 J. Biomedical Science and Engineering, 2013, 6, 1056-1061 JBiSE http://dx.doi.org/10.4236/jbise.2013.611132 Published Online November 2013 (http://www.scirp.org/journal/jbise/) GH treatment, BMI and different genotypes in patients with Prader-Willi syndrome and scoliosis: Is there any relationship? Tiziana Greggi1*, E. Pipitone1, K. Martikos1, F. Lolli1, F. Vommaro1, E. Maredi1, M. Di Silvestre1, S. Giacomini1, L. Sangiorgi2 1Spine Surgery Department, Rizzoli Orthopaedic Institute—I.O.R., Bologna, Italy 2Medical Genetics, Rizzoli Orthopaedic Institute—I.O.R., Bologna, Italy Email: *tiziana.greggi@ior.it Received 25 August 2013; revised 25 September 2013; accepted 9 October 2013 Copyright © 2013 Tiziana Greggi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The purpose of this study is to try to find a protocol de- fining a clinical diagnostic procedure for the patients to be admitted to the authors’ Institute to receive treat- ment for either suspected or confirmed diagnosis of spine deformity in Prader-Willi syndrome (PWS). The aim is to evaluate every subject from the diagnostic point of view, assessing variability of clinical expres- sion and evolution of spinal deformity in the light of the related genetic aspects, thus providing a univocal protocol. The present series only includes patients (18 cases) with PWS, 7 hospitalized for surgical treat- ment of scoliosis, 11 followed-up at the authors’ insti- tute only for conservative treatment of scoliosis. Both BMI tracks (weight/height2) and BMI Z-score (only for children older than 2 years) were assessed. More- over, the GH treatment was evaluated for each group of patients as follows: being administered, suspended or no treatment. Finally, the gene was compared with BMI. No relationship was observed either between GH treatment and mean BMI or between genetics and mean BMI. More patients should be seen by the authors to confirm or refute the current findings. Keywords: Scoliosis; Prader-Willi; GH; BMI 1. INTRODUCTION The PWS is a rare and complex genetic disorder consid- ered the most common genetic cause of morbid obesity, first described by Prader et al. back in 1956 [1]. The prevalence reported in epidemiological studies varies between 1 in 8000 and 1 in 134,000 whereas the number of births identified as being PWS cases varies be- tween 1 in 15,000 and 1 in 30,000. In 2010, the European Organization for Rare Disease in partnership with Orpha- net estimated the number of person living with PWS in Europe to be around 10.7 per 100,000 (1:9346) [2,3]. Genes associated with PWS are known to be express- ed from chromosome 15 derived from the father, but not from the mother. Loss of the normal contribution from paternally inherited genes in this interval leads to the PWS phenotype, since maternal genes are normally inac- tivated. This type of genetic regulation dependent on the sex of the transmitting parent is termed genomic imprint- ing [4]. The three genetic mechanisms involved in PWS are: interstitial deletions of the paternal chromosome 15, which are of two classes differing by their total length (T1 dele- tion larger than T2 deletion) [5]; maternal uniparental di- somy (mUPD, or two copies of chromosome 15 from mother and none from father), [6] which