 Materials Sciences and Applicatio ns, 2011, 2, 70-80 doi:10.4236/msa.2011.22010 Published Online February 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff Yang Li, Brigitte Helmreich, Harald Horn Institute of Water Quality Control, Technische Universität München, Garching, Germany. Email: y.li@bv.tu-muenchen.de Received December 20th, 2010; revised December 31st, 2010; accepted January 12th, 2011. ABSTRACT In this study, the ability of red alga (Palmaria palmata) and beer draff (brewery waste) for Cu(II) removal was investi- gated. The influence of factors, such as pH, initial copper concentrations, and contact time, were also studied. Results showed the adsorption process was strongly dependent on the pH value and initial concentration. The optimum pH value was in the range of 5-6. The Langmuir isotherm model performed better than other models, suggesting monolayer adsorption prevailed in the adsorption process. The theoretical adsorption capacities for Cu(II) were 12.7 mg/g and 9.01 mg/g for red alga and beer draff, respectively. The spectroscopy analyses and desorption studies showed that chemical bonding was the main mechanism in the adsorption process rather than ion exchange. The functional groups of amino, hydroxyl, carboxyl, phenolic hydroxyl, sulphonic group and C–O, –NH stretch might be involved in adsorp- tion. After adsorption, both materials were successfully regenerated by nitric acid at a concentration of 10 mmol/L. Furthermore, the phenomenon that only 7% of adsorbed Cu(II) on red alga and 11% on beer draff were desorbed by sodium chloride solution suggested potential alternative of both materials for the treatment of road runoff containing considerable amounts of de-icing salt. Keywords: Adsorption, Cu(II), Red Alga, Beer Draff, Chemical Bonding 1. Introduction Road runoff is considered a major anthropogenic source of heavy metals in the environment [1-4]. Rainfall can wash these pollutants from impervious surfaces directly into streams and lakes or they can infiltrate into the soil alongside roads [5]. Some of the heavy metals, e.g., cop- per (Cu), zinc (Zn), and nickel (Ni) are essential elements that are required to a varying degree in plants and ani- mals, but when present in elevated bioavailable concen- trations, these elements can become toxic [3]. Due to this concern, there has been a push to remove heavy metals from road runoff. There are a large number of potential materials that can be used for adsorption of dissolved heavy metals. However, not all of them are suitable for technical use in road runoff. Zeolithes are one typical instance [6,7]. With ion exchange as the main mechanism, zeolithes would release the adsorbed heavy metal in contact with de-icing salt (mostly sodium and potassium chloride) in winter time [7]. Clay minerals are also potential adsorption ma- terials for heavy metals especially due to their large sur- face area, which can range up to 800 m2/g. However, they swell in water and have a low permeability, causing problems in technical application [8]. Oxides or hydrox- ides are other possible materials. One application of granular ferrum hydroxide consisting of akaganeite (β- FeOOH) has been done in stormwater treatment [9]. However, akaganeite is not thermodynamically stable and can convert itself at high temperatures. Natural ma- terials like sawdust, which could be by-products from industry, are also known to adsorb heavy metals [10,11], but they also swell in contact with water. Besides the physical and chemical characteristics of materials, the suitable adsorption materials must be ab- undantly available and readily obtainable at a reasonable price. In this context, biosorbents like bacteria, yeast, fungi, algae and many by-products from industry are of great interest [12,13]. Thus, biosorption, which was de- fined as adsorption onto the surface of microbial biomass through passive metal-bonding of a biosorbent [12,14], is regarded as an effective option for heavy metal removal, especially at low concentrations [12,15,16]. Among various biosorbents, algae have a high capac-  Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff71 ity of metal-bonding, most likely due to the abundant existence of various functional groups as carboxyl, sul- phonic, amino and hydroxyl groups [12,17,18]. The main mechanisms of metal-bonding include ionic interactions and complex formation between metal cations and li- gands on the surface [19]. However, quality and quantity of mechanisms in biosorption vary from types of ad- sorbent, reaction conditions, and types of adsorbed metal ions. In many biosorption processes more than one of these mechanisms takes place simultaneously and it is difficult to distinguish between the single steps [20]. The aim of the following study was