Paper Menu >>
Journal Menu >>
 Vol.4, No.9B, 63-69 (2013) Agricultural Sciences http://dx.doi.org/10.4236/as.2013.49B011 Copyright © 2013 SciRes. OPEN ACCESS Water sorption isotherms of globe artichoke leaves Luis Mayor1, Alejandro Calvo1, Ramon Moreira2, Pedro Fito1, Esperanza Garcia-Castello1* 1Instituto Universitario de Ingeniería de Alimentos para el Desarrollo, Universitat Politécnica de València, Camino de Vera s/n, 46022 Valencia, Spain; *Corresponding Author: egarcia1@iqn.upv.es 2Departamento de Enxeñaría Química, Universidade de Santiago de Compostela, Rúa Lope Gómez de Marzoa s/n, E-15782 Santiago de Compostela, Spain Received August 2013 ABSTRACT One third of the artichoke production is used in industrial processes, where up to 70% - 85% of the initial raw material is transformed into solid wastes. For an adequate management of these wastes, it is necessary to know their water sorption properties, because physical, chemical and biological changes which occur during their storage depend on water-solid interactions. The objectives of this work are to experimentally determine equilibrium sorption (adsorption and desorption) data of artichoke wastes at different temperatures (25˚C - 55˚C), as well as correlate and predict water sorption isotherms using bib- liographic models. Equilibrium moisture content ranged 0 - 0.6 kg water/kg dry solid (water activ- ity 0.05 - 0.9). Water sorption isotherms were classified between Types II and III. Hysteresis phenomenon was not observed, neither w as the dependence of the equilibrium data with tem- perature. BET, GAB, Oswin and Peleg correla- tion models were satisfactorily fitted to experi- mental data. A predictive model based on com- position and physical state of artichoke waste components was also successfully used to re- produce experimental data. Keywords: Food Waste; Modeling; Moisture; Water Activity 1. INTRODUCTION Artichoke (Cynara scolymus L.) is an herbaceous plant from the Astaraceae family originally from the Mediter- ranean area, although currently is cultivated and con- sumed worldwide. The edible part is its unripe inflores- cence. The most appreciated parts are the soft internal leaves and the fleshy parts of its centre, and both parts together are called artichoke hearts. One third of the artichoke production is used in indus- trial processes, mainly for the production of canned or frozen artichoke hearts [1]. In both processes, food wastes make up to 70% - 85% of the initial raw material [2]. These wastes, made up of defective raw artichokes and above all stems and external leaves, present management problems. As high moisture organic materials are perfect substrate for fermentations or for the development of insects and rodents. By the other hand, the cost of their transportation is expensive due to their high moisture content. Currently these wastes are used for animal feed, although other alternatives are also compost production [2], methane [3] and bioalcohol production [4]. There are also other less explored possibilities, i.e. the extraction of functional compounds such as inulin and phenolics [5]. These compounds, extracted and purified, can be used as raw material for other industrial processes. For an adequate management and valorization of these wastes, it is necessary to know their water sorption iso- therms. These are plots of the equilibrium moisture con- tent (X, kg