can be either hetero- or isodisomy (meaning two different maternal chromosomes or two copies of the same maternal chro- mosome), and imprinting defects [7] and translocations. Recently it has been suggested that additional factors such as maternal age, environmental variables causing genetic errors, the use of reproductive technologies and diagnos- tic methodology have influenced the previously recogni- zed distribution of genetic subtypes, with mUPD occur- ring more frequently than previously recognized [8,9]. In Italy PWS is related to deletion for 69% of the cases, mUPD for 30% [10]. The original consensus diagnostic criteria of Holm et al., 1993 [11] was recently revised [12] to indicate when genetic analysis should be performed [13]. *Corresponding author. OPEN ACCESS  T. Greggi et al. / J. Biomedical Science and Engineering 6 (2013) 1056-1061 1057 In the foetus and newborn, PWS is generally charac- terized by decreased fetal movement, prenatal and post- natal hypotonia (or diminish muscular tone) and poor suckling, causing decreased movement, lethargy, abnor- mal cry, breathing difficulties requiring special feeding techniques and poor reflexes, and resulting in failure to thrive. Whereas hypotonia improves over time, and dif- ficulties in feeding are replaced by normal calorie intake with a beginning of central obesity during the toddler years [14,15]. Characteristic facial features including narrow bifron- tal diameter with dolichocephaly (head disproportionally long and narrow), almond-shaped eyes with ocular de- fects (estropia, myopia), thin upper lip with down-turned mouth and thick saliva. In preadolescence, mild to moderate mental deficiency, behavioral disorders (e.g. obsessive-compulsive charac- teristics, self-injury behavior, skin-picking, temper tan- trums, manipulative behavior, impulsivity, stubbornness) and cognitive disabilities also become manifest and in- tensify with age. Musculoskeletal problems may also be- come apparent, such as scoliosis (abnormal back curva- ture) [16-20]. Hyperkyphotic deformity can be observed in nearly 40% of the patients [21-23]. The syndrome is associated with growth hormone de- ficiency (GHD). According to cross-sectional, prospec- tive and retrospective studies, GHD prevalence ranged from 58% to 100% [24,25]. Similar prevalence rates (74%) were observed in the international, observational KIGS database [26]. Obesity is a primary cause of increased morbidity and premature mortality in PWS. It leads to complications such as type 2 diabetes mellitus, hypertension, atheros- clerosis, hyperlipidemia, cardiovascular diseases, respi- ratory insufficiencies, thrombophlebitis, leg edema, and sleep apnea. Increased rates of scoliosis are also observed. Although the pharmacological therapy with growth hormone (GH) provides satisfactory results, its impact on progression of spinal deformities still remains controver- sial [21,27]. According to a study conducted in the UK population, mortality rate of individuals with PWS not treated with GH was estimated at 3% per year between age 6 and 56 years and at 7% per year above 30 years of age. Epide- miological data from Italy noted 18 deaths over a 20-year period of observation (4.2%), occurring in both the pedi- atric