to find and evalu- ate a suitable filter material which could retain dissolved copper from road runoff. It should later become a com- ponent of an on-site treatment plant for highly polluted urban road runoff [4]. Based on the inexpensive cost, availability, and potential high adsorption capacity, the red alga (Palmaria palmate) and one kind of by-product from brewery (beer draff) were chosen to adsorb Cu(II) ions from aqueous solution in this study. Cu(II), a pol- lutant produced from the brake device and tyres of cars, is a representative heavy metal in road runoff. The effect of the pH, initial metal concentration and contact time on the adsorption behavior of these bio-sorbents was deter- mined. Two kinetic models (pseudo-first-order model and pseudo-second-order model), three isotherm models (Lang- muir, Freundlich and Dubinin-Radushkevich (D-R) mod- els), and two spectroscopy (Fourier transform infra-red spectroscopy (FTIR) and Energy dispersive X-ray emis- sion (EDX)) were used to explain the possible mecha- nisms of the adsorption process. A sodium chloride solu- tion and nitric acid were applied in desorption experi- ments. The former was used to clarify the influence of de-icing salt in the adsorption process and nitric acid was used to provide a method for recycling the materials after saturation with heavy metals. 2. Methods 2.1. Adsorbents Red alga (Palmaria palmata) provided by Cfm Oskar Tropitzsch e.K., Germany was used. The beer draff was from Bavarian State Brewery Weihenstephan (Germany). Adsorbents were first dried at 60˚C overnight before grinding into pieces. Afterwards, they were rinsed sev- eral times with deionized water until no more color was detached. Then they were dried again at 60˚C until con- stant weight. The size of red alga was under 450 μm while beer draff was kept at 450 μm-1.4 mm to remove the big grain. 2.2. Chemicals A stock solution from copper nitrate (CuNO3) containing 1,000 mg/L Cu(II) (Merck KGaA, Germany) was used in all experiments. The stock solution was diluted with de- ionized water to obtain desired concentrations. The pH value was adjusted by adding nitric acid (HNO3) or so- dium hydroxide (NaOH). All chemical reagents used in the experiments were of analytical grade. 2.3. Adsorption Experiments Adsorption experiments were carried out in a 50 mL polypropylene vessel. Batch experiments were conducted by varying the pH value (1, 2, 3, 4.5 and 6), contact time (5, 10, 20, 30, 50, 90, 120, 150, 180 min), and adsorbate concentration (5, 10, 25, 50, 100 mg/L). pH 6 was the maximum pH value in all experiments to avoid precipita- tion. For equilibrium experiments, 0.1 g adsorbent was dis- persed in a 50 mL aliquot of the metal ion solution at desired concentration and shaken on a rotary shaker at the speed of 40 rpm for 24 hours. All experiments were car- ried out at room temperature (22˚C) in duplicate. Except during equilibrium experiments, the Cu(II) con- centration and pH value were kept constant at 10 mg/L and pH 6 (unless otherwise stated). 2.4. Desorption Experiments In desorption experiments 0.1 g of biosorbent was first treated for 12 h with a 10 mg/L copper solution, filtered, and dried at 60˚C overnight before use. The loaded sor- bent was then agitated with 50 mL desorbing solution for 90 minutes at 40 rpm. For the desorbing solutions, HNO3 and NaCl were prepared in a wide range of concentra- tions (1 mmol/L, 10 mmol/L, and 100 mmol/L). To eli- minate the disturbance of water, deionized water was used as a blank. All experiments have been done twice. 2.5. Analyses Cu(II) analyses were carried out by atomic absorption spectrometry (Varian SpectrAA-240FS) according to the standard methods 3111 and 3113 [21].The samples were acidified prior to analysis to a pH below 2. The detection limit was 2 µg/L. The study of FTIR was carried out by the FTIR Vertex 70 (Bruker Company, with ATR-CELL). EDX analyses were done with an XL 30 FEG ESEM Instrument (Com- pany Philips, with EDX detector). The adsorbent samples used in FTIR and EDX analysis were first treated in 100 mg/L copper solution, agitating for 12 hours. The process of potentiometric and conductometric ti- tration includes protonation and titration. Protonation was achieved by dispersing 0.1 g of the adsorbents in 50 mL 0.01 M HNO3 for 2 hours. After filtration, washing and drying, the dried adsorbent was dispersed again in Copyright © 2011 SciRes. MSA 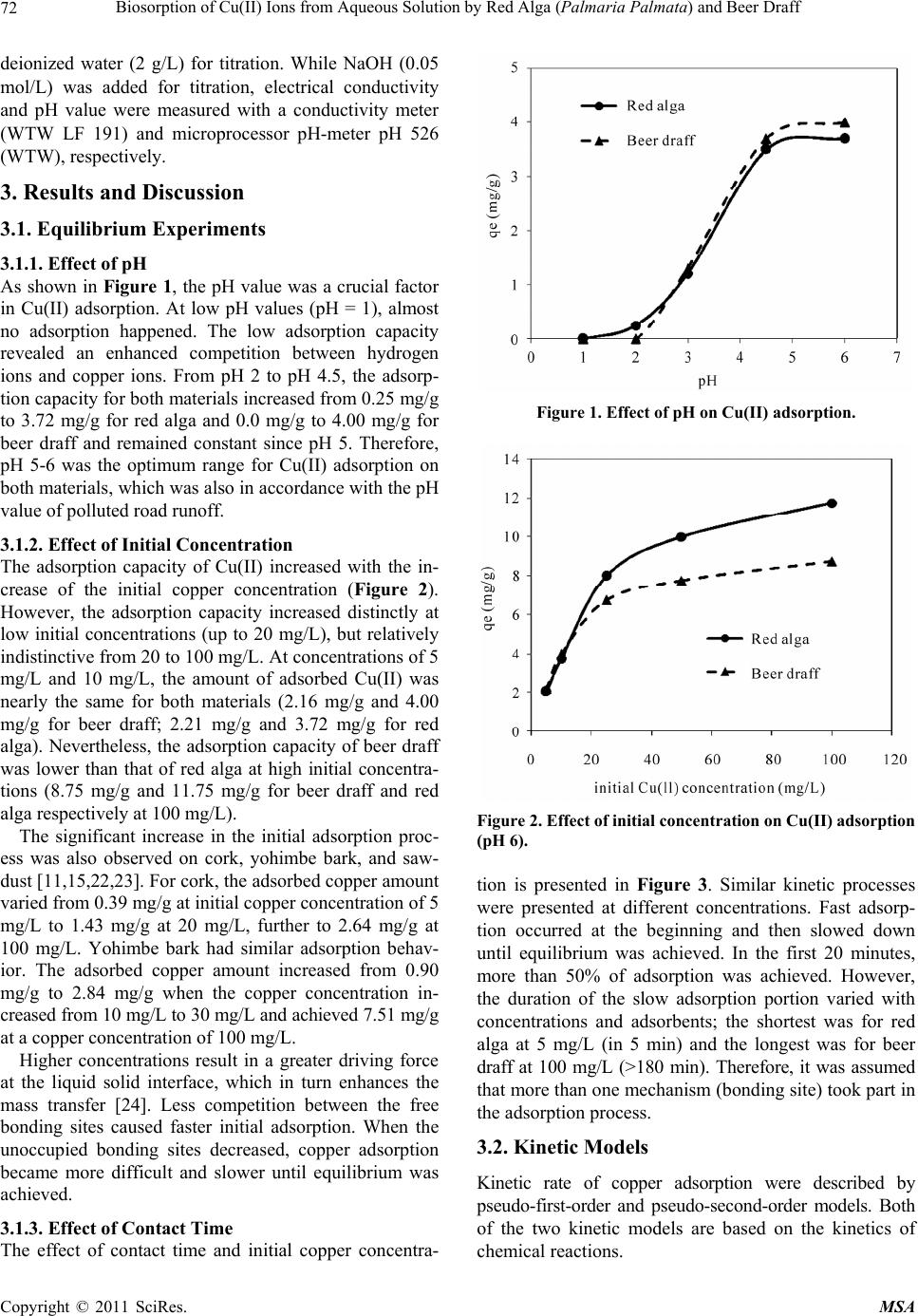 Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff 72 deionized water (2 g/L) for titration. While NaOH (0.05 mol/L) was added for titration, electrical conductivity and pH value were measured with a conductivity meter (WTW LF 191) and microprocessor pH-meter pH 526 (WTW), respectively. 