. http://dx.doi.org/10.1136/bmj.e2476 [19] C. G. Moertel, D. S. Childs Jr., R. J. Reitemeier, M. Y. Colby Jr., M. A. Holbrook, et al., “Combined 5-fluoro- uracil and Supervoltage Radiation Therapy of Locally Unresectable Gastrointestinal Cancer,” The Lancet, Vol. 2, No. 7626, 1969, pp. 865-867. http://dx.doi.org/10.1016/S0140-6736(69)92326-5 [20] J. B. Haslam, P. J. Cavanaugh and S. L. Stroup, “Radia- tion Therapy in the Treatment of Irresectable Adenocar- cinoma of the Pancreas,” Cancer, Vol. 32, No. 6, 1973, pp. 1341-1345. http://dx.doi.org/10.1002/1097-0142(197312)32:6<1341:: AID-CNCR2820320609>3.0.CO;2-A [21] M. H. Kaiser and S. S. Ellenberg, “Pancreatic Cancer. Adjuvant combined Radiation and Chemotherapy Fol- lowing Curative Resection,” Archives of Surgery, Vol. 120, No. 8, 1985, pp. 899-903. http://dx.doi.org/10.1001/archsurg.1985.01390320023003 [22] Gastrointestinal Tumor Study Group, “Further Evidence of Effective Adjuvant combined Radiation and Chemo- therapy Following Curative Resection,” Cancer, Vol. 59, No. 12, 1987, pp. 2006-2010. http://dx.doi.org/10.1002/1097-0142(19870615)59:12<20 06::AID-CNCR2820591206>3.0.CO;2-B [23] J. H. Klinkenbijl, J. Jeekel, T. Sahmoud, R. van Pel, M. L. Couvreur, et al., “Adjuvant Radiotherapy and 5-Fluorouracil after Curative Resection of Cancer of the Pancreas and Periampullary Region,” Annals of Surgery, Vol. 230, No. 6, 1999, pp. 776-782. http://dx.doi.org/10.1097/00000658-199912000-00006 [24] H. G. Smeenk, C. H. J. van Eijck, W. C. Hop, J. Erdmann, K. C. K. Tran, et al., “Long-term Survival and Metastatic Pattern of Pancreatic and Periampullary Cancer after Ad- juvant Chemoradiation or Observation: Long-term Re- sults of EORTC trial 40891,” Annals of Surgery, Vol. 246, No. 5, 2007, pp. 734-740. http://dx.doi.org/10.1097/SLA.0b013e318156eef3 [25] J. P. Neoptolemos, D. D. Stocken, H. Friess, C. Bassi, J. A. Dunn, et al., “A Randomized Trial of Chemoradio- therapy and Chemotherapy after Resection of Pancreatic Cancer,” The New England Journal of Medicine, Vol. 350, No. 12, 2004, pp. 1200-1210. http://dx.doi.org/10.1056/NEJMoa032295 [26] H. Oettle, S. Post, P. Neuhaus, K. Gellert, J. Langrehr, et al., “Adjuvant Chemotherapy with Gemcitabine vs Ob- servation in Patients Undergoing Curative-intent Resec- tion of Pancreatic Cancer: A Randomized Controlled Trial.,” JAMA: The Journal of the American Medical As- sociation, Vol. 297, No. 3, 2007, pp. 267-277. http://dx.doi.org/10.1001/jama.297.3.267 [27] J. P. Neoptolemos, D. D. Stocken, C. Bassi, P. Ghaneh, D. Cunningham, et al., “Adjuvant Chemotherapy with Fluorouracil plus Folinic Acid vs Gemcitabine Following Pancreatic Cancer Resection,” JAMA: The Journal of the American Medical Association, Vol. 304, No. 10, 2010, pp. 1073-1081. http://dx.doi.org/10.1001/jama.2010.1275 [28] H. A. Burris 3rd, M. J. Moore, J. Andersen, M. R. Green, M. L. Rothenberg, et al., “Improvements in Survival and Clinical Benefit with Gemcitabine as First-line Therapy for Patients with Advanced Pancreas Cancer: A Ran- domized Trial,” Journal of Clinical Oncology, Vol. 15, No. 6, 1997, pp. 