V. S. KYBLANOVSKY ET AL. 645
Table 2. Kinetic parameters of palladium(II) electroreduction from an iminodiacetate electrolyte (CPd2+/CL2 = 1:100, pH 3.8).
T (˚C) lg jd
(mA·m2)
Di·105
(cm2·s1) bk (V) α' lg jowb()
(mA·cm2)
lg jowa()
(mA·cm2)
26 1.47 0.69 0.136 0.42 3.47 3.19
35 1.30 1.01 0.146 0.41 3.28 2.94
40 1.26 1.12 0.151 0.41 3.22 2.71
50 1.21 1.26 0.157 0.40 3.14 2.47
60 1.09 1.54 0.164 0.40 3.04 2.25
jowb()—exchange current density of barrierless palladium(II) discharge; jowa()—exchange current density of activationless palladium(II) discharge.
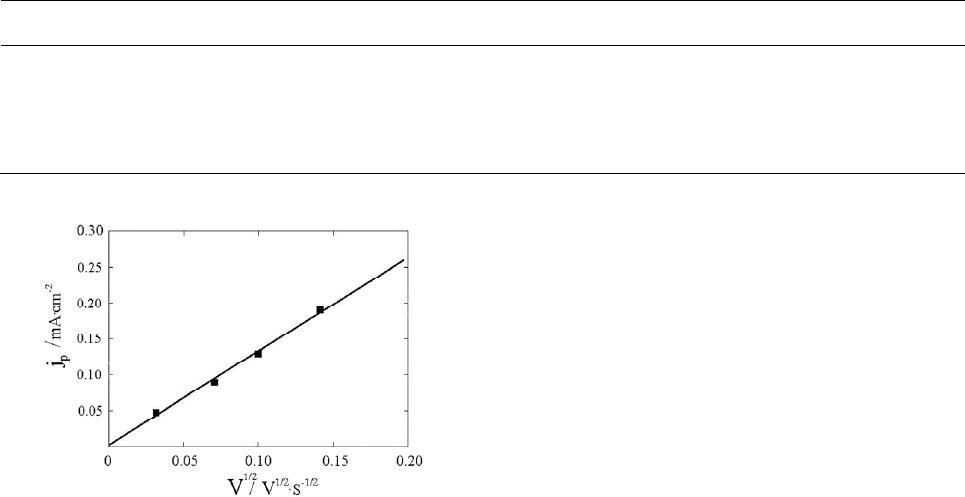
Figure 4. Dependence of limiting cathode current on square
root from sweep rate in an iminodiacetate electrolyte con-
taining (mol·l1): [PdL2]2 (5.11 × 104), H2L (5.11 × 102),
NaClO4 (1.0), pH 3.8, 26˚C.
tion in palladium(II) reduction from iminodiacetate elec-
trolyte containing a 100-fold excess of free ligand at pH
3.8 involves [PdL2]2 complexes. The mechanism of pal-
ladium(II) electroreduction from iminodiacetate electro-
lyte was proposed in Refs [4,5].
With allowance for ionic composition, mass transfer,
the kinetics and mechanism of palladium(II) electrore-
duction from iminodiacetate electrolyte and the nature of
the rate-determining step, the possibility of using this
complexonate electrolyte for the deposition of high-
quality fine-crystalline and adherent functional palladium
coatings has been examined.
The electrolyte under investigation contained at the
bottom of the electrolytic cell excess palladium(II) com-
plex in order that constant palladium ion concentration
might be maintained during the experiment. The palla-
dium(II) ion concentration in the electrolyte for palla-
dium plating was not over 6 × 104 mol·l1 at the 50-fold
excess of iminodiacetate due to the low solubility of pal-
ladium(II) iminodiacetate complex.
Palladium coatings were deposited from a prepared
iminodiacetate electrolyte containing (mol·l1): [PdLn]2
(6.0 × 104), [H2L] (3.0 × 102), NaClO4 (1.0) at 23˚C, pH
4.2 - 4.3 and a current density of 0.03 A·dm2. Palladium
was deposited on one side of a 20 μm thick polyethylene
film chemically coated with a 1 - 2 μm thick nickel layer.
The nickel layer on the other side of the polyethylene
film was previously stripped with a concentrated hydro-
chloric acid solution. The electrolysis time was calcu-
lated by the Faraday law with allowance for the current
yield of metal, which was 75% - 99% depending on coat-
ing thickness, and monitored by cathode weight increase.
A platinum plate was used as the anode.
Micrographs of the structure of palladium coatings
electrodeposited from the iminodiacetate electrolyte un-
der investigation as a function of coating thickness (0.5
μm and 0.75 μm) are shown in Figure 5. On the basis of
morphological data (Figure 5), the dependence of the
particle size distribution of palladium coatings, deposited
from an iminodiacetate electrolyte, on an electroless-
nickel-coated polyethylene film was studied.
The dependence of crystallite size distribution is
shown in Figure 6, from which it is seen that in 0.5 μm
thick palladium coatings (Figure 6(a)), 0.08 - 0.58 μm
crystallites predominate, though 0.08 - 1.20 μm crystal-
lites are also present. Palladium coatings 0.75 μm in
thickness (Figure 6(b)) consist of 0.25 - 1.70 μm crystal-
lites, but 0.60 - 1.20 μm crystallites predominate.
It follows from Figure 6 that the formation of palla-
dium electrodeposits on a polyethylene film precoated
with electroless nickel begins with the formation on the
substrate surface of crystallizing nuclei, from which
crystallites of definite size grow during the cathodic de-
position of the metal, which enlarge with the growth of
the electrodeposit.
The ductility tests of palladium coatings deposited
from an iminodiacetate electrolyte were carried out for
thicknesses of 0.50 μm and 0.75 μm. The 0.50 and 0.75
μm thick palladium coatings electrodeposited from an
iminodiacetate electrolyte withstand 4 and 5 bendings
respectively.
4. Conclusions
Allowing for ionic composition, the kinetics and mecha-
nism of the electroreduction of a palladium(II) bis-imi-
nodiacetate complex and the nature of the rate-deter-
mining step of the process, it has been shown that imino-
diacetate electrolyte can be used for the deposition of
fine-crystalline adherent and very ductile palladium coat-
ings.
Open Access AJAC