3. Results and Discussion 3.1. Equilibrium Experiments 3.1.1. Effect of pH As shown in Figure 1, the pH value was a crucial factor in Cu(II) adsorption. At low pH values (pH = 1), almost no adsorption happened. The low adsorption capacity revealed an enhanced competition between hydrogen ions and copper ions. From pH 2 to pH 4.5, the adsorp- tion capacity for both materials increased from 0.25 mg/g to 3.72 mg/g for red alga and 0.0 mg/g to 4.00 mg/g for beer draff and remained constant since pH 5. Therefore, pH 5-6 was the optimum range for Cu(II) adsorption on both materials, which was also in accordance with the pH value of polluted road runoff. 3.1.2. Effect of Initial Concentration The adsorption capacity of Cu(II) increased with the in- crease of the initial copper concentration (Figure 2). However, the adsorption capacity increased distinctly at low initial concentrations (up to 20 mg/L), but relatively indistinctive from 20 to 100 mg/L. At concentrations of 5 mg/L and 10 mg/L, the amount of adsorbed Cu(II) was nearly the same for both materials (2.16 mg/g and 4.00 mg/g for beer draff; 2.21 mg/g and 3.72 mg/g for red alga). Nevertheless, the adsorption capacity of beer draff was lower than that of red alga at high initial concentra- tions (8.75 mg/g and 11.75 mg/g for beer draff and red alga respectively at 100 mg/L). The significant increase in the initial adsorption proc- ess was also observed on cork, yohimbe bark, and saw- dust [11,15,22,23]. For cork, the adsorbed copper amount varied from 0.39 mg/g at initial copper concentration of 5 mg/L to 1.43 mg/g at 20 mg/L, further to 2.64 mg/g at 100 mg/L. Yohimbe bark had similar adsorption behav- ior. The adsorbed copper amount increased from 0.90 mg/g to 2.84 mg/g when the copper concentration in- creased from 10 mg/L to 30 mg/L and achieved 7.51 mg/g at a copper concentration of 100 mg/L. Higher concentrations result in a greater driving force at the liquid solid interface, which in turn enhances the mass transfer [24]. Less competition between the free bonding sites caused faster initial adsorption. When the unoccupied bonding sites decreased, copper adsorption became more difficult and slower until equilibrium was achieved. 3.1.3. Effect of Contact Time The effect of contact time and initial copper concentra- Figure 1. Effect of pH on Cu(II) adsorption. Figure 2. Effect of initial concentration on Cu(II) adsorption (pH 6). tion is presented in Figure 3. Similar kinetic processes were presented at different concentrations. Fast adsorp- tion occurred at the beginning and then slowed down until equilibrium was achieved. In the first 20 minutes, more than 50% of adsorption was achieved. However, the duration of the slow adsorption portion varied with concentrations and adsorbents; the shortest was for red alga at 5 mg/L (in 5 min) and the longest was for beer draff at 100 mg/L (>180 min). Therefore, it was assumed that more than one mechanism (bonding site) took part in the adsorption process. 3.2. Kinetic Models Kinetic rate of copper adsorption were described by pseudo-first-order and pseudo-second-order models. Both of the two kinetic models are based on the kinetics of chemical reactions. Copyright © 2011 SciRes. MSA 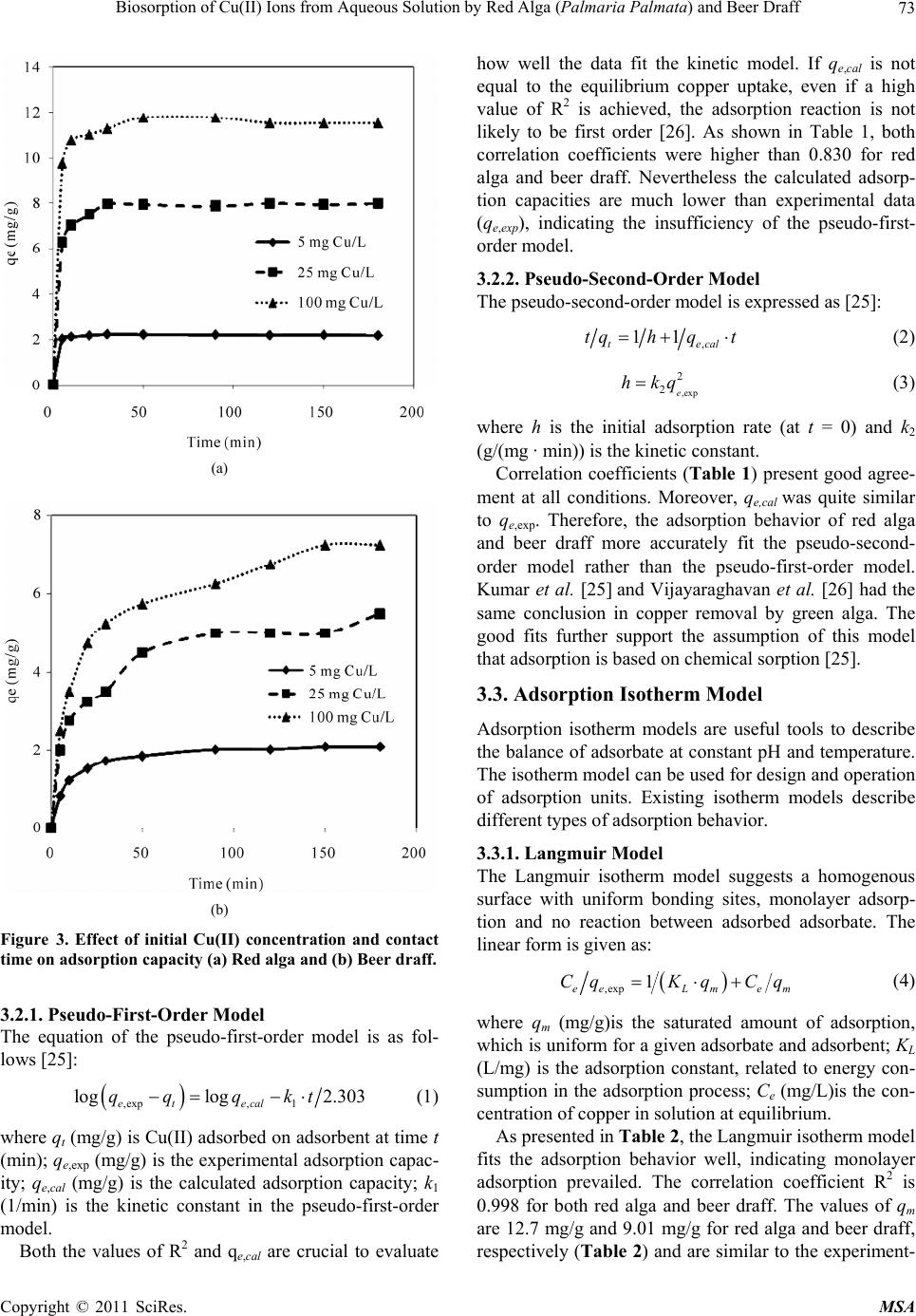 Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff73 (a) (b) Figure 3. Effect of initial Cu(II) concentration and contact time on adsorption capacity (a) Red alga and (b) Beer draff. 3.2.1. Pseudo - Fi r st-Order Model The equation of the pseudo-first-order model is as fol- lows [25]: ,exp, 1 loglog 2.303 et ecal qq qkt (1) where qt (mg/g) is Cu(II) adsorbed on adsorbent at time t (min); qe,exp (mg/g) is the experimental adsorption capac- ity; qe,cal (mg/g) is the calculated adsorption capacity; k1 (1/min) is the kinetic constant in the pseudo-first-order model. Both the values of R2 and qe,cal are crucial to evaluate how well the data fit the kinetic model. If qe,cal is not equal to the equilibrium copper uptake, even if a high value of R2 is achieved, the adsorption reaction is not likely to be first order [26]. As shown in Table 1, both correlation coefficients were higher than 0.830 for red alga and beer draff. Nevertheless the calculated adsorp- tion capacities are much lower than experimental data (qe,exp), indicating the insufficiency of the pseudo-first- order model. 3.2.2. Pseudo-Second-Order Model The pseudo-second-order model is expressed as [25]: , 11 te tqh qt cal (2) ,exp 2 2e hkq (3) where h is the initial adsorption rate (at t = 0) and k2 (g/(mg · min)) is the kinetic constant. Correlation coefficients (Table 1) present good agree- ment at all conditions. Moreover, qe,cal was quite similar to qe,exp. Therefore, the adsorption behavior of red alga and beer draff more accurately fit the pseudo-second- order model rather than the pseudo-first-order model. Kumar et al. [25] and Vijayaraghavan et al. [26] had the same conclusion in copper removal by green alga. The good fits further support the assumption of this model that adsorption is based on chemical sorption [25]. 