2403-2413. [29] W. F. Regine, K. A. Winter, R. A. Abrams, H. Safran, J. P. Hoffman, et al., “Fluorouracil vs Gemcitabine Che- motherapy before and after Fluorouracil-based Chemora- diation following Resection of Pancreatic Adenocarci- noma: A Randomized Controlled Trial,” JAMA: The Journal of the American Medical Association, Vol. 299, No. 9, 2008, pp. 1019-26. http://dx.doi.org/10.1001/jama.299.9.1019 [30] Fukutomi, K. Uesaka, N. Boku, H. Kanemoto, M. Kon- ishi, et al., “JASPAC 01: Randomized Phase III Trial of Adjuvant Chemotherapy with Gemcitabine versus S-1 for Patients with Resected Pancreatic Cancer,” Journal of Clinical Oncology, Vol. 31, Suppl. 4003, 2013. [31] D. D. Stocken, M. W. Büchler, C. Dervenis, C. Bassi, H. Jeekel, et al., “Meta-Analysis of Randomised Adjuvant Therapy Trials for Pancreatic Cancer,” British Journal of Cancer, Vol. 92, No. 8, pp. 1372-1381. http://dx.doi.org/10.1038/sj.bjc.6602513 [32] A. Vincent, J. Herman, R. Schulick, R. H. Hruban and M. Goggins, “Pancreatic Cancer,” The Lancet, Vol. 378, No. 9791, 2001, pp. 607-620. http://dx.doi.org/10.1016/S0140-6736(10)62307-0 [33] M. J. Moore, D. Goldstein, J. Hamm, A. Figer, J. R. Hecht, et al., “Erlotinib plus Gemcitabine Compared with Gemcitabine alone in Patients with Advanced Pancreatic Cancer: A Phase III Trial of the National Cancer Institute of Canada Clinical Trials Group,” Journal of Clinical Oncology, Vol. 25, No. 15, 2007, pp. 1960-1966. http://dx.doi.org/10.1200/JCO.2006.07.9525 [34] K. S. Gunturu, G. R. Rossi and M. W. Saif, “Immuno- therapy Updates in Pancreatic Cancer: Are We There Yet?” Therapeutic Advances in Medical Oncology, Vol. 5, No. 1, 2013, pp. 81-89. Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 25 http://dx.doi.org/10.1177/1758834012462463 [35] J. M. Hardacre, M. F. Mulcahy, et al., “Addition of Al- genpantucel-L Immunotherapy to Standard of Care (SOC) Adjuvant Therapy for Pancreatic Cancer,” Journal of Clinical Oncology, Vol. 30, Suppl. 4049, 2012. [36] E. Lutz, C. J. Yeo, K. D. Lillemoe, B. Biedrzycki, B. Kobrin, et al., “A Lethally Irradiated Allogeneic Granu- locyte-macrophage Colony Stimulating Factor-secreting Tumor Vaccine for Pancreatic Adenocarcinoma. A Phase II Trial of Safety, Efficacy, and Immune Activation,” Annals of Surgery, Vol. 253, No. 2, 2011, pp. 328-335. http://dx.doi.org/10.1097/SLA.0b013e3181fd271c [37] C. Belli, S. Cereda, S. Anand and M. Reni, “Neoadjuvant Therapy in Resectable Pancreatic Cancer: A Critical Re- view,” Cancer Treatment Reviews, Vol. 29, No. 5, 2013, pp. 518-524. http://dx.doi.org/10.1016/j.ctrv.2012.09.008 [38] S. Heinrich, B. C. Pestalozzi, M. Schäfer, A. Weber, P. Bauerfeind, et al., “Prospective Phase II Trial of Neoad- juvant Chemotherapy with Gemcitabine and Cisplatin for Resectable Adenocarcinoma of the Pancreatic Head,” Journal of Clinical Oncology, Vol. 26, No. 15, 2008, pp. 2526-2531. http://dx.doi.org/10.1200/JCO.2007.15.5556 [39] D. H. Palmer, D. D. Stocken, H. Hewitt, C. E. Markham, A. B. Hassan, et al., “A Randomized Phase 2 Trial of Neoadjuvant Chemotherapy in Resectable Pancreatic Cancer: Gemcitabine Alone versus Gemcitabine com- bined with Cisplatin,” Annals of