and adult subjects (age range: 0.5 - 39.3). Causes of death in children are usually associated with respiratory infection or insufficiency, whereas mortality in adults is found to be primarily circulatory and/or respiratory fail- ure [10]. The various different clinical and pathological condi- tions with onset during the pediatric and early adolescent age, and affecting the general aspect and health status, can change or delay treatment of spinal deformities and subsequently entail negative consequences in terms of life quality and expectation. Scoliosis is reported to re- quire active treatment in 15% to 20% of the cases and to behave as an idiopathic scoliosis, with high risk of pro- gression during adolescence [1,22]. Absent, inadequate or delayed treatment of spinal de- formities can jeopardize the patient’s respiratory and car- diocirculatory “compliance”, which is already severely compromised by the other remarkable phenotypic aspects of the syndrome. 2. MATERIALS AND METHODS The present case series includes 18 patients, 11 of which (9 males and 3 females) were seen at the Rare Disease Outpatients’ Unit for conservative treatment; mean age was 4.2 years (range, 0.5 to 16.1). Another 7 patients were surgically treated: they were 5 males and 2 females, aged 12.8 years on average (range, 10 to 14.6 years). Regarding the patients’ origin, 87% came from outside the region (Emilia-Romagna): 61% from the central south- ern area and 44% from Latium, in particular (Figure 1). For all of the 18 patients, both standard BMI (weight/ height2) and the BMI Z-score developed by the Centers for Disease Control and Prevention (CDC) were calculat- ed. The latter is to be preferred when assessing children [28,29] to allow for their classification into underweight/ normal weight/overweight/obesity (Table 1), although more recent studies tend to attach importance to the use of BMI also for younger patients [30]. As a matter of fact, the values of BMI Z-score are charted for specific Figure 1. Patients’ Origin Region of origin of the patients be- longing to the series with PWS. Copyright © 2013 SciRes. OPEN ACCESS 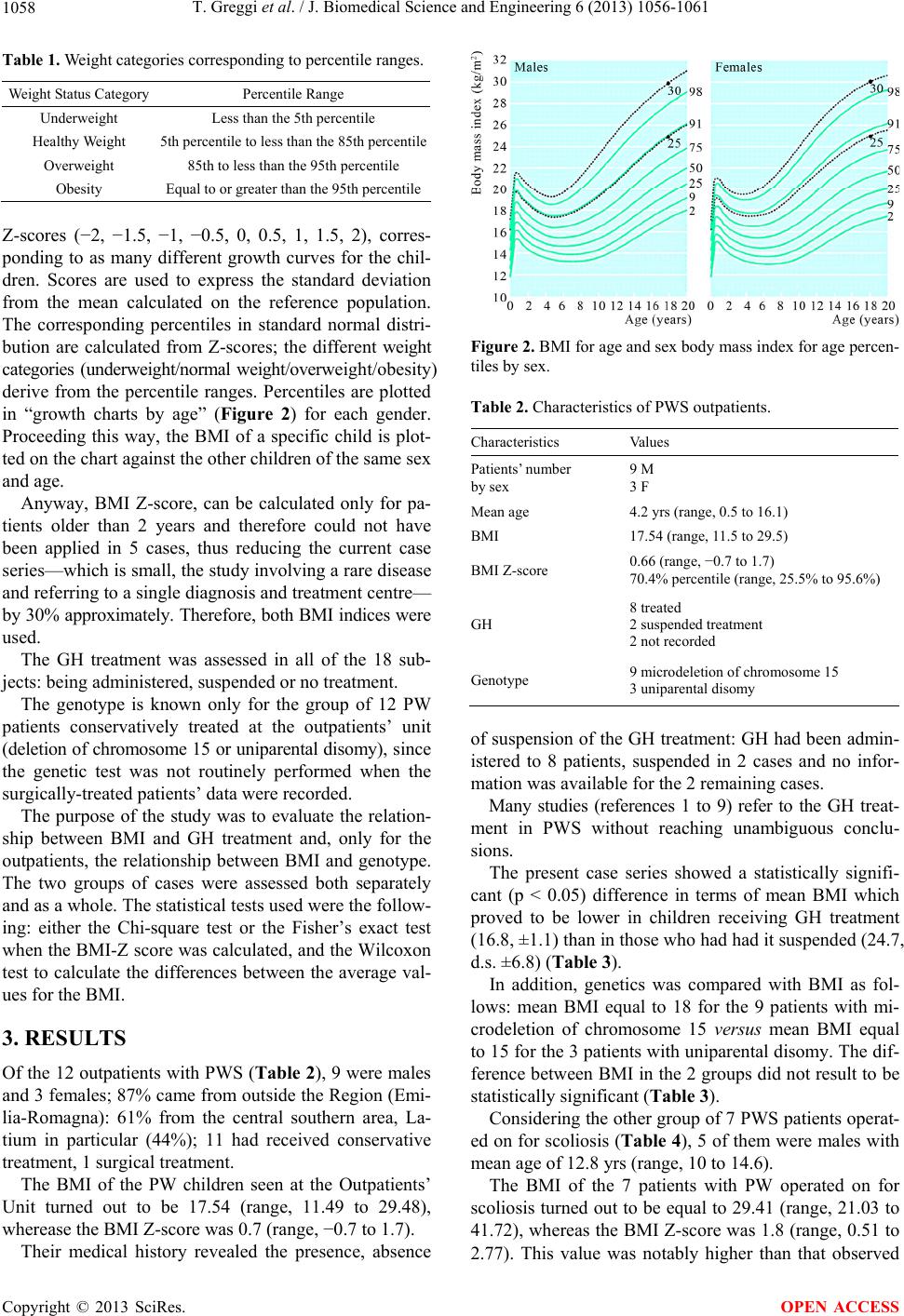 T. Greggi et al. / J. Biomedical Science and Engineering 6 (2013) 1056-1061 1058 Table 1. Weight categories corresponding to percentile ranges. Weight Status Category Percentile Range Underweight Less than the 5th percentile Healthy Weight 5th percentile to less than the 85th percentile Overweight 85th to less than the 95th percentile Obesity Equal to or greater than the 95th percentile Z-scores (−2, −1.5, −1, −0.5, 0, 0.5, 1, 1.5, 2), corres- ponding to as many different growth curves for the chil- dren. Scores are used to express the standard deviation from the mean calculated on the reference population. The corresponding percentiles in standard normal distri- bution are calculated from Z-scores; the different weight categories (underweight/normal weight/overweight/obesity) derive from the percentile ranges. Percentiles are plotted in “growth charts by age” (Figure 2) for each gender. Proceeding this way, the BMI of a specific child is plot- ted on the chart against the other children of the same sex and age. Anyway, BMI Z-score, can be calculated only for pa- tients