 Journal of Geoscience and Environment Protection 2013. Vol.1, No.2, 7-12 Published Online October 2013 in SciRes (http://www.scirp.org/journal/gep) http://dx.doi.org/10.4236/gep.2013.12002 Copyright © 2013 SciRes. Value Addition to Waste Material Supported by Removal of Available Phosphate from Simulated Brackish Water—A Low Cost Approach S. Malavipathirana1*, S. Wimalasiri2, N. Priyantha1†, S. Wickramasooriya1, A. Welagedara3, G. Renman3 1Postgraduate Institute of Science, University of Peradeniya, Peradeniya, Sri Lanka 2Department of Food Science and Technology, University of Perradeniya, Peradeniya, Sri Lanka 3Department of Land and Water Resources Engineering, Royal Institute of Technology, Stockholm, Sweden Email: *malavisarath@gmail.com, namalpriyantha@pdn.ac.lk Received August 2013 Phosphorus is one of the major nutrients that have been identified as a limited resource that would end up earlier than predicted at the rate of current consumption. Therefore, at te mpts to recover phosphorus from waste and its subsequent use are a concern of current researchers. Nevertheless, recovery of nut rie nts from wastewater is cumbersome because nutrients such as phosphates ( ) and nitrates ( ) prefer to remain in aqueous phase rather than being adsorbed on solid matrixes. Investigation of adsorption of available - P from simulated brackish water, on granulated solid waste material, prepared by crushed autoclaved aerated concrete (CAAC), and subsequent use of the material as phosphate fertilizer would be the focus of this research. Treatment of nutrient-rich brackish water is important because such water is discharged in huge volume at the time of harvesting of shrimp aquaculture ponds. Experiments conducted in simulated brackish water confirmed non-linear adsorption association with changing dis tri- bution coefficient (KD) which attributed the maximum removal of about 98% - P from 100 mg∙dm−3 solution at its value of 40. The non-linear adsorption supported by both the Langumuir and the Freundlich isotherm models simultaneously satisfied monolayer adsorption and multilayer adsorption de- picted by the regression coefficients of greater than .99 by the linearized forms of the isotherm models. Moreover, promising phosphate uptakes characteristics are exhibited by the adsorbent at the process of repetitive adsorption which resulted in 12 g/kg uptake of phosphate at 81% efficiency. The adsorbent seems to be used as a slow-released phosphorus fertilizer at the end of its life as an adsorbent. Keywords: Aquaculture; Adsorption; Distribution Coefficient; Nutrient Loading; Phosphate Fertilizer; Pollution; Potentially Toxic Elements Introduction Phosphate is an essential and major nutrient that promotes the growth of all types of flora. Use of phosphate fertilizer is therefore common in practic e in agriculture. Nevertheless, ap- plication of fertilizers in excess with the belief of obtaining high- er yield is a common trend in many agricultural based countries. In addition to agricultural based run off, nutrients are rich in domestic wastewater (Kuzawa, Jung, Kiso, Yamada, Nagai, & Lee, 2006). Population growth causes nutrient loading in the environment, irrespective of the route sources of such nutrients. Consequently, nutrient found in excess or presence of nutrients at inappropriate locations is considered as nutrient pollution (Malavipathirana, Wimalasiri, Priyantha, Wickramasooriya, We- lagedara, & Renman, (submitted)). Causes of pollution can either be due to geogenic characteristics of the specific area, or it may be a reason of anthropogenic inputs which diversified along with human activities. Nutrient pollution has been received immense