water/kg dry solid (ds)) vs. water activity (aw) at a certain temperature and pressure. Moisture content and water activity data are important to evaluate the phys- ical, chemical and biological changes which occur during processing and storage of biomaterials [6] in processes where water transfer exists, such as drying, packaging and rehydration of dehydrated products [7]. In this con- text, the knowledge of the water sorption isotherms of artichoke wastes will provide very useful information to select the best conditions of their storage. It will also be useful in the design and optimization of valorization processes where water removal is involved, such as dry- ing. Furthermore, it will be of interest to obtain, within the studied temperature and water activity ranges, mathe- matical models that relate water activity with moisture content and temperature. For this purpose are usually employed empirical or semi-empirical equations, being the parameters of these equations obtained through the fitting of experimental data. Examples of these correla- tion models are the BET model [8], the GAB model [9] and others [10-14]. Recently, some models have arisen  L. Mayor et al. / Agricultural Sciences 4 (2013) 63-69 Copyright © 2013 SciRes. OPEN ACCESS 64 that allow predicting food water sorption isotherms from the chemical composition. These models use the Ross equation [15] and account for the multiphasic nature of food systems [6,16]. The objectives of this work were to experimentally determine sorption (adsorption and desorption) isotherms of artichoke wastes (external leaves) at different temper- atures, as well as obtain water sorption isotherms through different models from the literature. For the last purpose, correlation models as well as those based on the food chemical composition were used. 2. MATERIALS AND METHODS 2.1. Sample Preparation Artichokes (Cynara scolymus L, “Blanca de Tudela” variety) were purchased in a local market, selecting those with similar color, size and compactness. Then they were stored in hermetic containers at 5˚C until use. Previously to the experiments, artichokes were separated in their different parts: stem, fleshy centre, internal and external leaves. External leaves were used as solid waste since they are 90% in weight of the total solid wastes. 2.2. Proximate Composition 2.2.1. Moisture Content Moisture content, X, was calculated by determining sample mass before and after dehydration in a vacuum oven (Vaciotem-T, SELECTA) at 60˚C and less than 13 kPa till constant weight. Determinations were performed in triplicate. 2.2.2. Reducing Sugars and Inulin Dehydrated leaves were ground (GVX 242, KRUPS) and 5 g of fine powder were extracted in 500 mL of wa- ter during 100 min at 80˚C [17]. The filtered extract was diluted and analyzed for reducing sugars (glucose, fruc- tose and sucrose) content by ionic chromatography (Me- trosep carb 1250/4.6 column, Ion Analysis cromatograph, Methrom). Inulin was indirectly calculated by determin- ing reducing sugars content in the filtered extract before and after acidic hydrolysis [18]. Inulin content was given by the following equation: mg eq fructoseΔsucrose Inulin0.9( fructose) L extract2 (1) where Δfructose (mg/L) is the increase of fructose and Δsucrose (mg/L) is the decrease of sucrose after hydroli- sis. 0.9 is a constant for the conversion of hydrated fruc- tose to anhidrofructose [19]. 