older than 2 years and therefore could not have been applied in 5 cases, thus reducing the current case series—which is small, the study involving a rare disease and referring to a single diagnosis and treatment centre— by 30% approximately. Therefore, both BMI indices were used. The GH treatment was assessed in all of the 18 sub- jects: being administered, suspended or no treatment. The genotype is known only for the group of 12 PW patients conservatively treated at the outpatients’ unit (deletion of chromosome 15 or uniparental disomy), since the genetic test was not routinely performed when the surgically-treated patients’ data were recorded. The purpose of the study was to evaluate the relation- ship between BMI and GH treatment and, only for the outpatients, the relationship between BMI and genotype. The two groups of cases were assessed both separately and as a whole. The statistical tests used were the follow- ing: either the Chi-square test or the Fisher’s exact test when the BMI-Z score was calculated, and the Wilcoxon test to calculate the differences between the average val- ues for the BMI. 3. RESULTS Of the 12 outpatients with PWS (Ta ble 2 ), 9 were males and 3 females; 87% came from outside the Region (Emi- lia-Romagna): 61% from the central southern area, La- tium in particular (44%); 11 had received conservative treatment, 1 surgical treatment. The BMI of the PW children seen at the Outpatients’ Unit turned out to be 17.54 (range, 11.49 to 29.48), wherease the BMI Z-score was 0.7 (range, −0.7 to 1.7). Their medical history revealed the presence, absence Figure 2. BMI for age and sex body mass index for age percen- tiles by sex. Table 2. Characteristics of PWS outpatients. Characteristics Values Patients’ number by sex 9 M 3 F Mean age 4.2 yrs (range, 0.5 to 16.1) BMI 17.54 (range, 11.5 to 29.5) BMI Z-score 0.66 (range, −0.7 to 1.7) 70.4% percentile (range, 25.5% to 95.6%) GH 8 treated 2 suspended treatment 2 not recorded Genotype 9 microdeletion of chromosome 15 3 uniparental disomy of suspension of the GH treatment: GH had been admin- istered to 8 patients, suspended in 2 cases and no infor- mation was available for the 2 remaining cases. Many studies (references 1 to 9) refer to the GH treat- ment in PWS without reaching unambiguous conclu- sions. The present case series showed a statistically signifi- cant (p < 0.05) difference in terms of mean BMI which proved to be lower in children receiving GH treatment (16.8, ±1.1) than in those who had had it suspended (24.7, d.s. ±6.8) (Table 3). In addition, genetics was compared with BMI as fol- lows: mean BMI equal to 18 for the 9 patients with mi- crodeletion of chromosome 15 versus mean BMI equal to 15 for the 3 patients with uniparental disomy. The dif- ference between BMI in the 2 groups did not result to be statistically significant (Table 3). Considering the other group of 7 PWS patients operat- ed on for scoliosis (Table 4), 5 of them were males with mean age of 12.8 yrs (range, 10 to 14.6) . The BMI of the 7 patients with PW operated on for scoliosis turned out to be equal to 29.41 (range, 21.03 to 41.72), whereas the BMI Z-score was 1.8 (range, 0.51 to 2.77). This value was notably higher than that observed Copyright © 2013 SciRes. OPEN