3.3. Adsorption Isotherm Model Adsorption isotherm models are useful tools to describe the balance of adsorbate at constant pH and temperature. The isotherm model can be used for design and operation of adsorption units. Existing isotherm models describe different types of adsorption behavior. 3.3.1. Langmuir Model The Langmuir isotherm model suggests a homogenous surface with uniform bonding sites, monolayer adsorp- tion and no reaction between adsorbed adsorbate. The linear form is given as: ,exp 1 eeLm em CqK qCq (4) where qm (mg/g)is the saturated amount of adsorption, which is uniform for a given adsorbate and adsorbent; KL (L/mg) is the adsorption constant, related to energy con- sumption in the adsorption process; Ce (mg/L)is the con- centration of copper in solution at equilibrium. As presented in Table 2, the Langmuir isotherm model fits the adsorption behavior well, indicating monolayer adsorption prevailed. The correlation coefficient R2 is 0.998 for both red alga and beer draff. The values of qm are 12.7 mg/g and 9.01 mg/g for red alga and beer draff, respectively (Tabl e 2) and are similar to the experiment- Copyright © 2011 SciRes. MSA  Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff Copyright © 2011 SciRes. MSA 74 Table 1. Constants in kinetic models for (a) Red alga and (b) Beer draff at different concentrations of copper. (a) Pseudo-first-order Pseudo-second-order Co (mg/L) qe,exp (mg/g) qe,cal (mg/g) k1 (1/min) R2 h (mg/(g · min))k2 (g/(mg · min)) qe,cal (mg/g) R2 5 2.21 0.30 0.09 0.978 4.12 0.84 2.22 1.000 25 8.00 2.42 0.08 0.977 5.68 0.09 8.00 1.000 100 11.75 2.31 0.06 0.830 14.71 0.11 11.49 0.999 (b) Pseudo-first-order Pseudo-second-order Co (mg/L) qe,exp (mg/g) qe,cal (mg/g) k1 (1/min) R2 h (mg/(g · min))k2 (g/(mg · min)) qe,cal (mg/g) R2 5 2.16 1.06 0.01 0.838 0.19 0.05 2.01 0.999 25 6.75 3.99 0.005 0.888 0.42 0.01 5.68 0.995 100 8.75 5.08 0.01 0.928 0.56 0.01 7.75 0.996 Table 2. Coefficients in four isotherm models for red alga and beer draff. Materials Materials Materials Constants Red alga Beer draff Constants Red algaBeer draff Constants Red alga Beer draff Langmuir Freundlich D-R qm (mg/g) 12.7 9.01 KF (mg/g)(L/mg)1/n 2.63 3.05 qm (mg/g) 39.4 19.3 KL (L/mg) 0.178 0.323 n 2.519 3.676 KD (mol2/kJ2) 0.003 0.002 E (kJ/mol) 12.9 15.8 R2 0.998 0.998 R2 0.905 0.902 R2 0.943 0.941 where KF (mg/g) (L/mg)1/n and n (n > 1) are constants, dependent on temperature, adsorbate, and adsorbent. KF is related to the maximum adsorption capacity while n is a measurement of adsorption intensity. tal adsorption capacity, qe,exp. Although KL is a simple fitting parameter rather than a true adsorption constant, the high value of KL can indicate a high affinity for ad- sorption [14]. The higher value of KL on beer draff might be related with the high amount of total acidic groups. The correlation coefficients R2 of the Freundlich mo- del (Table 2) are 0.905 and 0.902 for red alga and beer draff, respectively. Compare to the Langmuir isotherm model, the Freundlich model did not fit the adsorption behavior on red alga and beer draff well. The good performance of the Langmuir model can also be found in other studies [15,22,23,25]. Compared with the qm values of other materials, red alga and beer draff present better adsorption capacities than cork (3.01 mg/g) or sawdust (1.79 mg/g). However, beer draff ad- sorbed less than yohimbe bark (9.54 mg/g) and grape stalks (10.2 mg/g), while red alga (Palmaria palmata) performed worse than one green alga (Ulva fasciata sp.), which adsorbed a maximum 26.9 mg/g Cu(II). 3.3.3. D-R Isotherm Model The D-R isotherm model was applied to identify physical or chemical processes in adsorption [27,28]. The related equations [29] are expressed as Equations (6-8): 2 ,exp ln ln em qqK D (6) 3.3.2. Freundl i ch Model Different from the Langmuir isotherm model, the Freun- dlich isotherm model considers the inhomogeneous sur- face of an adsorbent. The linear form is given as: where KD (mol2/kJ2) is the constant which is related to the calculated average sorption energy E, and ε (kJ · mol) is the Polanyi potential, which is described as: ,exp lnln1 ln eF qKn C e (5) Rln11 e TC (7) 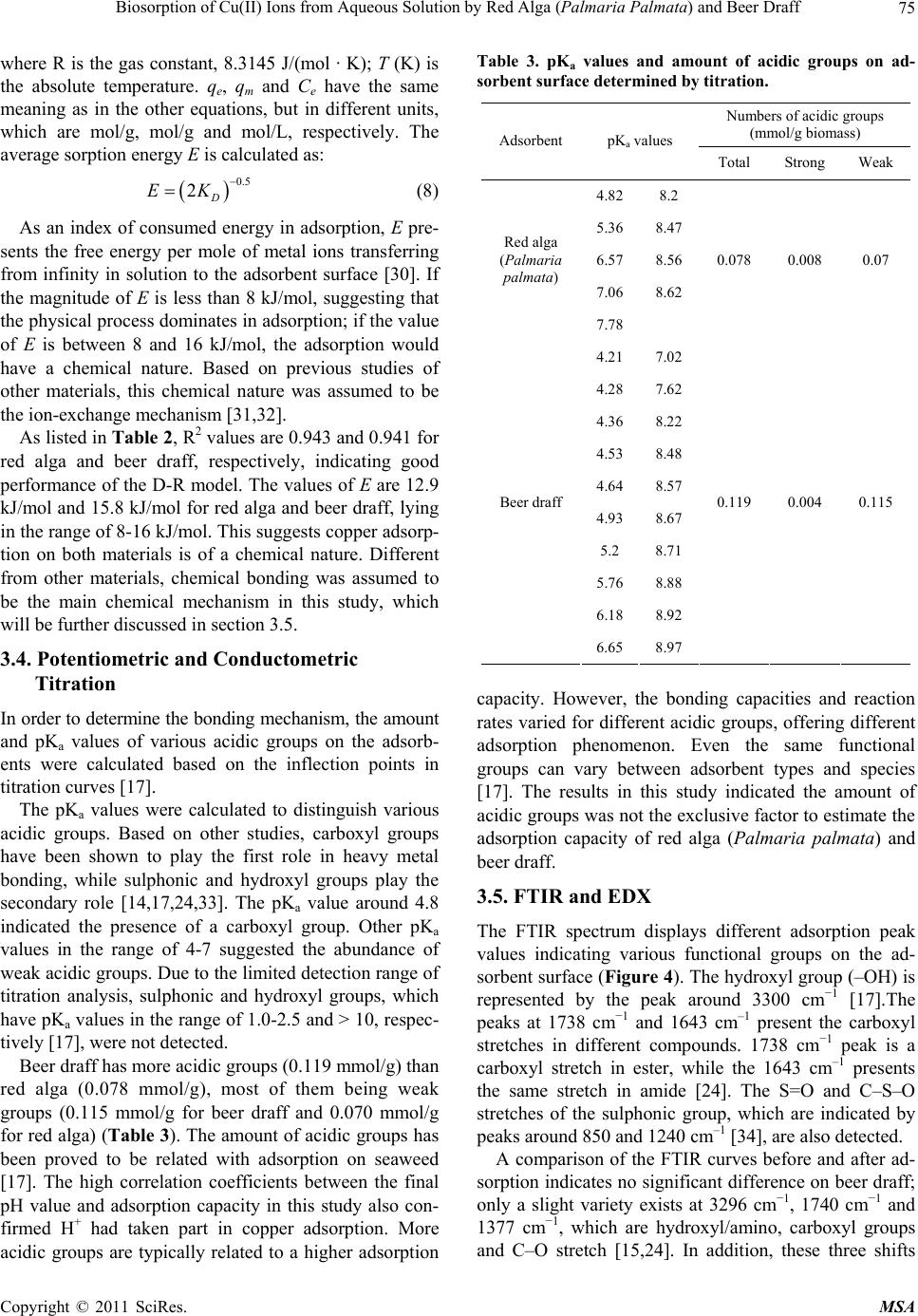 Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff75 where R is the gas constant, 8.3145 J/(mol · K); T (K) is the absolute temperature. qe, qm and Ce have the same meaning as in the other equations, but in different units, which are mol/g, mol/g and mol/L, respectively. The average sorption energy E is calculated