2.2.3. Other Proximal Components from Food Composition databases Protein, fat, insoluble fiber and inorganic salts were determined by consulting food composition databases from the literature. The consulted food databases were: National Nutrient Database for Standard Reference [20], Danish Food Composition Databank [21] and Spanish Food Composition Database [22]. 2.3. Water Sorption Isotherms Equilibrium moisture content of artichoke leaves at different water activities and temperatures (25˚C, 35˚C, 45˚C and 55˚C) were determined by the static method [23]. All determinations were performed in triplicate using ca. 1.5 g of fresh leaves for the desorption iso- therms and 0.5 of freeze-dried leaves (LyoAlfa 6/−50, Telstar) for the adsorption isotherms. Determinations were performed in a range of water activity from 0.05 to 0.90 using different saturated saline solutions. Selected salts were KOH, LiCl, MgCl2, K2CO3, Mg(NO3)2, NH4NO3, NaCl, KCl and BaCl2. Each saturated solution was in- troduced in a hermetic container, along with vials con- taining the sample. For water activities above 0.65 a vial with thymol was also introduced to avoid microbial growth. Closed containers were introduced in an oven (Conterm 2000201, JP selecta) at the selected temperature. Equili- brium between samples and their environment was at- tained when weight variations measured each 7 days were lower than that 0.1%. Moisture content of equili- brated samples was determined as described in 2.2.1. 2.4. Sorption Isotherms Modeling 2.4.1. Correlation Models Bibliographic models with different number of para- meters were selected for the fitting sorption data. The models are as follows: - BET [8] 111 w ww aba Xaba (2) - GAB [9] 1(11) w ww abca Xcab ca (3) - Halsey [10] 1 ln b w a Xa (4) - Henderson [11] 1 ln 1b w a Xa (5) - Oswin [12]  L. Mayor et al. / Agricultural Sciences 4 (2013) 63-69 Copyright © 2013 SciRes. OPEN ACCESS 65 () 1 b w w a Xa a (6) - Peleg [13] bd ww Xaaca (7) - Smith [14] ln(1 ) w X ab a (8) The fitting of these models to the experimental data was performed through the nonlinear optimization tool Solver of Excel 2007 (Microsoft, Redmond, WA, USA). 2.4.2. Predictive Model Based on Food Composition For simplification in the calculations only the proxim- al composition of the artichoke leaves was used: soluble sugars (glucose, fructose and sucrose), inulin, proteins, insoluble fiber (as cellulose) and soluble inorganic salts (as KCl). In this algorithm, previously described in detail [6], water activity of the food material is determined as the product of the water activity of each component, as- suming that each component has an independent beha- vior and considering their solubilities in water. Water activity of soluble compounds was determined in the case of non-electrolitic compounds (glucose, fructose and sucrose) by the Norrish equation [24] and for electrolytic compounds by the Pitzer equation [25]. Water activity of non-soluble compounds (fiber, protein, inulin) and non- solubilized fraction of solubles (sugars and KCl) was determined through water sorption isotherms from the literature [26,27]. To simplify the model it was consi- dered that in the studied temperature range sugar sorp- tion isotherms are constant. Solubility was calculated through specific equations for each soluble component [16]. The prediction algorithm consists in an