Surgical Oncology, Vol. 14, No. 7, 2007, pp. 2088-2089. http://dx.doi.org/10.1245/s10434-007-9384-x [40] M. S. Talamonti, W. Small Jr., M. F. Mulcahy, J. D. Wayne, V. Attaluri, et al., “A Multi-Institutional Phase II Trial of Preoperative Full-dose Gemcitabine and Concur- rent Radiation for Patients with Potentially Resectable Pancreatic Carcinoma,” Annals of Surgical Oncology, Vol. 13, No. 7, 2006, pp. 150-158. http://dx.doi.org/10.1245/ASO.2006.03.039 [41] G. R. Varadhachary, R. A. Wolff, C. H. Crane, C. C. Sun, J. E. Lee, et al., “Preoperative Gemcitabine and Cisplatin Followed by Gemcitabine-Based Chemoradiation for Re- sectable Adenocarcinoma of the Pancreatic Head,” Jour- nal of Clinical Oncology, Vol. 26, 2008, pp. 3487-3495. http://dx.doi.org/10.1200/JCO.2007.15.8642 [42] S. Gillen, T. Schuster, C. Meyer Zum Büschenfelde, H. Friess and J. Kleeff, “Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta- Analysis of Response and Resection Percentages,” PLoS Medicine, Vol. 7, No. 4, 2010. http://dx.doi.org/10.1371/journal.pmed.1000267 [43] S. MacKenzie, H. Zeh, L. E. McCahill, T. D, Sielaff, N. Bahary, et al., “A Pilot Phase II Multicenter Study of Nab-paclitaxel (Nab-P) and Gemcitabine (G) as Preop- erative Therapy for Potentially Resectable Pancreatic Cancer (PC),” Journal of Clinical Oncology, Vol. 31 Suppl. 4038, 2013. [44] D. Habermehl, K. Kessel, T. Welzel, H. Hof, A. Abdol- lahi, et al., “Neoadjuvant Chemoradiation with Gemcit- abine for Locally Advanced Pancreatic Cancer,” Journal of Radiation Oncology, Vol. 7, No. 28, 2012, p. 28. http://dx.doi.org/10.1186/1748-717X-7-28 [45] M. Nakamura, T. Kayashima, K. Fujiwara, Y. Nagayoshi and H. Kono, “Combination Therapy of Portal Vein Re- section and Adjuvant Gemcitabine Improved Prognosis of Advanced Pancreatic Cancer,” Hepatogastroenterology, Vol. 60, No. 122, 2013, pp. 354-357. http://dx.doi.org/10.5754/hge12614 [46] B. Chauffert, F. Mornex, F. Bonnetain, P. Rougier and C. Mariette, “Phase III Trial Comparing Intensive Induction Chemoradiotherapy (60 Gy, Infusional 5-FU and Inter- mittent Cisplatin) Followed by Maintenance Gemcitabine with Gemcitabine Alone for Locally Advanced Unre- sectable Pancreatic Cancer. Definitive Results of the 2000-01 FFCD/SFRO Study,” Annals of Oncology, Vol. 19, No. 9, 2008, pp. 1592-1599. http://dx.doi.org/10.1093/annonc/mdn281 [47] P. J. Loehrer Sr, Y. Feng, H. Cardenes, L. Wagner, J. M. Brell, et al., “Gemcitabine Alone versus Gemcitabine plus Radiotherapy in Patients With Locally Advanced Pancre- atic Cancer: An Eastern Cooperative Oncology Group Trial,” Journal of Clinical Oncology, Vol. 29, No. 31, 2011, pp. 4105-4112. http://dx.doi.org/10.1200/JCO.2011.34.8904 [48] S. Mukherjee, C. Hurt, G. Griffiths, J. A. Bridgewater, T. Crosby, et al., “SCALOP: Results of a Randomized Phase II Study of Induction Chemotherapy Followed by Gem- citabine (G) or Capecitabine (Cap) based Chemoradiation (CRT) in Locally Advanced Pancreatic Cancer (LAN PC),” Journal of Clinical Oncology, Vol. 30, Suppl. 