as: 0.5 2D EK (8) As an index of consumed energy in adsorption, E pre- sents the free energy per mole of metal ions transferring from infinity in solution to the adsorbent surface [30]. If the magnitude of E is less than 8 kJ/mol, suggesting that the physical process dominates in adsorption; if the value of E is between 8 and 16 kJ/mol, the adsorption would have a chemical nature. Based on previous studies of other materials, this chemical nature was assumed to be the ion-exchange mechanism [31,32]. As listed in Table 2 , R2 values are 0.943 and 0.941 for red alga and beer draff, respectively, indicating good performance of the D-R model. The values of E are 12.9 kJ/mol and 15.8 kJ/mol for red alga and beer draff, lying in the range of 8-16 kJ/mol. This suggests copper adsorp- tion on both materials is of a chemical nature. Different from other materials, chemical bonding was assumed to be the main chemical mechanism in this study, which will be further discussed in section 3.5. 3.4. Potentiometric and Conductometric Titration In order to determine the bonding mechanism, the amount and pKa values of various acidic groups on the adsorb- ents were calculated based on the inflection points in titration curves [17]. The pKa values were calculated to distinguish various acidic groups. Based on other studies, carboxyl groups have been shown to play the first role in heavy metal bonding, while sulphonic and hydroxyl groups play the secondary role [14,17,24,33]. The pKa value around 4.8 indicated the presence of a carboxyl group. Other pKa values in the range of 4-7 suggested the abundance of weak acidic groups. Due to the limited detection range of titration analysis, sulphonic and hydroxyl groups, which have pKa values in the range of 1.0-2.5 and > 10, respec- tively [17], were not detected. Beer draff has more acidic groups (0.119 mmol/g) than red alga (0.078 mmol/g), most of them being weak groups (0.115 mmol/g for beer draff and 0.070 mmol/g for red alga) (Table 3). The amount of acidic groups has been proved to be related with adsorption on seaweed [17]. The high correlation coefficients between the final pH value and adsorption capacity in this study also con- firmed H+ had taken part in copper adsorption. More acidic groups are typically related to a higher adsorption Table 3. pKa values and amount of acidic groups on ad- sorbent surface determined by titration. Numbers of acidic groups (mmol/g biomass) Adsorbent pKa values Total Strong Weak 4.828.2 5.368.47 6.578.56 7.068.62 Red alga (Palmaria palmata) 7.78 0.078 0.008 0.07 4.217.02 4.287.62 4.368.22 4.538.48 4.648.57 4.938.67 5.2 8.71 5.768.88 6.188.92 Beer draff 6.658.97 0.119 0.004 0.115 capacity. However, the bonding capacities and reaction rates varied for different acidic groups, offering different adsorption phenomenon. Even the same functional groups can vary between adsorbent types and species [17]. The results in this study indicated the amount of acidic groups was not the exclusive factor to estimate the adsorption capacity of red alga (Palmaria palmata) and beer draff. 3.5. FTIR and EDX The FTIR spectrum displays different adsorption peak values indicating various functional groups on the ad- sorbent surface (Figure 4). The hydroxyl group (–OH) is represented by the peak around 3300 cm−1 [17].The peaks at 1738 cm−1 and 1643 cm–1 present the carboxyl stretches in different compounds. 1738 cm−1 peak is a carboxyl stretch in ester, while the 1643 cm–1 presents the same stretch in amide [24]. The S=O and C–S–O stretches of the sulphonic group, which are indicated by peaks around 850 and 1240 cm–1 [34], are also detected. A comparison of the FTIR curves before and after ad- sorption indicates no significant difference on beer draff; only a slight variety exists at 3296 cm−1, 1740 cm−1 and 1377 cm−1, which are hydroxyl/amino, carboxyl groups and C–O stretch [15,24]. In addition, these three shifts Copyright © 2011 SciRes. MSA  Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff Copyright © 2011 SciRes. MSA 76 (a) (b) Figure 4. FTIR of (a) Red alga and (b) Beer draff before and after Cu(II) adsorption. happened in the first 4 hours of adsorption and shifted in 12 hours (Figure 4), suggesting these functional groups and stretch conduced to a fast and flexible adsorption. Compared to the results of potentiometric and conduc- tometric titration, the abundant weak acidic groups that exist in beer draff seem to have a large contribution to the affinity of Cu(II) in adsorption process. Therefore, the copper adsorption on beer draff was assumed to be a combination of several functional groups. On the con- trary, the FTIR curve of red alga presented significant fluctuations at wavenumbers of 3281 cm−1 (changed to 3296 cm−1), 1333 cm−1 (changed to 1311 cm–1), 1230 