iterative process started with a supposed water activity value for insoluble compounds and food equilibrium moisture content. Then for this moisture content it is determined the fraction of soluble compounds, and water activity of soluble and insoluble compounds are calculated at each step accord- ing to the following convergence criteria: a) aw of soluble compounds ≥ aw of insoluble compounds; b) if aw of so- luble compounds < initially supposed aw for insoluble compounds, then it is necessary to add an amount of wa- ter till both water activities are equal. Finally, the results of the application of the algorithm are pairs of data water activity and its corresponding equilibrium moisture content. The algorithm has to be repeated as times as pairs of data are required to build the sorption isotherm. All calculations were performed through the software Excel 2007. 2.5. Statistical Analysis Experimental sorption data shown were the average value of three experimental determinations. Goodness of fit of the models was evaluated by the following para- meters: mean relative deviation (E), median of the rela- tive deviations, coefficient of determination (R2) and the root mean square deviation (ERMS): exp exp 1 100 cal Nii ii XX ENX (9) 12 2 exp 1 1N RMS cal i EXX N (10) Where cal i X y exp i X are the calculated and experi- mental moisture content values, respectively, and N is the number of experimental data. Calculations were per- formed with the Excel 2007 software. 3. RESULTS AND DISCUSSION 3.1. Proximal Composition of Artichoke External Leaves Ta b l e 1 shows the proximal composition of artichoke wastes (external leaves), obtained from moisture content, sugars and inulin determinations, as well as information of food composition databases. Data from Tabl e 1 were obtained as follows: a) Mois- ture content was determined experimentally; b) reducing sugars and inulin were also experimentally determined; c) protein, fat and inorganic salts are the average values of the three consulted food composition databases. Since potassium is by far the main inorganic cation, inorganic salts are given in KCl equivalents. This simplification makes easier the use of the model described in 2.4.2; d) finally, cellulose as insoluble fiber was obtained through a mass balance between total solids content and the other proximal components calculated previously. Table 1. Proximal composition of artichoke external leaves. Component % (wet basis) % (dry basis) Water 83.74 - Protein 3.16 19.41 Insoluble fiber (cellulose) 8.74 53.78 Inulin 1.93 11.86 Fat 0.18 1.13 Inorganic salts (KCl) 1.02 6.27 Glucose 0.23 1.41 Fructose 0.51 3.12 Sucrose 0.49 3.00 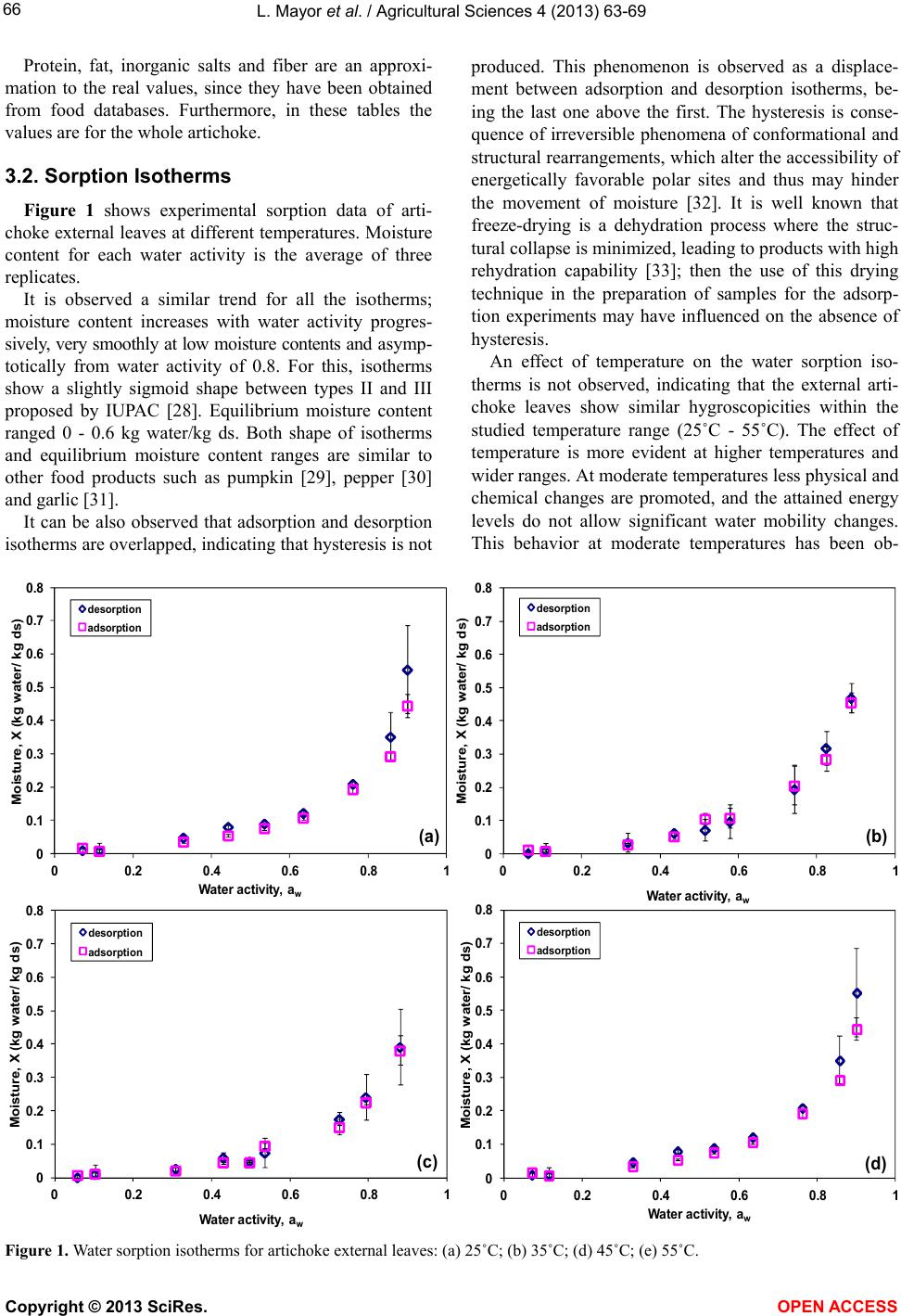 L. Mayor et al. / Agricultural Sciences 4 (2013) 63-69 Copyright © 2013 SciRes. OPEN ACCESS 66 Protein, fat, inorganic salts and fiber are an approxi- mation to the real values, since they have been obtained from food databases. Furthermore, in these tables the values are for the whole artichoke. 