attention as it can impair the quality of the receiving environment while de- cline and/or stop the quantity of output from the receiving en- vironment. In other words, it may result in complete loss of sustainability of the recipient. The recipient, most of time, would be a w at e r body such as a river, stream, and even brackish wa- ter environment. This is because, nearly infinite solubility of many nutrients in aqueous phase enabling them to easy mobili- zation with water. Some attempts made to prevent entering of nutrients into water ways, have emerged undesirable conse- quences. For instance, contamination of milk powder with di- cyandia mi de (DCD) used in pasture to manage nitrate forma- tion and subsequent leaching into water bodies (Editorial, 2013). In the context of marine environment and associated activi- ties such as aquaculture, harbor activities, attribute heavy pollu- tion loads which leads to eutrophication, silting, oxygen deple- tion and contribution of heavy metals, residues of pesticides and antibiotics, disinfectant by-products and many other organ- ic and inorganic pollutants broadly de fined as potentially toxic elements (PTEs) (Le & Munekage, 2004; Marhaba, Mangmee- chai, Chaiwatpongsakom, & Payasant, 2006; Colt, 2006; Sapko- ta(a), Sapkota(b), Kucharski, Burke, McKenzie, Walker, & Law- *Corresponding author. † This author was assigned to facilitate domestic funding requirements.  S. MALAVIPATHIRANA ET AL. Copyright © 2013 SciRes. rence, 2008; Wu & Yang, 2010; Paul & Vogal, 2011; Malavi- pathirana, Mubarak, & Perera, 2013). The costal belt of the many Asian countries is inherent with saltwater (brackish) lagoon, estuaries, mangrove and costal mud flats which are potential grounds for brackish water shrimp aq- uaculture. For this reason, Asian countries, China being the first and India, Vietnam, Indonesia, Thailand, Bangladesh and Phi- lippine among many other countries, dominate the tropical and sub-tropical aquaculture that provides more than 90% of the world aquaculture production (Sapkota(a), Sapkota(b), Kucharski, Burke, McKenzie, Walker & Lawrence, 2008; Rico, Sataporn- vanit, Haque, & van den Brink, 2010). Simultaneously, the in- dustry generates and discharges huge volume of polluted water at the cost of quality and sustainability of the receiving envi- ronment that may lead to many social problems. Moreover, nutrient like phosphorus is known to be a lasting resource, and no alternative has been so far identified in place of phosphorus (Ebie, kondo, Kadoy a, Mouri, Maruyama, Nori- take, Inamori, & Xu, 2008) for agricultural and other uses. Even though phosphorus in the world will be end up within next 345 years, at the rat e of present consumption, a “peak phosphorus” is predicted to be occurred in 30 years, thereby end up of world phosphorus reserves has been advanced to the next 50 to 100 years (Malavipathirana, Wimalasiri, Priyantha, Wickramasooriya, Welagedara, & Renman, (submitted)). Therefore, scarcity of nu- trient such as phosphorus in the future deserves attention of scientists (Ebie, kondo, Kadoya, Mouri, Maruyama, Noritake, Inamori, & Xu, 2008; Kawasaki, Ogata, & Tominaga, 2010). Consequently, many innovative approaches have been in progress in different ways and means. For instance, recovery (treatment) of wastewater rich in nutrients and/or reuse of either such re- covered nutrients or the adsorbent itself used for nutrient ad- sorption or both. The need of removal of nutrients from waste- water is, thus confirmed. Accordingly, research is in need for devel opment of sustainable and affordable technologies for treat- ment of waste containing nutrients, and reuse of them as much as possible, especially