ACCESS  T. Greggi et al. / J. Biomedical Science and Engineering 6 (2013) 1056-1061 1059 Table 3. BMI relationship with GH and genotype in PW outpa- tients. GH treated GH interrupted p Mean BMI 16.8 (±1.1) 24.7 (±6.8) 0.05 Microdeletion of chromosome 15 Uniparental disomy Mean BMI 18 (±4.1) 15 (±2.8) n.s. Table 4. Characteristics of PWS surgically-treated patients. Characteristics Values Patients’ number by sex 5 M 2 F Mean age 12.8 yrs (range, 10 to 14.6) BMI 29.41 (range, 21.0 to 41.7) BMI Z-score 1.8 (range 0.5 to 2.8). GH 4 treated 3 not treated in outpatients who also were much younger. The BMI Z-score could be calculated for all of the pa- tients belonging to this group; subsequently, using growth charts by age percentiles, the weight categories could be assessed and the following results achieved: normal weight in 2 cases, overweight in 1 and obesity in 4. Focus was put on the relationship between weight categories (obesity and overweight were considered as a whole, due to the low number of subjects to be included in the latter) and the corresponding GH treatment: of the 4 cases receiving Gh treatment, 3 (75%) were obese and one overweight; of the 3 who did not receive the GH treatment, 2 were normal weight (75%) and 1 obese. The test proved to be not significant. Besides, since surgery cannot be considered as an ef- fect modifier of this relationship, outpatients and surgi- cally-treated patients were assessed as a whole. In this case, the authors compared the average BMI for the 2 groups instead of the weight categories deriving from the BMI Z-score: no significant difference was found (Table 5). 4. DISCUSSION AND CONCLUSIONS Before to begin GH treatment, many steps have to be accomplished, starting from genetic confirmation of PWS, On GH treatment regular clinical assessment of height, weight, BMI, body composition, pubertal status, scolio- sis, and side effects every 2 - 6 months should be done. At cessation of GH, treatment uncontrolled progression of obesity should be present in terms of how it has been re- ported in the International—Second Expert Meeting of the Comprehensive Care of Patients with PWS (2008) [31]. Table 5. Mean BMI relationship with GH in PWS patients. GH administered GH suspended No GH treatment GH Not recordedp Mean BMI 20.7 (±6.1) 24.7 (±6.8) 31.4 (±14.6) 13.3 (±2.6) n.s. The only report that state scoliosis evolution and GH treatment is a two-year open label study of Sode-Carlsen et al. (2011) [32]. This study reported that scoliosis pro- gressed in six of 38 patients, whereas three patients had a decrease of Cobb angle of more than 5˚. It is unclear if these observations are the results of GH treatment or the natural history of progression of scoliosis in PWS adults. Similar observations were reported for edema which is often seen in clinical studies of GH treatment in adults [33-35]. In our study, no significant difference in terms of BMI was observed between patients receiving GH treatment and patients who did not receive it. No relationship be- tween GH treatment and mean BMI was seen. Anyway, additional studies can be performed creating a subgroup from the present case series which includes the patients younger than 2 yrs, and replacing the BMI with the BMI