cm−1 (changed to 1219 cm−1) and 841 cm−1 (replaced by 852 cm−1 and 839 cm−1), which are hydroxyl, –NH stretch  Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff77 (amino), phenolic hydroxyl, and sulphonic group [24]. The diversified performances of adsorption revealed that ad- sorption capacity varies among various functional groups and adsorbents. Energy dispersive X-ray emission (EDX) was used to investigate the characteristics of element abundance on the adsorbent. EDX spectrums of red alga before and after adsorption are shown in Figure 5. It can be seen that copper appeared after adsorption, from 0% to 3.24%. Furthermore, no other significant fluctuations of other elements were detected. Potassium (K) and Magnesium (Mg) slightly decreased and were ignored when taking account of measurement error. The EDX results of beer draff (not presented) provide similar results, in which copper appeared significantly after adsorption and most elements had a smaller decrease than that in red alga. Therefore, it is assumed that chemical bonding was the main mechanism in the adsorption process rather than ion-exchange. 3.6. Thermodynamic Study of Adsorption Gibbs free energy () is one of the main parameters in thermodynamic studies, suggesting spontaneity of ad- o G (a) (b) Figure 5. EDX spectrum of red alga (a) before and (b) after Cu(II) adsorption. sorption. The equation for this index is expressed as fol- lows: R ln o GTK (9) Since the Langmuir model fit better than the other iso- therm models, the coefficient KL in the Langmuir model was chosen to be the equilibrium constant K (L/mmol) for this calculation. The values of o G for red alga and beer draff are −22.9 kJ/mol and −24.4 kJ/mol, respectively. The nega- tive values of o G indicate the adsorption on red alga and beer draff was spontaneous. Compared to other bio- sorbents, many negative values of have been found, −23.54 kJ/mol, −12.60 kJ/mol and −21.37 kJ/mol for rubber leaves, green alga (Ulothrix zonata) and saw- dust, respectively [10,24,35]. This is advantageous as there is no energy requirement for adsorption on these materials. o G 3.7. Desorption Experiments Desorption experiments were carried out not only to evaluate if the materials can be regenerated after satura- tion with heavy metals, but also to clarify the stability of the adsorbed heavy metal on the surface of the materials in the presence of de-icing salt. The concentrations of sodium chloride used in desorption experiments were also in the range of normal de-icing salt concentrations in road runoff [4]. As shown in Table 4, HNO3 solution performed very well in the desorption process. At the lowest concentra- tion (1 mmol/L), more than 66% of adsorbed Cu(II) was removed by HNO3 from both biosorbents. At a concen- tration of 10 mmol/L HNO3 nearly 100% of adsorbed Cu(II) was set free. On the contrary, the treatment of the Cu(II) loaded Table 4. Desorbed copper amount in HNO3 and NaCl solu- tion. Desorption (%) Desorption reagent Concentration (mmol/L) Red alga Beer draff 1 66.4 68.2 10 96.3 100 100 100 100 HNO3 Blank (deionized water) <1 <1 1 <1 3.1 10 <1 4.1 100 7.0 11.1 NaCl Blank (deionized water) <1 <1 Copyright © 2011 SciRes. MSA  Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff 78 materials with sodium chloride (NaCl) solution, which was used as a de-icing salt, resulted in quite low desorp- tion efficiency, especially for red alga. At the highest concentration of NaCl (100 mmol/L), only 7% of the adsorbed Cu(II) on red alga was desorbed. Meanwhile, beer draff attained 11% Cu(II) desorption at the same concentration. Therefore, red alga seems more suitable to be the adsorbent in road runoff treatment. From these results, HNO3 at the concentration of 10 mmol/L was chosen to be the effective desorption re- agent. Red alga performed better in holding adsorbed Cu(II) in the presence of de-icing salt. Furthermore, the performance of HNO3 and NaCl in the desorption study also revealed ion-exchange was not the main mechanism in the adsorption of copper on red alga and beer draff. 4. Conclusions According to the results, red alga and beer draff can be used to remove copper ions from aqueous solutions. The adsorption capacity of the materials for Cu(II) was strongly dependent on the pH value and initial Cu(II) concentration. The optimum pH range was 5-6 for both materials. The relationship between final pH and adsorp- tion capacity revealed hydrogen ions had taken part in the copper adsorption process. The pseudo-second-order model was in accordance with adsorption behavior on both materials, showing the chemical characteristic of the adsorption process. The Langmuir isotherm model fit well with the adsorption phenomenon, indicating that the maximum adsorption capacities were 12.7 mg/g and 9.01 