in consideration of financial constrains for environmental remediation in developing countries. This paper explains the ability of a solid wast e material (crushed autoclaved aer ate d concrete - CAAC) that consists of SiO2 and CaO, for repeated applications in order to remove available - P from simulated brackish water. Thereby, this ap- proach functions in several means by value addition to the solid waste mater i al , management of pollution load cause d by in wastewater, and concurrently explore the viability of the adsorbent to use as a phosphorus-based fertilizer. Materials and Methods Materials and Chemicals Scrap material from the production of autoclaved aerated concrete (AAC) was crushed and graded into 2 - 4 mm (CAAC), used in this study was obtained from Royal Institute of Tech- nology, Sweden. Solutions of phosphate at different concentra- tions were prepared by analytical grade potassium dihydrogen orhtophosphate dried at 105˚C until it reached a constant mass, obtained from Hopkin and Williams Ltd., England. Ammonium metavanadate (Hopkin and Will iams Ltd., England), Ammo- nium molybdate tetrahydrate and hydrochloric acid (Fluka, UK) were used as purchased in order to prepare the Vanada te -M oly- bdate reagent. Sodium chloride was purchased from Loba Che- mie, India and used to simulate brackish water condition. All the standards and samples were prepared in simulated brackish water. Instrumentation SK 600 Lab Companion orbital shaker was used to perform contact batch experiments. The absorbance of working stan- dards and filter ed solutions was measured at 470 nm by means of Shimadzu UV 1800 UV/Vis spectrophotometer. Research Design Preparation of Working Standards: Serial dilutions of the stock phosphate standard solution were made in order to pre- pare working standards in the range of 1 to 16 mg ∙dm−3 - P. Determination of sorption capacity: The sorption capacity of the adsorbent was ascertained via batch experiments. A con- stant amount of the adsorbent was made into contact with a known volume of the phosphate solution of predetermined con- centration. The amount of P sorbed to the adsorbent was ex- pressed in unit mass of P per unit mass of the adsorbent. Opti- mized contact time of 240 min at 150 rpm, and the settling time of 20 min were remained constant as identified by the previous research of the same researchers (Malavipathirana, Wimalasi r i, Priya ntha , Wickramasooriya, Welagedara, & Renman, (sub- mitted)). Isotherm Studies: Under the optimized conditions, replicate experiments were carried out at different - P concentra- tions so that modelling of isotherm characteristics would be established. Spectrophotometric Measurements: Phosphorus content of all samples was determine d at 470 nm as per the vanadomolybdo- phosphoric acid colorimetric method. Results and Discussion In the way of finding a good adsorbent, optimization of ad- sorption characteristics is a must (Lim , Priyantha, Tennakoon, & Dahri, 2012; Pillai, Mullassery, Fernandez, Giri j a, Geetha, & Koshy, 2013). An adsorbent that takes a little time to attain its optimum performance, and if it can be repeatedly used or it can uptake higher sorbate amount, such a material can be consid- ered as a good adsorbent. CAAC used in the s tudy was exhib- ited reasonably acceptable optimum contact time of 240 min followed by 20 min settling time for its best performance. Thus, all subsequent experiments were carried out at these optimum conditions. Distribution Coefficient Distribution coefficient (KD) defined as the ratio between the concentration of an analyte in the solid phase (adsorbent) to that of the aqueous phase at the equilibrium, is an