Z-score. No relationship between genetics and mean BMI was found in the outpatients’ group. However, our case series is being too small, as it al- ways happens with rare diseases, and for recording of da- ta incomplete, more patients need to be examined by the authors to confirm or refute these findings. REFERENCES [1] Grugni, G., Crinò, A., Bertocco, P. and Marzullo, P. (2009) Body fat excess and stimulate growth hormone levels in adult patients with Prader-Willi syndrome. American Jour- nal of Medical Genetics, 149A, 726-731. [2] Orphanet (2010). Prevalence of rare diseases: Bibliogra- phic data. [3] Feigerlová, E., Diene, G., Conte-Auriol, F., Molinas, C., Gennero, I., Salles, J.P., Arnaud, C. and Tauber, M. (2008) Hyperghrelinemia precedes obesity in Prader-Willi syn- drome. The Journal of Clinical Endocrinology & Meta- bolism, 93, 2800-2805. [4] Horsthemke, B. and Wagstaff, J. (2008) Mechanisms of imprinting of the Prader-Willi/Angelman region. Ameri- can Journal of Medical Genetics, 146A, 2041-2052. http://dx.doi.org/10.1002/ajmg.a.32364 [5] Ledbetter, D.H., Riccardi, V.M., Airhart, S.D., Strobel, R.J., Keenan, B.S. and Crawford, J.D. (1981) Deletions of chromosome 15 as a cause of the Prader-Willi syndrome. The New England Journal of Medicine, 304, 325-329. http://dx.doi.org/10.1056/NEJM198102053040604 [6] Nicholls, R.D., Knoll, J.H., Butler, M.G., Karam, S. and Lalande, M. (1989) Genetic imprinting suggested by ma- Copyright © 2013 SciRes. OPEN ACCESS  T. Greggi et al. / J. Biomedical Science and Engineering 6 (2013) 1056-1061 1060 ternal heterodisomy in nondeletion Prader-Willi syndrome. Nature, 342, 281-285. http://dx.doi.org/10.1038/342281a0 [7] Buiting, K., Saitoh, S., Gross, S., Dittrich, B., Schwartz, S., Nicholls, R.D., et al. (1995) Inherited microdeletions in the Angelman and Prader-Willi syndromes define an im- printing centre on human chromosome 15. Nature Genet- ics, 9, 395-400. http://dx.doi.org/10.1038/ng0495-395 [8] Sinnema, M., van Roozendaal, K.E., Maaskant, M.A., Smeets, H.J., Engelen, J.J., Jonker-Houben, N., et al. (2010) Different distribution of the genetic subtypes of the Pra- der-Willi syndrome in the elderly. European Journal of Human Genetics, 18, 993-998. http://dx.doi.org/10.1038/ejhg.2010.67 [9] Whittington, J.E., Butler, J.V. and Holland, A.J. (2007) Changing rates of genetic subtypes of Prader-Willi syn- drome in the UK. European Journal of Human Genetics, 15, 127-130. http://dx.doi.org/10.1038/sj.ejhg.5201716 [10] Grugni, G., Crino, A., Bosio, L., Corrias, A., Cuttini, M., De Toni, T., et al. (2008) The Italian National Survey for Prader-Willi syndrome: An epidemiologic study. Ameri- can Journal of Medical Genetics, 146, 861-872. http://dx.doi.org/10.1002/ajmg.a.32133 [11] Holm, V.A., Cassidy, S.B., Butler, M.G., Hanchett, J.M., Greenswag, L.R., Whitman, B.Y., et al. (1993) Prader- Willi syndrome: Consensus diagnostic criteria. Pediatrics, 91, 398-402. [12] Gunay-Aygun, M., Schwartz, S., Heeger, S., O’Riordan, M.A. and Cassidy, S.B. (2001) The changing purpose of Prader-Willi syndrome clinical diagnostic criteria and pro- posed revised criteria. Pediatrics, 108, E92. http://dx.doi.org/10.1542/peds.108.5.e92 [13] Goldstone, A.P., Holland, A.J., Hauffa, B.P., Hokken-Ko- elega, A.C. and