34, 2012. [49] P. Hammel, F. Huguet, J-L. Van Laethem, D. Goldstein, B. Glimelius, et al., “Comparison of Chemoradiotherapy (CRT) and Chemotherapy (CT) in Patients with a Locally Advanced Pancreatic Cancer (LAPC) Controlled after 4 Months of Gemcitabine with or without Erlotinib: Final Results of the International Phase III LAP 07 study,” Journal of Clinical Oncology, Vol. 31, Suppl. LBA4003, 2013. [50] J. M. Herman, A. T. Wild, H. Wang, P. T. Tran, K. J. Chang, et al., “Randomized Phase III Multi-Institutional Study of TNFerade Biologic with Fluorouracil and Ra- diotherapy for Locally Advanced Pancreatic Cancer,” Journal of Clinical Oncology, Vol. 31, 2013, pp. 886-894. http://dx.doi.org/10.1200/JCO.2012.44.7516 [51] J. D. Berlin, P. Catalano, J. P. Thomas, J. W Kugler, D. G. Haller, et al., “Phase III Study of Gemcitabine in Combination with Fluorouracil versus Gemcitabine alone in Patients with Advanced Pancreatic Carcinoma: Eastern Cooperative Oncology Group Trial E2297,” Journal of Clinical Oncology, Vol. 20, No. 15, 2002, pp. 3270-3275. http://dx.doi.org/10.1200/JCO.2002.11.149 [52] R. Herrmann, G. Bodoky, T. Ruhstaller, B. Glimelius, E. Bajetta E, et al., “Gemcitabine plus Capecitabine Com- pared with Gemcitabine alone in Advanced Pancreatic Cancer: A Randomized, Multicenter, Phase III Trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group,” Journal of Clinical Oncology, Vol. 25, No. 16, 2007, pp. 2212- 2217. http://dx.doi.org/10.1200/JCO.2006.09.0886 [53] C. Louvet, R. Labianca, P. Hammel, G. Lledo, M. G. Zampino, et al., “Gemcitabine in Combination with Ox- Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer 26 aliplatin Compared with Gemcitabine alone in Locally Advanced or Metastatic Pancreatic Cancer: Results of a GERCOR and GISCAD phase III Trial,” Journal of Clinical Oncology, Vol. 23, No. 15, 2005, pp. 3509-3516. http://dx.doi.org/10.1200/JCO.2005.06.023 [54] V. Heinemann, D Quietzsch, F. Gieseler, M. Gonnermann, H. Schönekäs, et al., “Randomized Phase III trial of Ge- mcitabine plus Cisplatin compared with Gemcitabine alone in Advanced Pancreatic Cancer,” Journal of Clini- cal Oncology, Vol. 24, No. 24, 2006, pp. 3946-3952. http://dx.doi.org/10.1200/JCO.2005.06.023 [55] C. M. Rocha Lima, M. R Gree, R Rotche, W. H. Miller, G. M. Jeffrey, et al., “Irinotecan plus Gemcitabine Re- sults in no Survival Advantage Compared with Gemcit- abine Monotherapy in Patients with Locally Advanced or Metastatic Pancreatic Cancer despite Increased Tumor Response Rate,” Journal of Clinical Oncology, Vol. 22, No. 18, 2004, pp. 3776-3783. http://dx.doi.org/10.1200/JCO.2004.12.082 [56] H. Oettle, D. Richards, R. K. Ramanathan, J. L. van Laethem, M. Peeters, et al., “A Phase III Trial of Pe- metrexed plus Gemcitabine versus Gemcitabine in Pa- tients with Unresectable or Metastatic Pancreatic Can- cer,” Annals of Oncology, Vol. 16, No. 10, 2005 pp. 1639- 1645. http://dx.doi.org/10.1093/annonc/mdi309 [57] A. Sultana, C. T. Smith, D. Cunningham, N. Starling, J. P. Neoptolemos, et al., “Meta-analyses of Chemotherapy for Locally Advanced and Metastatic Pancreatic Cancer”. Journal of Clinical Oncology, Vol. 25, No. 18, 2007, pp. 2607-1265. http://dx.doi.org/10.1200/JCO.2006.09.2551 [58] V. Heinemann, S. Boeck, A. Hinke, R. Labianca, C. Louvet, et al., “Meta-Analysis of Randomized Trials: Evaluation of Benefit from Gemcitabine-based Combina- tion Chemotherapy Applied in Advanced Pancreatic Cancer,” BMC Cancer, Vol. 8, 2008, p. 82. http://dx.doi.org/10.1186/1471-2407-8-82 [59] E. van Cutsem, W. L. Vervenne, J. Bennouna, Y. Hum- blet Y, S. Gill, et al., “Phase III Trial of Bevacizumab in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer,” Journal of Clinical Oncology, Vol. 27, No. 13, 2009 pp. 2231-2237. http://dx.doi.org/10.1200/JCO.2008.20.0238 [60] H. L. Kindler, D. Niedzwiecki, D. Hollis, S. Sutherland, D. Schrag, et al., “Gemcitabine plus Bevacizumab Com- pared with Gemcitabine plus Placebo in Patients with Advanced Pancreatic Cancer: Phase III Trial of the Can- cer and Leukemia Group B (CALGB 80303),” Journal of Clinical Oncology, Vol. 28, No. 22, pp. 3617-3622. http://dx.doi.org/10.1200/JCO.2010.28.1386 [61] P. A. Philip, J. Benedetti, C. L. Corless, R. Wong, E. M. O'Reill, et al., “Phase III Study Comparing Gemcitabine plus Cetuximab versus Gemcitabine in Patients with Ad- vanced Pancreatic Adenocarcinoma: Southwest Oncology Group-directed Intergroup Trial S0205,” Journal of Clini- cal Oncology, Vol. 28, No. 22, 2010, pp. 3605-3610. http://dx.doi.org/10.1200/JCO.2009.25.7550 [62] J. P. Spano, C. Chodkiewicz, J. Maurel, R. Wong, H. Wasan, et al., “Efficacy of Gemcitabine Plus Axitinib Compared with Gemcitabine Alone in Patients with Ad- vanced Pancreatic Cancer: An Open-label Randomised phase II Study,” Lancet, Vol. 371, No. 9630, pp. 2101- 2108. http://dx.doi.org/10.1016/S0140-6736(08)60661-3 [63] A. Gonçalves, M. Gilabert, E. François, L. Dahan, H. Perrier, et al., “BAYPAN Study: A Double-blind Phase III Randomized Trial Comparing Gemcitabine plus Sorafenib and Gemcitabine plus Placebo in Patients with Advanced Pancreatic Cancer,” Annals of Oncology, Vol. 23, No 11, pp. 2799-2805. http://dx.doi.org/10.1093/annonc/mds135 [64] T. Conroy, F. Desseigne, M. Ychou, O. Bouché, R. Guim- baud, et al., “FOLFIRINOX versus Gemcitabine for Me- tastatic Pancreatic Cancer,” The New England Journal of Medicine, Vol. 364, No. 19, pp. 1817-1825. http://dx.doi.org/10.1056/NEJMoa1011923 [65] P. J. Hosein, J. Macintyre, C. Kawamura, J. C. Mal- donado, V. Ernani, et al., “A Retrospective Study of Neo- adjuvant FOLFIRINOX in Unresectable or Borderline- Resectable Locally Advanced Pancreatic Adenocarci- noma,” BMC Cancer, Vol. 12, No. 199, 2012. http://dx.doi.org/10.1186/1471-2407-12-199 [66] T. Conroy, C. Gavoille, E. Samalin, M. Ychou, M. Ducreux, et al., “The Role of the FOLFIRINOX Regimen for Advanced Pancreatic Cancer,” Current Oncology Re- ports, Vol. 15, No. 2, 2013, pp. 182-189. http://dx.doi.org/10.1007/s11912-012-0290-4 [67] A. Neesse, P. Michl, K. K. Frese, C. Feig, N. Cook, et al., “Stromal Biology and Therapy in Pancreatic Cancer,” Gut, Vol. 60, No. 6, 2011, pp. 861-868. http://dx.doi.org/10.1136/gut.2010.226092 [68] J. R. Infante, H. Matsubayashi, N. Sato, J. Tonascia, A. P. Klein, et al., “Peritumoral Fibroblast SPARC Expression and Patient Outcome with Resectable Pancreatic Adeno- carcinoma,” Journal