indication of the relative effectiveness of an adsorbent. Therefore, CAAC as an adsorbent for removal of phosphate can be expressed with re- spect to the distribution coefficient (KD) of the sys tem. The KD is compelled to be a linear adsorption model with the assump- tion that adsorption of the sorbate of interest is essentially in- dependent of its concentration in the aqueous phase. (Goldberg, 1995), Nevertheless, the truth behind practical situation is that adsorption of a sorbate can deviate from the linear relationship  S. MALAVIPATHIRANA ET AL. Copyright © 2013 SciRes. required by the KD. Because, solution ph ase concentration of a sorbate may increase to a level high enough to occupy all ad- sorption sites where the linear relationship between the sorbate already adsorbed to that remains in bulk solution would no longer exist, an d leading the system to satisfy non-linear adsorption. Thus, in the context of non-linear adsorption, KD of a system varies with the concentration of the sorbate (Grathwohl, 2005/06). Distribution coefficients always greater than 1, as depicted in Table 1, wit h respect to different initial concentrations, indicate that concentration of the analyte (phosphate) at the sorbent - water interface is higher than that in the continuous aqueous phase. CAAC as an effective adsorbent towards phosphate re- moval from si mulated brackish water is thus, established. The maximum value of KD (at 100 mg∙dm−3 - P) was ac- companied by 10 fold variation with respect to its value at the initial concentration (10 mg∙dm−3 - P). In contrast to a small change in the values of KD associated with concentration that indicates non-complex interactions, the change in concen- tration as same order as of KD, indicates complex interaction between the adsorbent and the adsorbate (Okieimen, Okundia, & Ogbeifun, 1991). Recovery of adsorbed phosphate is not, thus practi ca l ly simple. Nevertheless, the maximum adsorption of 98% attributed at the highest KD of 40 at 100 mg∙dm−3 - P implies that CAAC is an effective adsorbent towards phos- phate removal from simulated brackish water. Isotherm Studies In the event that adsorption occurs deviation from linearity, isotherm models are in place to describe such non-linear ad- sorption. Consequently, existence of equilibrium of an adsor- bate between the solution phase and the soli d phase (adsorbent) can be explained in terms of an isotherm (Lichtfouse, Schwarz- bauer, & Robert, 2005). As evident by the values of KD, non- linear adsorption of - P on CAAC was apparent. There- fore, the simplest and the most usually used isotherms models, the Langmuir and the Freundlich isotherm models are to be satisfied by the adsorption of - P from liquid phase onto solid phase. Langmuir Isotherm Model: This is a simple and widely used model. The validity of the model is based on the following as- sumptions; • The sorbate upon adsorption is associated within a well defined site of the adsorbent. • The individual site of the adsorbent gets only one adsorbed species. • The same energy of adsorption is associated with all the sites and it is independent of the occurrence of the adsorbed species on the adjacent sites. This model explains monomolecular adsorption on the sur- face of an adsorbent. Table 1. Distribution coefficient (KD) for phosphate adsorption on CAAC. Initial concentration (mg∙dm−3) Remaining concentration (mg∙dm−3) Distribution coefficient (KD) 10 2 4.07 20 3 6.77 30 3 10.50 50 2 20.24 100 2 40.60 The empirical data can be fitted with the linearized form of the Langmuir modes as given be low. max max 111 1 e eq qbq Cq = + (1) where qe is the amount adsorbed (mg∙g−1) from the bulk solu- tion and Ceq