Tauber, M. (2008) Recommendations for the diagnosis and management of Prader-Willi syndrome. The Journal of Clinical Endocrinology & Metabolism, 93, 4183-4197. http://dx.doi.org/10.1210/jc.2008-0649 [14] Fillion, M., Deal, C. and Van Vliet, G. (2009) Retrospec- tive study of the potential benefits and adverse events du- ring growth hormone treatment in children with Prader- Willi syndrome. Journal of Pediatrics, 154, 230-233. [15] Lin, H.Y., Lin, S.P., Tsai, L.P., Chao, M.C., Chen, M.R., Chuang, C.K., Huang, C.Y., Tsai, F.J., Chou, I.C., Chiu, P.C., Huang, C.H., Yen, J.L., Lin, J.L. and Kuo, P.L. (2008) Effects of growth hormone treatment on height, weight, and obesity in Taiwanese patients with Prader-Willi syn- drome. Journal of the Chinese Medical Association, 71, 305-309. [16] de Lind van Wijngaarden, R.F., de Klerk, L.W., Festen, D.A. and Hokken-Koelega, A.C. (2008) Scoliosis in Pra- der-Willi syndrome: Prevalence, effects of age, gender, body mass index, lean body mass and genotype. Archives of Disease in Childhood, 93, 1012-1016. http://dx.doi.org/10.1136/adc.2007.123836 [17] Nakamura, Y., Nagai, T., Iida, T., Ozeki, S. and Nohara, Y. (2009) Epidemiological aspects of scoliosis in a cohort of Japanese patients with Prader-Willi syndrome. Spine Journal, 9, 809-816. http://dx.doi.org/10.1016/j.spinee.2009.06.017 [18] Sinnema, M., Maaskant, M.A., van Schrojenstein Lant- man-de Valk, H.M., Caroline, V.N.I., Drent, M.L., Curfs, L.M., et al. (2011) Physical health problems in adults with Prader-Willi syndrome. American Journal of Medical Genetics, 155, 2112-2124. http://dx.doi.org/10.1002/ajmg.a.34171 [19] Butler, M.G., Haber, L., Mernaugh, R., Carlson, M.G., Price, R. and Feurer, I.D. (2001) Decreased bone mineral density in Prader-Willi syndrome: Comparison with obese subjects. American Journal of Medical Genetics, 103, 216-222. http://dx.doi.org/10.1002/ajmg.1556 [20] Kroonen, L.T., Herman, M., Pizzutillo, P.D. and Mace- wen, G.D. (2006) Prader-Willi Syndrome: Clinical con- cerns for the orthopaedic surgeon. Journal of Pediatric Orthopaedics, 26, 673-679. http://dx.doi.org/10.1097/01.bpo.0000226282.01202.4f [21] Festen, D.A., de Lind van Wijngaarden, R., van Eekelen, M., Otten, B.J., Wit, J.M., Duivenvoorden, H.J. and Hok- ken-Koelega, A.C. (2008) Randomized controlled GH trial: Effects on anthropometry, body composition and body proportions in a large group of children with Prader-Willi syndrome. Clinical Endocrinology, 69, 443-451. [22] Crinò, A., Di Giorgio, G., Schiaffini, R., Fierabracci, A., Spera, S., Maggioni, A. and Gattinara, G.C. (2008) Cen- tral precocious puberty and growth hormone deficiency in a boy with Prader-Willi syndrome. European Journal of Pediatrics, 167, 1455-1458. [23] Galassetti, P., Saetrum Opgaard, O., Cassidy, S.B. and Pontello, A. (2007) Nutrient intake and body composition variables in Prader-Willi syndrome—Effect of growth hormone supplementation and genetic subtype. Journal of Pediatric Endocrinology and Metabolism, 20, 491-500. [24] Costeff, H., Holm, V.A., Ruvalcaba, R. and Shaver, J. (1990) Growth hormone secretion in Prader-Willi syn- drome. Acta paediatrica Scandinavica, 79, 1059-1062. http://dx.doi.org/10.1111/j.1651-2227.1990.tb11383.x [25] Myers, S.E., Whitman, B.Y., Carrel, A.L., Moerchen, V., Bekx, M.T. and Allen, D.B. (2007) Two years of growth hormone therapy in young children with Prader-Willi syn- drome: Physical and neurodevelopmental benefits. Ame- rican Journal of Medical Genetics, 143, 443-448. http://dx.doi.org/10.1002/ajmg.a.31468 [26] Tauber, M. and Cutfield, W. (2007) KIGS highlights: Growth hormone treatment in Prader-Willi Syndrome. Hormone Research, 68, 48-50. [27] Caixàs, A., Giménez-Palop, O., Broch, M., Vilardell, C., Megía, A., Simón, I.,Giménez-Pérez, G., Mauricio, D., Ven- drell, J., Richart, C. and González-Clemente, J.M. (2008) Adult subjects with Prader-Willi syndrome show more low-grade systemic inflammation than matched obese sub- jects. Journal of Endocrinological Investigation, 31, 169- 175. [28] Frankenfield et al. (2001) Limits of body mass index to detect obesity and predict body composition. Nutrition, 17, 26-30. http://dx.doi.org/10.1016/S0899-9007(00)00471-8 [29] Deurenberg-Yap et al. (2000) The paradox of low body mass index and high body fat percentage among Chinese, Malays, and Indians in Singapore. International Journal Copyright © 2013 SciRes. OPEN ACCESS  T. Greggi et al. / J. Biomedical Science and Engineering 6 (2013) 1056-1061 Copyright © 2013 SciRes. 1061 OPEN ACCESS of Obesity and Related Metabolic Disorders, 24, 1011- 1017. http://dx.doi.org/10.1038/sj.ijo.0801353 [30] Berkey, C.S. and Colditz, G.A. (2007) Adiposity in ado- lescents: Change in actual BMI works better than change in BMI z score for longitudinal studies. Annals of Epide- miology, 17, 44-50. [31] Goldstone, A.P., Holland, A.J., Hauffa, B.P., Hokken- Koelega, A.C., Tauber, M. and Speakers Contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS (2008) Recommendations for the di- agnosis and management of Prader-Willi syndrome. The Journal of Clinical Endocrinology & Metabolism, 93, 4183-4197. [32] Sode-Carlsen, R., Farholt, S., Rabben, K.F., Bollerslev, J., Schreiner, T., Jurik, A.G., et al. (2010) One year of growth hormone treatment in adults with Prader-Willi syndrome improves body composition: Results from a randomized, placebo-controlled study. The Journal of Clinical Endo- crinology & Metabolism, 95, 4943-4950. http://dx.doi.org/10.1210/jc.2010-0907 [33] Hoybye, C., Hilding, A., Jacobsson, H. and Thoren, M. (2003) Growth hormone treatment improves body com- position in adults with Prader-Willi syndrome. Clinical Endocrinology, 58, 653-661. http://dx.doi.org/10.1046/j.1365-2265.2003.01769.x [34] Bertella, L., Mori, I., Grugni, G., Pignatti, R., Ceriani, F., Molinari, E., et al. (2007) Quality of life and psychologi- cal well-being in GH-treated, adult PWS patients: A lon- gitudinal study. Journal of Intellectual Disability Resear- ch, 51, 302-311. http://dx.doi.org/10.1111/j.1365-2788.2006.00878.x [35] Mogul, H.R., Lee, P.D., Whitman, B.Y., Zipf, W.B., Frey, M., Myers, S., et al. (2008) Growth hormone treatment of adults with Prader-Willi syndrome and growth hormone deficiency improves lean body mass, fractional body fat, and serum triiodothyronine without glucose impairment: results from the United States multicenter trial. The Jour- nal of Clinical Endocrinology & Metabolism, 93, 1238- 1245. http://dx.doi.org/10.1210/jc.2007-2212
|