of Clinical Oncology, Vol. 25, No. 3, 2007, pp. 319-325. http://dx.doi.org/10.1200/JCO.2006.07.8824 [69] V. Trieu, J. Hwang and N. Desai, “Nanoparticle Albu- minbound (nab) Technology may Enhance Antitumour Activity via Targeting of SPARC Protein,” Proceedings New Targets and Delivery System for Cancer Diagnosis and Treatment conference, Sidney Kramer Cancer Center, San Diego, 5-7 March 2007. [70] D. D. Von Hoff, R. K. Ramanathan, M. J. Borad, D. A. Laheru, L. S. Smith, et al., “Gemcitabine Plus Nab-Pa- clitaxel is an Active Regimen in Patients with Advanced Pancreatic Cancer: A Phase I/II Trial,” Journal of Clini- cal Oncology, Vol. 29, No. 34, 2011, pp. 4548-4554. http://dx.doi.org/10.1200/JCO.2011.36.5742 [71] D. D. von Hoff, T. J. Ervin, F. P. Arena, E. G. Chiorean, R. J. Infante, et al., “Randomized Phase III Study of Weekly Nab-Paclitaxel plus Gemcitabine versus Gemcit- abine alone in Patients with Metastatic Adenocarcinoma of the Pancreas (MPACT),” Journal of Clinical Oncology, Vol. 30, Suppl. 34, 2012. [72] M. J. Moore, D. D. Von Hoff, T. J. Ervin, F. P. Arena, E. G. Chiorean, et al., “Prognostic Factors (PFs) of Survival in a Randomized Phase III Trial (MPACT) of Weekly Nab-paclitaxel (nab-P) plus Gemcitabine (G) versus G alone in Patients (pts) with Metastatic Pancreatic Cancer Open Access JCT  Recent Developments and Current Issues in the Treatment of Pancreatic Cancer Open Access JCT 27 (MPC),” Journal of Clinical Oncology, Vol. 31, Suppl. 4059, 2013. [73] U. Pelzer, K. Kubica, J. Stieler, I. Schwaner, G. Heil, et al., “A Randomized Trial in Patients with Gemcitabine Refractory Pancreatic Cancer. Final Results of the CONKO 003 Study,” Journal of Clinical Oncology, Vol. 26, No. 15S, Suppl. 4508, 2008. [74] U. Pelzer, I. Schwaner, J. Stieler, M. Adler, J. Seraphin J., et al., “Best Supportive Care (BSC) versus Oxaliplatin, Folinic Acid and 5-Fluorouracil (OFF) Plus BSC in Pa- tients for Second-line Advanced Pancreatic Cancer: A Phase III-study from the German CONKO-Study Group,” European Journal of Cancer, Vol. 47, No. 11, 2011, pp. 1676-1681. http://dx.doi.org/10.1016/j.ejca.2011.04.011 [75] H. Q. Xiong, G. R. Varadhachary, J. C. Blais, K. R. Hess, J. L. Abbruzzese, et al., “Phase 2 Trial of Oxaliplatin plus Capecitabine (XELOX) as Second-line therapy for Pa- tients with Advanced Pancreatic Cancer,” Cancer, Vol. 113, No. 8, 2008, pp. 2046-2052. http://dx.doi.org/10.1002/cncr.23810 [76] N. Mizuno, K. Yamao, Y. Komatsu, M. Munakata, A. Ishiguro, et al., “Randomized Phase II Study of Best Available Fluoropyrimidine Compared with Continuation of Gemcitabine (Gem) Monotherapy in Patients with Gem-Refractory Pancreatic Cancer,” Journal of Clinical Oncology, Vol. 30, Suppl. 34, Abstr. 263, 2012. [77] T. Ioka, K. Katayama, N. Ishida, R. Takada, T. Yamai, et al., “Randomized Phase II Trial of S-1 versus S-1 Plus Irinotecan (IRIS) in Patients with Gemcitabine-Refractory Pancreatic Cancer,” Journal of Clinical Oncology, Vol. 30, Suppl. 34, Abstr. 