is the analyte concentration (mg∙dm−3) at the equi- librium. The maximum adsorption of an adsorbate per unit mass of an adsorbent is given by qmax, and b is the Langmuir constant. Accordingly, qmax and b can be calcula ted by means of the intercept and the gradient of the plot of 1/qe against 1/ Ceq. Moreover, separation factor (RL) is an important parameter that indicates characteristics of the adsorption. The RL is a unitless constant as given below. (2) where C0 is the initial concentration and b is the Langmuir con- stant. Depending on the value assigned to RL, based on the Langmuir model, the adsorption can be explained as irreversi- ble, favourable, linear and unfavourable if RL = 0, 0 < RL < 1, RL = 1 and RL > 1 respectively (Dada, Olalekan, Olatunya, & Dada, 2012). Results c alculated based on the Langmuir model as given in Table 2 explain that the maximum phosphate removal (adsorp- tion) capacity through monolayer coverage is 14.29 mg∙g−1 of CAAC, Further, being the separation factor in between 0 and 1(RL = .474) confirms favourable adsorption that best explained by the Langmuir isotherm model with the regression coefficient of .996. Freundlich Isotherm Model: This model associates with the surface heterogeneity and the exponential distribution of active sites and their energies related to adsorption (Njoku, Oguzie, Obi, Bello , & Ayuk, 2011). Understanding of compliance of the model is important because, the present st udy considered only single sorbate (phosphate) in contact with the sorbent (CAAC) (Palanisamy & Sivakumar, 2009). In addition to the relevance of the Freundlich model with a single solute system, it is a practical depiction of the adsorption of an analyte in an aqueous medium onto the surface of an adsorbent (Arfaoui, Frini-Srasra, & Srasra, 2008). The Freundlich equation can be expressed as; (3) where kf and n are empirical constants to be found, Ce is the equilibrium concentration of an adsorbate (mg∙dm−3) while qe is the amount of an adsorbate adsorbed per gram of the adsorbent at equilibrium (mg∙g−1). Despite monolayer adsorption at the sites with identical energy, adsorption on multiple sites with diverse energies is demonstrated by the Freundlich model. The model, therefore, depicts real time closeness of a sorbate in a solution in contact with a sorbent (Priyantha & Bandaranayaka, 2011). The linear form of the Freundlich equation can be ex- pressed as follows: Table 2. Characteristic parameters of the Langmuir and the Freundlich models for the sorption of phosphate on CAAC. Langmuir Model Freundlich Mode l qmax (mg∙g ) b (dm ∙mg ) RL R 1/n kf R 14.29 .0011 .474 .996 .94 22.2 .995 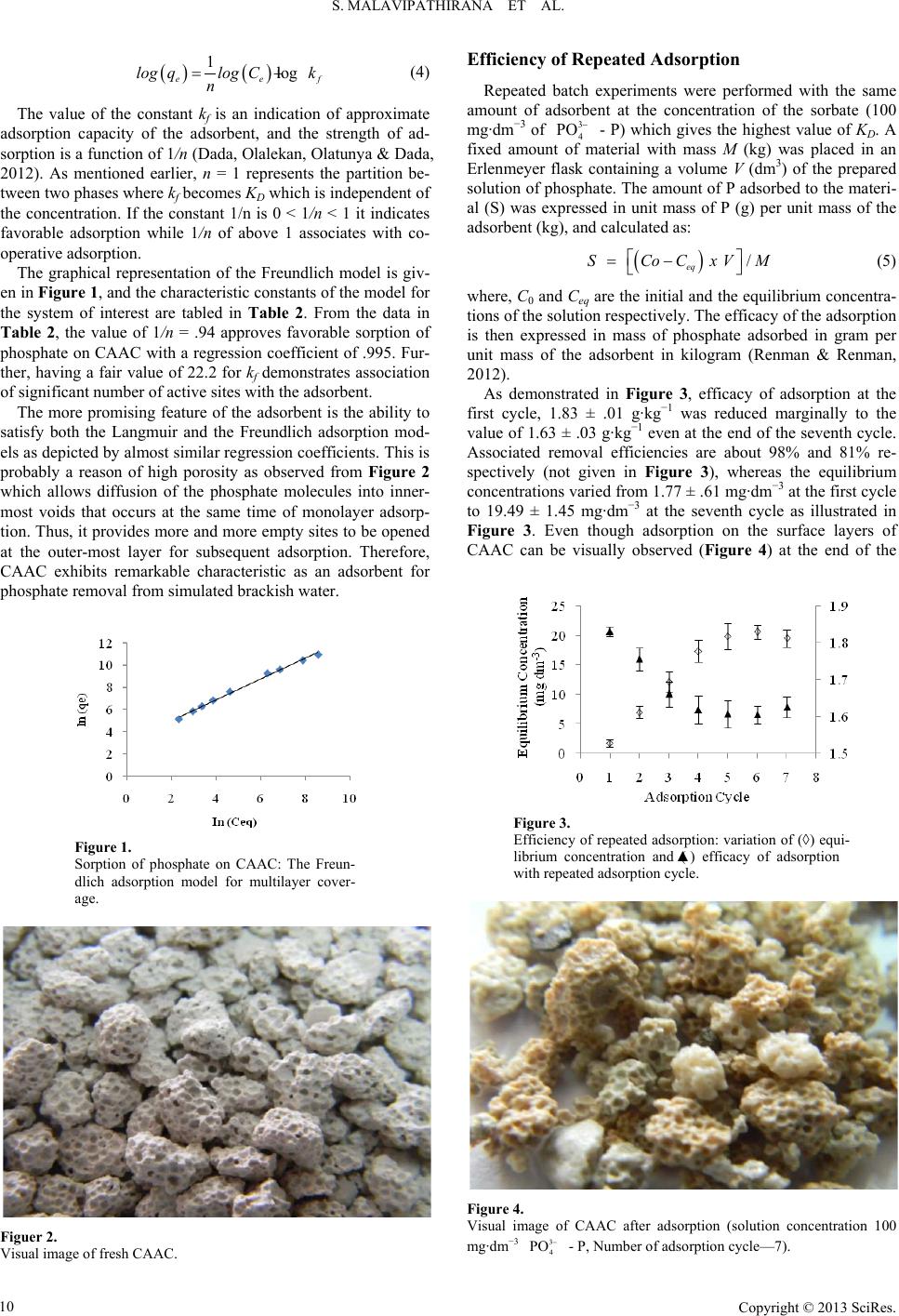 S. MALAVIPATHIRANA ET AL. Copyright © 2013 SciRes. ( )() 1 log e ef logqlog Ck n = + (4) The value of the constant kf is an indication of approximate adsorption capacity of the adsorbent, and the strength of ad- sorption is a function of 1/n (Dada, Olalekan, Olatunya & Dada, 2012). As mentioned earl ie r , n = 1 represents the partition be- tween two phases where kf becomes KD which is independent of the concentration. If the constant 1/n is 0 < 1/n < 1 it indicate s favorable adsorption while 1/n of above 1 associates with co- operative adsorption. The graphical representation of the Freundlich model is giv- en in Figure 1, and the characteristic constants of the model for the system of interest are tabled in Table 2. From the data in Table 2, the value of 1/n = .94 approves favorable sorption of phosphate on CAAC with a regression coefficient of .995. Fur- ther, having a f ai r value of 22.2 for kf demonstrates association of significant number of active sites with the adsorbent. The more