3.2. Sorption Isotherms Figure 1 shows experimental sorption data of arti- choke external leaves at different temperatures. Moisture content for each water activity is the average of three replicates. It is observed a similar trend for all the isotherms; moisture content increases with water activity progres- sively, very smoothly at low moisture contents and asymp- totically from water activity of 0.8. For this, isotherms show a slightly sigmoid shape between types II and III proposed by IUPAC [28]. Equilibrium moisture content ranged 0 - 0.6 kg water/kg ds. Both shape of isotherms and equilibrium moisture content ranges are similar to other food products such as pumpkin [29], pepper [30] and garlic [31]. It can be also observed that adsorption and desorption isotherms are overlapped, indicating that hysteresis is not produced. This phenomenon is observed as a displace- ment between adsorption and desorption isotherms, be- ing the last one above the first. The hysteresis is conse- quence of irreversible phenomena of conformational and structural rearrangements, which alter the accessibility of energetically favorable polar sites and thus may hinder the movement of moisture [32]. It is well known that freeze-drying is a dehydration process where the struc- tural collapse is minimized, leading to products with high rehydration capability [33]; then the use of this drying technique in the preparation of samples for the adsorp- tion experiments may have influenced on the absence of hysteresis. An effect of temperature on the water sorption iso- therms is not observed, indicating that the external arti- choke leaves show similar hygroscopicities within the studied temperature range (25˚C - 55˚C). The effect of temperature is more evident at higher temperatures and wider ranges. At moderate temperatures less physical and chemical changes are promoted, and the attained energy levels do not allow significant water mobility changes. This behavior at moderate temperatures has been ob- Figure 1. Water sorption isotherms for artichoke external leaves: (a) 25˚C; (b) 35˚C; (d) 45˚C; (e) 55˚C. 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 00.2 0.4 0.6 0.81 Moisture, X (kg water/ kg ds) W ater activity, a w desorption adsorption (a) 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 00.2 0.4 0.6 0.81 Moisture, X (kg water/ kg ds) Water act i vi t y, a w desorption adsorption (b) 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 00.2 0.4 0.6 0.81 Moisture, X (kg water/ kg ds) W ater activity , a w desorption adsorption (c) 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 00.2 0.4 0.6 0.81 Moisture, X (kg water/ kg ds) W ater activity, a w desorption adsorption (d) 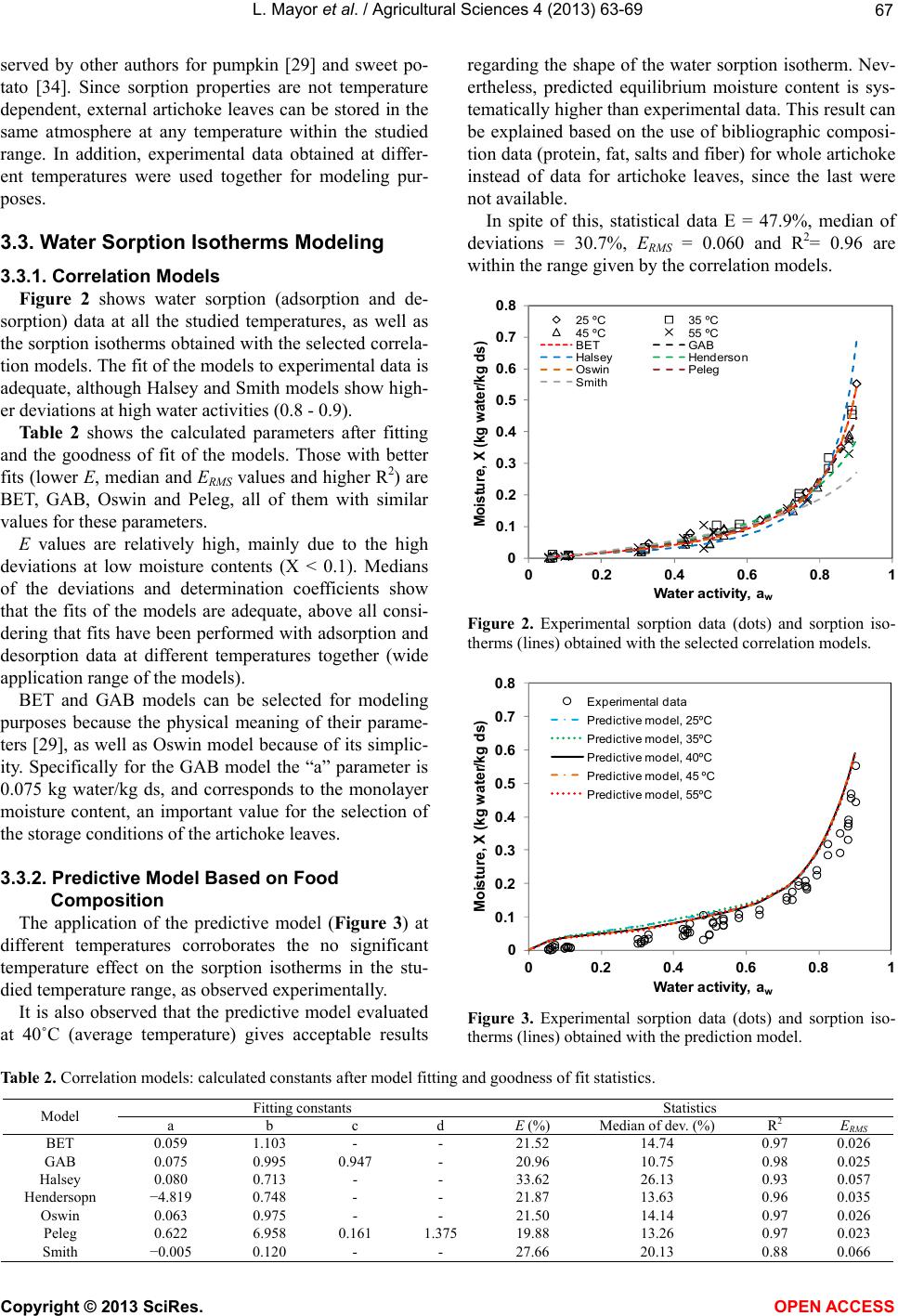 L. Mayor et al. / Agricultural Sciences 4 (2013) 63-69 Copyright © 2013 SciRes. OPEN ACCESS 67 served by other authors for pumpkin [29] and sweet po- tato [34]. Since sorption properties are not temperature dependent, external artichoke leaves can be stored in the same atmosphere at any temperature within the studied range. In addition, experimental data obtained at differ- ent temperatures were used together for modeling pur- poses. 3.3. Wa ter Sorption Isotherms Modeling 3.3.1. Correlation Models Figure 2 shows water sorption (adsorption and de- sorption) data at all the studied temperatures, as well as the sorption isotherms obtained with the selected correla- tion models. The fit of the models to experimental data is adequate, although Halsey and Smith models show high- er deviations at high water activities (0.8 - 0.9). Table 2 shows the calculated parameters after fitting and the goodness of fit of the models. Those with better fits (lower E, median and ERMS values and higher R2) are BET, GAB, Oswin and Peleg, all of them with