287, 2012. [78] C. N. Oldenhuis, S. F. Oosting, J. A. Gietema and E. G. de Vries, “Prognostic versus Predictive Value of Bio- markers in Oncology,” European Journal of Cancer, Vol. 44, No. 7, 2008, pp. 946-953. http://dx.doi.org/10.1016/j.ejca.2008.03.006 [79] A. C. Berger, K. Winter, J. P. Hoffman, W. F. Regine, R. A. Abrams, et al., “Five Year Results of US Intergroup/ RTOG 9704 with Postoperative CA 19-9 ≤90 U/mL and Comparison to the CONKO-001 Trial,” International Journal of Radiation Oncology Biology Physics, Vol. 84, No. 3, 2012, pp. 291-297. http://dx.doi.org/10.1016/j.ijrobp.2012.04.035 [80] E. G. Chiorean, D. D. Von Hoff, T. J. Ervin, F. P. Arena, J. R. Infante, et al., “CA19-9 Decrease at 8 Weeks as a Predictor of Overall Survival (OS) in a Randomized Phase III Trial (MPACT) of Weekly Nab-paclitaxel (nab- P) plus Gemcitabine (G) versus G alone in Patients with Metastatic Pancreatic Cancer (MPC),” Journal of Clinical Oncology, Vol. 31, Suppl. 4058, 2013. [81] C. Verslype, W. Verwvenne, J. Bennouna, Y. Humblet, J. Cosaert J, et al., “Rash as a Marker for the Efficacy of Gemcitabine plus Erlotinib-based Therapy in Pancreatic Cancer: Results from the AViTA Study,” Journal of Clinical Oncology, Vol. 27, Suppl. 4532, 2009. [82] J. Manzano, F. Rivera, M. Gala, M. Valladares, C. Pericay, et al., “A Phase II, Open Label Study to Evaluate the Relationship between Skin Rash and Survival in Pa- tients with Unresectable and/or Metastatic Pancreatic Cancer Treated with Erlotinib combined with Gemcit- abine,” Journal of Clinical Oncology, Vol. 28, Abstr. 4094. [83] E. Aranda, J. L. Manzano, F. Rivera, M. Galán, M. Val- ladares-Ayerbes, et al., “Phase II Open-label Study of Erlotinib in Combination with Gemcitabine in Unre- sectable and/or Metastatic Adenocarcinoma of the Pan- creas: Relationship between Skin Rash and Survival (Pan- tar Study),” Annals of Oncology, Vol. 23, No. 7, 2012, pp. 1919-1925. http://dx.doi.org/10.1093/annonc/mdr560 [84] V. Heinemann, U. Vehling-Kaiser, D. Waldschmidt, E. Kettner, A. Märten, et al., “Gemcitabine Plus Erlotinib followed by Capecitabine versus Capecitabine plus Er- lotinib followed by Gemcitabine in Advanced Pancreatic Cancer: Final Results of a Randomised Phase 3 Trial of the ‘Arbeitsgemeinschaft Internistische Onkologie’ (AIO- PK0104),” Gut, Vol. 62, No. 5, 2013, pp. 751-762. http://dx.doi.org/10.1136/gutjnl-2012-302759 [85] J. García-Manteiga, M. Molina-Arcas, F. J. Casado, A. Mazo and M. Pastor-Anglada, “Nucleoside Transporter Profiles in Human Pancreatic Cancer Cells: Role of hCNT1 in 2’,2’-Difluorodeoxycytidine-Induced Cytotox- icity,” Clinical Cancer Research, Vol. 9, No. 13, 2003, pp. 5000-5008. [86] J. P. Neoptolemos, W. Greenhalf, P. Ghaneh, D. H. Pal- mer, T. F. Cox, et al., “HENT1 Tumor Levels to Predict Survival of Pancreatic Ductal Adenocarcinoma Patients who Received Adjuvant Gemcitabine and Adjuvant 5FU on the ESPAC Trials,” Journal of Clinical Oncology, Vol. 31, Suppl. 4006, 2013. [87] J. J. Farrell, H. Elsaleh, M. Garcia, R. Lai, A. Ammar, et al., “Human Equilibrative Nucleoside Transporter 1 Lev- els Predict Response to Gemcitabine in Patients with Pancreatic Cancer,” Gastroenterology, Vol. 136, No. 1, 2009, pp. 187-195. http://dx.doi.org/10.1053/j.gastro.2008.09.067
|