promising feature of the adsorbent is the ability to satisfy both the Langmuir and the Freundlich adsorption mod- els as depicted by almost simila r regression coefficients. This is probably a reason of high porosity as observed from Figure 2 which allows diffusion of the phosphate mole cules into inner- most voids t ha t occurs at the same time of monolayer adsorp- tion. Thus, it provides more and more empty sites to be opened at the outer-most layer for subsequent adsorption. Therefore, CAAC exhibits remarkable characteristic as an adsorbent for phosphate removal from simulate d brackish water. Figure 1. Sorption of phosphate on CAAC: The Freun- dlich adsorption model for multilayer cover- age. Figuer 2. Visual image of fresh CAAC. Efficiency of Repeated Adsorp tion Repeated batch experiments were performed with the same amount of adsorbent at the concentration of the sorbate (100 mg∙dm−3 of - P) which gives the highest value of KD. A fixed amount of material with mass M (kg) was placed in an Erlenmeyer flask containing a volume V (dm3) of the prepared solution of phosphate. The amount of P adsorbed to the mater i- al (S) was expressed in unit mass of P (g) per unit mass of the adsorbent (kg), and calculated as: (5) where, C0 and Ceq are the initial and the equilibrium concentra- tions of the solution respectively. The efficacy of the adsorption is then expressed in mass of phosphate adsorbed in gram per unit mass of the adsorbent in kilogram (Renman & Renman, 2012). As demonstrated in Figure 3, efficacy of adsorption at the first cycle, 1.83 ± .01 g∙kg−1 was reduced marginally to the value of 1.63 ± .03 g∙kg−1 even at the end of the seventh cycle. Associated removal efficiencies are about 98% and 81% re- spectively (not given in Figure 3), whereas the equilibrium concentrations varied from 1.77 ± .61 mg∙dm−3 at the first cycle to 19.49 ± 1.45 mg∙dm−3 at the seventh cycle as illustrated in Figure 3. Even though adsorption on the surface layers of CAAC can be visually observed (Figu r e 4) at the end of the Figure 3. Efficiency of repeated ad sorption: variation of (◊) equi- librium concentration and (▲) efficacy of adsorption with repeated adsorption cycle. Figure 4. Visual image of CAAC after adsorption (solution concentration 100 mg∙dm−3 - P, Number of adsorption cycle—7). 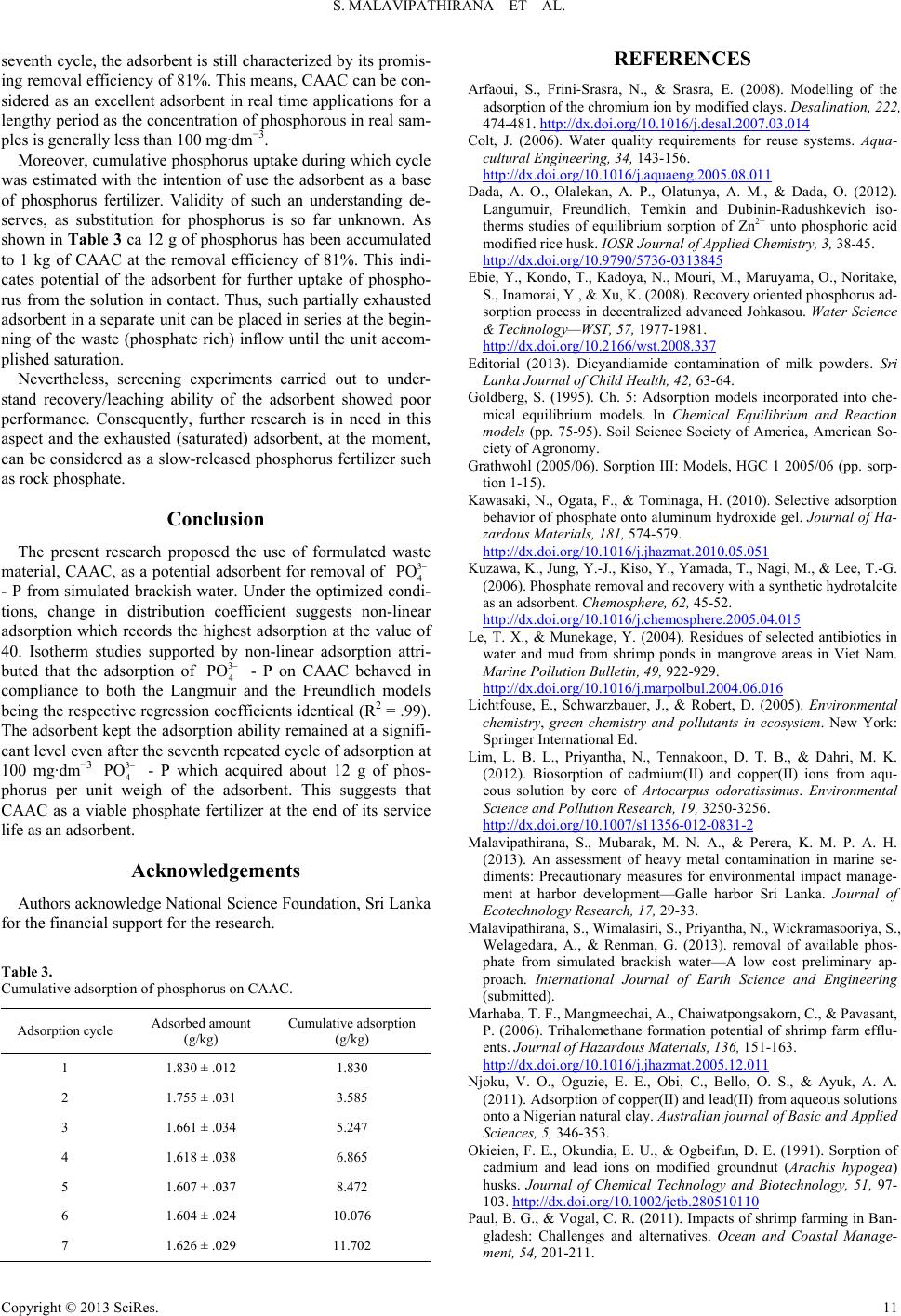 S. MALAVIPATHIRANA ET AL. Copyright © 2013 SciRes. seventh cycle, the adsorbent is still characterized by its promis- ing removal efficiency of 81%. This means, CAAC can be con- sidered as an excellent adsorbent in real time applications for a lengthy period as the concentration of phosphorous in real sam- ples is generally less than 100 mg∙dm−3. Moreover, cumulative phosphorus uptake during which cycle was estimated with the intention of use the adsorbent as a base of phosphorus fertilizer. Validity of such an understanding de- serves, as substitution for phosphorus is so far unknown. As shown in Table 3 ca 12 g of phosphorus has been accumulated to 1 kg of CAAC at the removal efficiency of 81%. This indi- cates potential of the adsorbent for further uptake of phospho- rus from the solution in contact. Thus, such partially exhausted adsorbent in a separate uni t can be placed in series at the begin- ning of the waste (phosphate rich) inflow until the unit accom- plished saturation. Nevertheless, screening experiments carried out to under- stand recovery/leaching ability of the adsorbent showed poor performance. Consequently, further research is in need in this aspect and the exhausted (saturated) adsorbent, at the moment, can be considered as a slow-rel eased phosphorus fertilizer such as rock phosphate. Conclusion The