similar values for these parameters. E values are relatively high, mainly due to the high deviations at low moisture contents (X < 0.1). Medians of the deviations and determination coefficients show that the fits of the models are adequate, above all consi- dering that fits have been performed with adsorption and desorption data at different temperatures together (wide application range of the models). BET and GAB models can be selected for modeling purposes because the physical meaning of their parame- ters [29], as well as Oswin model because of its simplic- ity. Specifically for the GAB model the “a” parameter is 0.075 kg water/kg ds, and corresponds to the monolayer moisture content, an important value for the selection of the storage conditions of the artichoke leaves. 3.3.2. Predictive Model Based on Food Composition The application of the predictive model (Figure 3) at different temperatures corroborates the no significant temperature effect on the sorption isotherms in the stu- died temperature range, as observed experimentally. It is also observed that the predictive model evaluated at 40˚C (average temperature) gives acceptable results regarding the shape of the water sorption isotherm. Nev- ertheless, predicted equilibrium moisture content is sys- tematically higher than experimental data. This result can be explained based on the use of bibliographic composi- tion data (protein, fat, salts and fiber) for whole artichoke instead of data for artichoke leaves, since the last were not available. In spite of this, statistical data E = 47.9%, median of deviations = 30.7%, ERMS = 0.060 and R2= 0.96 are within the range given by the correlation models. Figure 2. Experimental sorption data (dots) and sorption iso- therms (lines) obtained with the selected correlation models. Figure 3. Experimental sorption data (dots) and sorption iso- therms (lines) obtained with the prediction model. Table 2. Correlation models: calculated constants after model fitting and goodness of fit statistics. Model Fitting constants Statistics a b c d E (%) Median of dev. (%) R2 E R MS BET 0.059 1.103 - - 21.52 14.74 0.97 0.026 GAB 0.075 0.995 0.947 - 20.96 10.75 0.98 0.025 Halsey 0.080 0.713 - - 33.62 26.13 0.93 0.057 Hendersopn −4.819 0.748 - - 21.87 13.63 0.96 0.035 Oswin 0.063 0.975 - - 21.50 14.14 0.97 0.026 Peleg 0.622 6.958 0.161 1.375 19.88 13.26 0.97 0.023 Smith −0.005 0.120 - - 27.66 20.13 0.88 0.066 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 00.20.40.60.81 Moisture, X (kg water/kg ds) Water act ivit y, a w 25 ºC35 ºC 45 ºC55 ºC BET GAB Halsey Henders on Oswin Peleg Smith 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 00.2 0.4 0.6 0.81 Moisture, X (kg water/kg ds) W ater activity, a w Experimental dat a Pre dictive model, 25 ºC Pre dictive model, 35 ºC Pre dictive model, 40 ºC Pre dictive model, 45 ºC Pre dictive model, 55 ºC  L. Mayor et al. / Agricultural Sciences 4 (2013) 63-69 Copyright © 2013 SciRes. OPEN ACCESS 68 4. CONCLUSIONS The shape of the sorption isotherms of artichoke leaves was between types II and III (slight sigmoidal curve). Within the studied temperature range, hysteresis phe- nomenon was not observed, neither was the equilibrium data dependence on temperature. BET, GAB, Oswin and Peleg correlation models were satisfactorily fitted to experimental data. The predictive model based on composition and physical state of arti- choke waste components was also successfully used to reproduce experimental values. An advantage of this last model is its independence of experimental water sorption values to obtain predicted data and experimental time saving. 