A. H. Abdelrahman et al. / Natural Science 5 (2013) 1183-1188 1185
Lasing wavelength and energy are very sensitive to the
choice of solvent. Most laser dyes are polar molecules,
and excitation into their lowest-lying singlet state is ac-
companied by an increase in the dipole moment. Ac-
cordingly, solvent polarity plays an important role in
shifting the lasing wavelength. In a majority of circum-
stances, increasing solvent polarity will shift the gain
curve toward longer wavelength. In the case of more polar
dyes, the shift can be as high as 20 - 60 nm.
The output power of dye lasers is strongly dependent on
the purity of the solvent. Impurities and additives may
strongly affect upper state lifetime of the dye or may
catalyse photochemical reactions. Therefore, for best
results, only high quality solvents are to be recommended
[11].
With the exception of water, all solvents should be
considered hazardous. In many instances, the solvent in
which the dye is dissolved plays a major role in the hazard
presented by the final solution.
Water is called the universal solvent because more
substances dissolve in water than in any other chemical.
This has to do with the polarity of each water molecule.
The hydrogen side of each water (H2O) molecule carries a
slight positive electric charge, while the oxygen side car-
ries a slight negative electric charge. This helps water
dissociate ionic compounds into their positive and nega-
tive ions. The positive part of an ionic compound is at-
tracted to the oxygen side of water while the negative
portion of the compound is attracted to the hydrogen side
of water.
These hazards must be addressed carefully in dye han-
dling and solution preparation. Nearly all solvents are
highly flammable. Therefore, a small fire extinguisher
should be installed near the laser in a readily accessible
and unobstructed area.
A particular fire hazard that is not commonly known
occurs with nonpolar and, hence, nonconductive solvents.
If these solvents are circulated at a high speed through
plastic tubings, the pump unit acts as a van de Graff gen-
erator, producing up to 100 kV, and sparks may pierce the
tubing and ignite the solvent. The dye selectors use
grounding wires inside the plastic tubings to eliminate
these problems. However, when using such solvents,
check first for static electricity before opening the reser-
voir. Static electricity is present when hair on the back of
your hand or forearm is attracted to the plastic tubing. do
not circulate dye solutions made with such solvents for
more than a minute, unless the cuvette has been placed
into the crate and is grounded [12].
4. BEET ROOTS AND BEET DYES
The beet (Beta vulgaris) is a plant in the amaranth
family. It is best known in its numerous cultivated varie-
ties, the most well-known of which is probably the red
root vegetable known as the beetroot or garden beet.
However, other cultivated varieties include the leaf
vegetables chard and spinach beet, as well as the root
vegetables sugar beet, which is important in the produc-
tion of table sugar, and mangel-wurzel, which is a fodder
crop. Three subspecies are typically recognized. All cul-
tivated varieties fall into the subspeciesBeta vulgaris
subsp. vulgaris, while Beta vulgaris subsp. maritima,
commonly known as the sea beet, is the wild ancestor of
these and is found throughout the Mediterranean, the
Atlantic coast of Europe, the Near East, and India. A
second wild subspecies, Beta vulgaris subsp. adanensis ,
occurs from Greece to Syria.