present research proposed the use of formulated waste material, CAAC, as a potential adsorbent for removal of - P from simulated brackish water. Under the optimized condi- tions, change in distribution coefficient suggests non-linear adsorption which records the highest adsorption at the value of 40. Isotherm studies supported by non-linear adsorption attri- buted that the adsorption of - P on CAAC behaved in compliance to both the Langmuir and the Freundlich models being the respective regression coefficients identical (R2 = .99). The adsorbent kept the adsorption abil i ty remained at a signifi- cant level even after the seventh repeated cycle of adsorption at 100 mg∙dm−3 - P which acquired about 12 g of phos- phorus per unit weigh of the adsorbent. This suggests that CAAC as a viable phosphate fertilizer at the end of its service life as an adsorbent. Acknowledgemen t s Authors acknowledge National Science Foundation, Sri Lanka for the financial support for the research. Table 3. Cumulative adsorption of phosphorus on CAAC. Adsorption cycle Adsorbed amount (g/kg) C umulative adsorption (g/kg) 1 1.830 ± .012 1.830 2 1.755 ± .031 3.585 3 1.661 ± .034 5.247 4 1.618 ± .038 6.865 5 1.607 ± .037 8.472 6 1.604 ± .024 10.076 7 1.626 ± .029 11.702 REFERENCES Arfaoui, S., Frini-Srasra, N., & Srasra, E. (2008). Modelling of the adsorption of the chr omium ion by modified clays. Desalination, 222, 474-481. http://dx.doi.org/10.1016/j.desal.2007.03.014 Colt, J. (2006). Water quality requirements for reuse systems. Aqua- cultural Engineering, 34, 143-156. http://dx.doi.org/10.1016/j.aquaeng.2005.08.011 Dada, A. O., Olalekan, A. P., Olatunya, A. M., & Dada, O. (2012). Langumuir, Freundlich, Temkin and Dubinin-Radushkevich iso- therms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR Journal of Applied Chemistry, 3, 38-45. http://dx.doi.org/10.9790/5736-0313845 Ebie, Y., Kondo, T., Kadoya, N., Mouri, M., Maruyama, O., Noritake, S., Inamorai, Y., & Xu, K. (2008). Recovery oriented phosphorus ad- sorption process in decentralized advanced Johkasou. Water Science & Technology—WST, 57, 1977-1981. http://dx.doi.org/10.2166/wst.2008.337 Editorial (2013). Dicyandiamide contamination of milk powders. Sri Lanka Journal of Child Health, 42, 63-64. Goldberg, S. (1995). Ch. 5: Adsorption models incorporated into che- mical equilibrium models. In Chemical Equilibrium and Reaction models (pp. 75-95). Soil Science Society of America, American So- ciety of Agronomy. Grathwohl (2005/06). Sorption III: Models, HGC 1 2005/06 (pp. sorp- tion 1-15). Kawasaki, N., Ogata, F., & Tominaga, H. (2010). Selective adsorption behavior of phosphate onto aluminum hydroxide gel. Journal of Ha- zardous Materials, 181, 574-579. http://dx.doi.org/10.1016/j.jhazmat.2010.05.051 Kuzawa, K., Jung, Y.-J., Kiso, Y., Yamada, T., Nagi, M., & Lee, T.-G. (2006). Phosphate removal and recovery with a synthetic hydrotalcite as an adsorbent. Chemosphere, 62, 45-52. http://dx.doi.org/10.1016/j.chemosphere.2005.04.015 Le, T. X., & Munekage, Y. (2004). Residues of selected antibiotics in water and mud from shrimp ponds in mangrove areas in Viet Nam. Marine Pollution Bulletin, 49, 922-929. http://dx.doi.org/10.1016/j.marpolbul.2004.06.016 Lichtfouse, E., Schwarzbauer, J., & Robert, D. (2005). Environmental chemistry, green chemistry and pollutants in ecosystem. New York: Springer International Ed. Lim, L. B. L., Priyantha, N., Tennakoon, D. T. B., & Dahri, M. K. (2012). Biosorption of cadmium(II) and copper(II) ions from aqu- eous solution by core of Artocarpus odoratissimus. Environmental Science and Pollution Research, 19, 3250-3256. http://dx.doi.org/10.1007/s11356-012-0831-2 