5. ACKNOWLEDGEMENTS Author Luis Mayor acknowledges JCI2009-04923 grant to MINECO (Spain). REFERENCES [1] Arthey, D. and Dennis, C. (1992) Procesado de hortalizas. Editorial Acribia, Zaragoza. [2] Rodriguez Lopez, J.N. (2009) Aprovechamiento de Resi- duos de alcachofa. http://hdl.handle.net/10201/6303 [3] Mata, J. (1998) Plantas de biometanización para la fracción orgánica de los RSU: II Tecnologías. Residuos, 42, 72-75. [4] Lázaro, L. and Arauzo, J. (1994) Aprovechamiento de residuos de la industria de conservas vegetales. Hidrólisis enzimática, 12, 227-240. [5] Lattanzio, V., Kroon, P.A., Linsalata, V. and Cardinali, A. (2009) Globe artichoke: A functional food and source of nutraceutical ingredients. Journal of Functional Foods, 1, 131-144. http://dx.doi.org/10.1016/j.jff.2009.01.002 [6] Moreira, R., Chenlo, F. and Torres, M.D. (2009) Simpli- fied algorithm for the prediction of water sorption iso- therms of fruits, vegetables and legumes based upon che- mical composition. Journal of Food Engineering, 94, 334-343. http://dx.doi.org/10.1016/j.jfoodeng.2009.03.026 [7] Singh, R.P. and Heldman, D.R. (1993) Introduction to food engineering. 2nd Edition, Academic Ress. Inc., San Diego. [8] Brunauer, S., Emmett, P.H. and Teller, E. (1938) Adsorp- tion of gases in multimolecular layers. Journal of the American Chemical Society, 60, 309-319. http://dx.doi.org/10.1021/ja01269a023 [9] Van der Berg, C. and Bruin, S. (1981) Water activity and its estimation in food systems. In: Rockland, L.B. and Stewarts, G.F., Eds., Theorical Aspects in Water Activity: Influence on Food Quality, Academic Press, New York, 12-45. [10] Halsey, G. (1948) Physical adsorption on non-uniform surfaces. Journal of Chemical Physics, 16, 931-937. http://dx.doi.org/10.1063/1.1746689 [11] Henderson, S.M. (1952) A basic concept of equilibrium moisture. Agricultural Engineering, 33, 29-32. [12] Oswin, G.R. (1946) The kinetics of package life. Interna- tional Chemical Industry, 65, 419-421. http://dx.doi.org/10.1002/jctb.5000651216 [13] Peleg, M. (1993) Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. Journal of Food Process Engineering, 16, 21- 37. http://dx.doi.org/10.1111/j.1745-4530.1993.tb00160.x [14] Smith, S. E. (1947) The sorption of water vapour by high polymers. Journal of the American Chemical Society, 69, 646-651. http://dx.doi.org/10.1021/ja01195a053 [15] Ross, K. D. (1975) Estimation of water activity in inter- mediate moisture foods. Food Technology, 3, 26-34. [16] Roman, A.D., Herman-y-Lara, E., Salgado-Cervantes, M. A. and García-Alvarado, M.A. (2004) Food sorption iso- therms prediction using the Ross equation. Drying Tech- nology, 22, 1829-1843. http://dx.doi.org/10.1081/DRT-200032802 [17] Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A. and Smith, F. (1956) Colorimetric method for determina- tion of sugars and related substances. Analytical Chemi- stry, 28, 350-356. http://dx.doi.org/10.1021/ac60111a017 [18] Saengkanuk, A., Nuchadomrong, S., Jogloy, S., Patano- thai, A. and Srijaranai, S. (2011) A simplified spectro- photometric method for the determination of inulin in Je- rusalem artichoke (Helianthus tuberosus L.) tubers. Eu- ropean