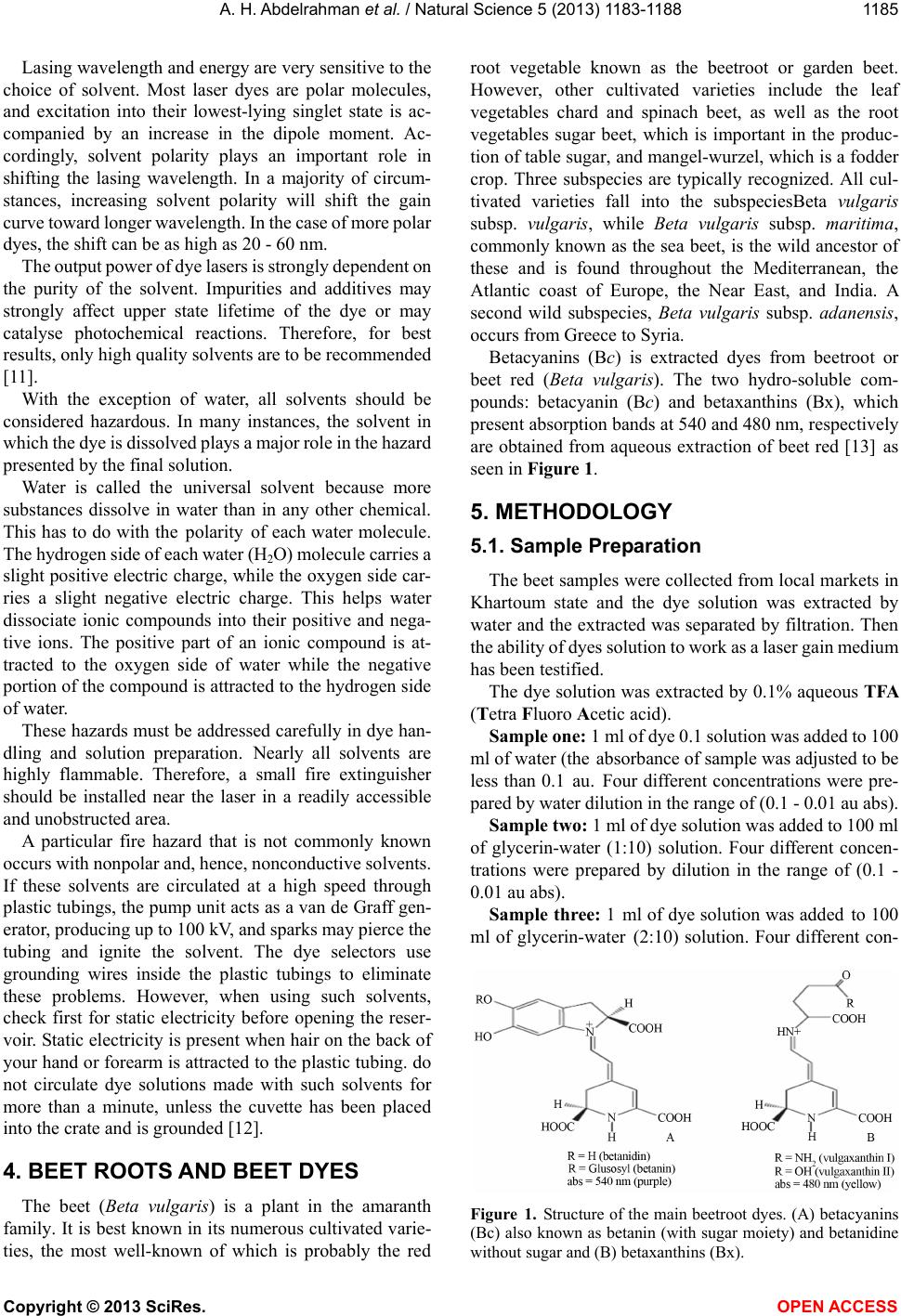
Betacyanins (Bc) is extracted dyes from beetroot or
beet red (Beta vulgaris). The two hydro-soluble com-
pounds: betacyanin (Bc) and betaxanthins (Bx), which
present absorption bands at 540 and 480 nm, respectively
are obtained from aqueous extraction of beet red [13] as
seen in Figur e 1.
5. METHODOLOGY
5.1. Sample Preparation
The beet samples were collected from local markets in
Khartoum state and the dye solution was extracted by
water and the extracted was separated by filtration. Then
the ability of dyes solution to work as a laser gain medium
has been testified.
The dye solution was extracted by 0.1% aqueous TFA
(Tetra Fluoro Acetic acid).
Sample one: 1 ml of dye 0.1 solution was added to 100
ml of water (the absorbance of sample was adjusted to be
less than 0.1 au. Four different concentrations were pre-
pared by water dilution in the range of (0.1 - 0.01 au abs).
Sample two: 1 ml of dye solution was added to 100 ml
of glycerin-water (1:10) solution. Four different concen-
trations were prepared by dilution in the range of (0.1 -
0.01 au abs).
Sample three: 1 ml of dye solution was added to 100
ml of glycerin-water (2:10) solution. Four different con-
Figure 1. Structure of the main beetroot dyes. (A) betacyanins
(Bc) also known as betanin (with sugar moiety) and betanidine
without sugar and (B) betaxanthins (Bx).
Copyright © 2013 SciRes. OPEN A CCESS