Malavipathirana, S., Mubarak, M. N. A., & Perera, K. M. P. A. H. (2013). An assessment of heavy metal contamination in marine se- diments: Precautionary measures for environmental impact manage- ment at harbor development—Galle harbor Sri Lanka. Journal of Ecotechnology Research, 17, 29-33. Malavipathirana, S., Wimalasiri, S., Priyantha, N., Wickramasooriya, S., Welagedara, A., & Renman, G. (2013). removal of available phos- phate from simulated brackish water—A low cost preliminary ap- proach. International Journal of Earth Science and Engineering (submitted). Marhaba, T. F., Mangmeechai, A., Chaiwatpongsakorn, C., & Pavasant, P. (2006). Trihalomethane formation potential of sh rimp farm efflu- ents. Journal of Hazardous Materials, 136, 151-163. http://dx.doi.org/10.1016/j.jhazmat.2005.12.011 Njoku, V. O., Oguzie, E. E., Obi, C., Bello, O. S., & Ayuk, A. A. (2011). Adsorption of copper(II) and lead(II) from aqueous solutions onto a Nigerian natural clay. Australian journal of Basic and Applied Sciences, 5, 346-353. Okieien, F. E., Okundia, E. U., & Ogbeifun, D. E. (1991). Sorption of cadmium and lead ions on modified groundnut (Arachis hypogea) husks. Journal of Chemical Technology and Biotechnology, 51, 97- 103. http://dx.doi.org/10.1002/jctb.280510110 Paul, B. G., & Vogal, C. R. (2011). Impacts of shrimp farming in Ban- gladesh: Challenges and alternatives. Ocean and Coastal Manage- ment, 54, 201-211.  S. MALAVIPATHIRANA ET AL. Copyright © 2013 SciRes. http://dx.doi.org/10.1016/j.ocecoaman.2010.12.001 Palanisamy, P. N., & Sivakumar, P. (2009). Kinetic and isotherm stu- dies of the adsorption of Acid Blue 92 usi ng a low-cost non-conven- tional activated carbon. Desalination, 249, 388-397. http://dx.doi.org/10.1016/j.desal.2009.09.006 Pillai, S. S., Mullassery, M. D., Fernandez, N. B., Girija, N., Geetha, P., & Koshy, M. (2013). Biosorption of Cr(VI) from aqueous solution by chemically modified potato starch: Equilibrium and kinetic stu- dies. Ecotoxicology and Environmental Safety, 92, 199-205. http://dx.doi.org/10.1016/j.ecoenv.2013.01.020 Priyantha, N., & Bandaranayaka, A. (2011). Interaction of Cr(VI) spe- cies with thermally treated brick clay. Environmental Science and Pollution Research, 18, 75-81. http://dx.doi.org/10.1007/s11356-010-0358-3 Renman, G., & Renman, A. (2012). WASCON 2012 Conference pro- ceedings. Sustainable use of crushed autoclaved concrete (CAAC) as a filter medium in wastewater purification. Rico, A., Satapornvanit, K., Haque, M. M., & van den Brink, P. (2010). Contamination risks: Situation appraisal, Use of chemicals and bio- logical products in (sub)tropical Asian Aquaculture: current situa- tion and research needs for an environmental risk assessment. SEAT Deliverable Ref: D 2.5b. University of Stirling, Institute of Aquacul- ture, United Kingdom, University of Copenhagen, Faculty of Life Sciences, Denmark, Shanghai Ocean University College of Fisheries and life Sciences, China, can Tho University College of Aquaculture and Fisheries, Vietnam and The Food and Agriculture organization, Italy. Sapkota, A., Sapkota, A. R., Kucharski, M., Burke, J., McKenzie, S., Walker, P., & Lawrence, R. (2008). Aquaculture practices and po- tential human health risks: Current knowledge and futu re priorities. Environmental International, 34, 1215-1226. http://dx.doi.org/10.1016/j.envint.2008.04.009 Wu, X.-Y., & Yang, Y.-F. (2010). Accumulation of heavy metals and total phosphorus in intensive aquatic farm sediments: Comparison of tilapia Oreochromis niloticus × oreochromis aureu, Asian seabass Lates calcarifer and white shrimp Litopenaeus vannamei farms. Aq- uaculture Research, 41, 1377-1386. http://dx.doi.org/10.1111/j.1365-2109.2009.02427.x
|