Food Research & Technology, 233, 609-616. http://dx.doi.org/10.1007/s00217-011-1552-3 [19] McCleary, B.V., Murphy, A. and Mugford, D.C. (2000) Measurement of total fructan in foods by enzymatic/ spectrophotometric method: Collaborative study. Journal of AOAC International, 83, 356-364. [20] United States Department of Agriculture (2013) National nutrient database for standard reference. http://ndb.nal.usda.gov [21] Danish National Food Institution (2013) Danish food composition databank. http://www.foodcomp.dk [22] Spanish Agency of Food Safety and Nutrition (2013) Spanish database of food composition. http://www.bedca.net [23] Wolf, W., Spiess, W.E.L. and Jung, G. (1985) Standardi- zation of isotherm measurements (Cost project 90 and 90 bis). In Simatos, D. and Multon, J.L., Eds., Properties of Water in Foods, Martinus Nijhoff, Dordrecht, 661-679. http://dx.doi.org/10.1007/978-94-009-5103-7_40 [24] Norrish, R.S. (1966) An equation for the activity coeffi- cients and equilibrium relative humidities of water in confectionary syrups. Journal of Food Technology, 1, 25- 39. http://dx.doi.org/10.1111/j.1365-2621.1966.tb01027.x [25] Pitzer, K.S. (1973) Electron repulsion integrals and sym- metry adapted charge distributions. Journal of Chemical Physics, 59, 3308-3312. http://dx.doi.org/10.1063/1.1680474 [26] Salgado, M.A., Garcia, M.A. and Waliszewski, K.N. (1994) Modeling of water activity and enthalpy of water sorption  L. Mayor et al. / Agricultural Sciences 4 (2013) 63-69 Copyright © 2013 SciRes. OPEN ACCESS 69 in cassava chips. Drying Technology, 12, 1743-1752. http://dx.doi.org/10.1080/07373939408962197 [27] Makower, B. and Dye, W.B. (1956) Equilibrium moisture content and crystallisation of amorphous sucrose and glucose. Journal of Agriculture and Food Chemistry, 4, 72-77. http://dx.doi.org/10.1021/jf60059a010 [28] Sing, K.S.W., Everett, D.H., Haul, R.A.W., Moscou, L., Pierotti, R.A., Rouquerol, J. and Siemienievwska, T. (1985) Reporting physisorption data for gas/solid systems. Pure Applied Chemistry, 57, 603-619. http://dx.doi.org/10.1351/pac198557040603 [29] Mayor, L., Moreira, R., Chenlo, F. and Sereno, A.M. (2005) Water sorption isotherms of fresh and partially osmotic dehydrated pumpkin parenchyma and seeds at several temperatures. European Food Research and Te- chnology, 220, 163-167. http://dx.doi.org/10.1007/s00217-004-1065-4 [30] Chenlo, F., Moreira, R., Chaguri, L. and Santos, F. (2005) Desorption isotherms of Padron peppers (Capsicum an- nuum L. Var Longum). Ciencia y Tecnología Alimentari a, 5, 18-24. [31] Vázquez, G., Chenlo, F., Moreira, R. and Costoyas, A. (1999) The dehydration of garlic. 1. Desorption isotherms and modelling of drying kinetics. Drying Technology, 17, 1095-1108. http://dx.doi.org/10.1080/07373939908917596 [32] Rizvi, S. S. (2005) Thermodynamic properties of foods in dehydration. In: Rao, M.A., Rizvi, S.S.H. and Datta A.K. Eds., Engineering properties of foods, 3rd Edition, Taylor & Francis, Boca Ratón, 259-346. http://dx.doi.org/10.1201/9781420028805.ch7 [33] Lin, T.M., Durance, T.D. and Scaman, C.H. (1998) Cha- racterization of vacuum microwave, air and freeze dried carrot slices. Food Research International, 31, 111-117. http://dx.doi.org/10.1016/S0963-9969(98)00070-2 [34] Chen, C. (2002) Sorption isotherms of sweet potato slices. Biosystems Engineering, 83, 85-95. http://dx.doi.org/10.1006/bioe.2002.0093 |