mg/g for red alga and beer draff, respectively. The D-R model demonstrated the chemical nature of the adsorption process. The nega- tive values of revealed that the adsorption process on both materials was spontaneous. o G Beer draff was found to have more weak acidic groups than red alga in the study of potentiometric and conduc- timetric titration. Considering the differences between the adsorption behavior of red alga and beer draff, the bonding capacities and reaction rates were assumed to be varied among different acidic groups. FTIR, EDX and desorption studies proved that chemical bonding was the main mechanism in adsorption rather than ion-exchange. The functional groups, such as amino, hydroxyl, car- boxyl, phenolic hydroxyl group, sulphonic group and C–O, –NH stretch, might be involved in adsorption. De- sorption experiments with NaCl indicated that the ad- sorption of Cu(II) to both materials mainly resulted from chemical bonding, not from ion exchange. Red alga and beer draff are inexpensive and widely available materials. Based on their satisfied adsorption capacities and low desorption efficiency with NaCl shown in this study, these two materials, especially the red alga, are possible alternative to remove copper from road runoff. REFERENCES [1] M. A. House, J. B. Ellis, E. E. Herricks, T. Hvitved- Jacobsen, J. Seager, L. Lijklema, R. H. Aalderink and I. T. Clifforde, “Urban Drainage Impact on Receiving Water Quality,” Water Science Technology, Vol. 27, No. 12, 1993, pp. 117-158. [2] K. Sriyaraj and R. B. E. Shutes, “An Assessment of the Im- pact of Motorway Runoff on a Pond, Wetland and Stream,” Environment International, Vol. 26, No. 5-6, 2001, pp. 433-439. doi:10.1016/S0160-4120(01)00024-1 [3] K. C. Rice, K. M. Conko and G. M. Hornberger, “An- thropogenic Sources of Arsenic and Copper in Sediments in Suburban Lake, Northern Virginia,” Environment Sci- ence Technology, Vol. 32, No. 23, 2002, pp. 4962-4967. doi:10.1021/es025727x [4] B. Helmreich, R. Hilliges, A. Schriewer and H. Horn, “Runoff Pollutants of a Highly Trafficked Urban Road— Correlation Analysis and Seasonal Influences,” Chemos- phere, Vol. 80, No. 9, 2010, pp. 991-997. doi:10.1016/j.chemosphere.2010.05.037 [5] K. Perdikaki and C. F. Mason, “Impact of Road Run-off on Receiving Streams in Eastern England,” Water Re- search, Vol. 33, No. 7, 1998, pp. 1627-1633. doi:10.1016/S0043-1354(98)00396-0 [6] V. J. Inglezakis, M. D. Loizidou and H. P. Grigoropoulou, “Equilibrium and Kinetic Ion Exchange Studies of Pb2+, Cr3+, Fe3+ and Cu2+ on Natural Clinoptilolite,” Water Re- search, Vol. 36, No. 11, 2002, pp. 2784-2792. doi:10.1016/S0043-1354(01)00504-8 [7] K. Athanasiadis and B. Helmreich, “Influence of Chemi- cal Conditioning on the Ion Exchange Capacity and on Ki- netic of Zinc Uptake by Clinoptilolite,” Water Research, Vol. 39, No. 1, 2005, pp. 1527-1532. doi:10.1016/j.watres.2005.01.024 [8] S. E. Bailey, T. J. Olin, R. M. Bricka and D. D. Adrian, “A Review of Potentially Low-Cost Sorbents for Heavy Metals,” Water Research, Vol. 33, No. 11, 1999, pp. 2469- 2479. doi:10.1016/S0043-1354(98)00475-8 [9] M. Steiner, “Adsorption of Copper from Stormwater Run- off onto Granulated Iron Hydroxide,” Dissertation, Zürich, 2003. [10] M. Ajmal, H. A. Khan, S. Ahmad and A. Ahmad, “Role of Sawdust in the Removal of Copper(II) from Industrial Wastes,” Water Research, Vol. 32, No. 10, 1998, pp. 3085- 3091. doi:10.1016/S0043-1354(98)00067-0 [11] A. Ahmad, M. Rafatulla and M. Danish, “Sorption Stud- ies of Zn(II) and Cd(II) Ions from Aqueous Solution on Treated Sawdust of Sissoo Wood,” European Journal of Wood Products, Vol. 65, No. 6, 2007, pp. 429-436. doi:10.1007/s00107-007-0175-7 Copyright © 2011 SciRes. MSA  Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff79 [12] B. Volesky, “Review: Biosorption and Me,” Water Research, Vol. 41, No. 18, 2007, pp. 4017-4029. doi:10.1016/j.watres.2007.05.062 [13] V. K. Gupta and I. Ali, “Removal of Lead and Chromium from Wastewater Using Bagasse Fly Ash—A Sugar In- dustry Waste,” Journal of Colloid Interface Science, Vol. 271, No. 2, 2004, pp. 321-328. doi:10.1016/j.jcis.2003.11.007 [14] T. A. Davis, B. Volesky and A. Mucci, “A Review of the Biochemistry of Heavy Metal Biosorption by Brown Alga,” Water Research, Vol. 37, No. 18, 2003, pp. 4311-4330. doi:10.1016/S0043-1354 (03)002 93-8 [15] I. Villaescusa, N. Fiol, M. Martinez, N. Miralles, J. Poch and J. Serarols, “Removal of Copper and Nickel Ions from Aqueous Solutions by Grape Stalks Wastes,” Water Research, Vol. 38, No. 4, 2004, pp. 992-1002. doi:10.1016/j.watres.2003.10.040 [16] G. Yan and T. Viraraghavan, “Heavy-Metal Removal from Aqueous Solution by Fungus Mucor Rouxii,” Water Research, Vol. 37, No. 18, 2003, pp. 4486-4496. doi:10.1016/S0043-1354(03)00409-3 [17] V. Murphy, H. Hughes and P. McLoughlin, “Cu(II) Bond- ing by Dried Biomass of Red, Green and Brown Macro- algae,” Water Research, Vol. 41, No. 4, 2007, pp. 731- 740. doi:10.1016/j.watres.2006.11.032 [18] Z. R. Holan, B. Volesky and I. Prasetyo, “Biosorption of Cadmium by Biomass of Marine Algae,” Biotechnology Bioengineering, Vol. 41, No. 8, 1993, pp. 819-825. doi:10.1002/bit.260410808 [19] Y. S. Yun, D. Park, J. M. Park and B. Volesky, “Biosorp- tion of Trivalent Chromium on the Brown Seaweed Bio- mass,” Environment Science Technology, Vol. 35, No. 21, 2001, pp. 4353-4358. doi:10.1021/es010866k [20] C. Lacher and R. W. Smith, “Sorption of Hg(II) by Po- tamogeton Natans Dead Biomass,” Minerals Engineering, Vol. 15, No. 3, 2002, pp. 187-191. doi:10.1016/S0892-6875(01)00212-6 [21] A. D. Eaton, L. S. Clesceri, E. W. Rice, A. E. Greenberg and M. A. H. Franson, “Standard Methods for the Ex- amination of Water & Wastewater,” 21st Edition, Ameri- can Public Health Association (APHA), American Water Works Association (AWWA) & Water Environment Federation (WEF), Washington, 2005. [22] I. Villaescusa, M. Martinez and N. Miralles, “Heavy Metal Uptake from Aqueous Solution by Cork and Yo- himbe Bark Wastes,” Journal of Chemical Technology and Biotechnology, Vol. 75, No. 9, 2000, pp. 812-816. doi:10.1002/1097-4660(200009)75:9<812::AID-JCTB28 4>3.0.CO;2-B [23] B. Yu, Y. Zhang, A. Shukla, S. S. Shukla and L. K. Dor- ris, “The Removal of Heavy Metals from Aqueous Solu- tions by Sawdust Adsorption-Removal of Lead and Com- parison of Its Adsorption with Copper,” Journal of Haz- ardous Materials, Vol. 84, No. 1, 2001, pp. 83-94. doi:10.1016/S0304-3894(01)00198-4 [24] W. S. W. Ngah and M. A. K. M. Hanafiah, “Surface Modi- fication of Rubber (Hevea Rasiliensis) Leaves for the Ad- sorption of Copper Ions: Kinetic, Thermodynamic and Bonding Mechanisms,” Journal of Chemical Technology and Biotechnology, Vol. 84, No. 2, 2008, pp. 192-201. doi:10.1002/jctb.2024 [25] Y. P. Kumar, P. King and V. S. R. K. Prasad, “Removal of Copper from Aqueous Solution Using Ulva Fasciata sp.—A Marine Green Algae,” Journal of Hazardous Ma- terials, Vol. 137, No. 1, 2006, pp. 367-373. doi:10.1016/j.jhazmat.2006.02.010 [26] K. Vijayaraghavan, R. J. Jegan, K. Palanivelu and M. Velan, “Copper Removal from Aqueous Solution by Ma- rine Green Alga Ulva Reticulate,” Electronic Journal of Biotechnology, Vol. 7, No. 1, 2004, pp. 61-71. [27] A. Benhammou, A. Yaacoubi, L. Nibou and B. Tanouti, “Adsorption of Metal Ions onto Moroccan Stevensite: Kinetic and Isotherm Studies,” Journal of Colloid Inter- face Science, Vol. 282, No. 2, 2005, pp. 320-326. doi:10.1016/j.jcis.2004.08.168 [28] S. T. Akar, T. Akar and A. Çabuk, “Decolorization of a Textile Dye, Reactive Red 198 (rr198), by Aspergillus Parasiticus Fungal Biosorbent,” Brazilian Journal of Che- mical Engineering, Vol. 26, No. 2, 2009, pp. 399-405. doi:10.1590/S0104-6632 200900 02000 18 [29] M. M. Dubinin and L. V. Radushkevich, “Equation of the Characteristic Curve of Activated Charcoal,” Proceedings of the Academy of Sciences, Physical Chemistry Section, U.S.S.R. 55, 1947, pp. 331-333. [30] M. S. Onyango, Y. Kojima, O. Aoyi, E. C. Bernardo and H. Matsuda, “Adsorption Equilibrium Modeling and So- lution Chemistry Dependence of Fluoride Removal from Water by Trivalent-Cation-Exchanged Zeolite F-9,” Jour- nal of Colloid Interface Science, Vol. 279, No. 2, 2004, pp. 341-350. doi:10.1016/j.jcis.2004.06.038 [31] S. S. Dubey and R. K. Gupta, “Removal Behavior of Ba- bool Bark (Acacia Nilotica) for Submicro Concentrations of Hg2+ from Aqueous Solutions: A Radiotracer Study,” Seperation and Purification Technology, Vol. 41, No. 1, 2005, pp. 21-28. doi:10.1016/j.seppur.2004.03.012 [32] A. Benhammou, A. Yaacoubi, L. Nibou and B. Tanouti, “Adsorption of Metal Ions onto Moroccan Stevensite: Kinetics and Isotherm Studies,” Journal of Colloid Inter- face Science, Vol. 282, No. 2, 2005, pp. 320-326. doi:10.1016/j.jcis.2004.08.168 [33] E. Fourest and B. Volesky, “Alginate Properties and Heavy Metal Biosorption by Marine Alga,” Application of Biochemistry and Biotechnology, Vol. 67, No. 3, 1997, pp. 215-226. doi:10.1007/BF02788799 [34] E. Fourest and B. Volesky, “Contribution of Sulfonate Groups and Alginate to Heavy Metal Biosorption by the Dry Biomass of Sargassum Fluitans,” Environment Sci- ence and Technology, Vol. 30, No. 1, 1996, pp. 277-282. Copyright © 2011 SciRes. MSA  Biosorption of Cu(II) Ions from Aqueous Solution by Red Alga (Palmaria Palmata) and Beer Draff Copyright © 2011 SciRes. MSA 80 doi:10.1021/es950315s [35] Y. Nuhoglu, E. Malkoc, A. Gürese and N. Canpolat, “The Removal of Cu(II) from Aqueous Solutions by Ulothrix Zonata,” Bioresource Technology, Vol. 85, No. 3, 2002, pp. 331-333. doi:10.1016/S0960-8524(02)00098-6
|