 Vol.2, No.4, 57-65 (2013) Modern Chemotherapy http://dx.doi.org/10.4236/mc.2013.24007 Allogeneic and autologous stem cell transplantation with busulfan, cyclophosphamide, and etoposide conditioning therapy for relapsed/refractory non-Hodgkin lymphoma Neelima Vidula1*, Andrew M. Evens2*, Irene B. Helenowski3, Borko Jovanovic3, Jane N. Winter4, Jayesh Mehta4, Seema Singhal4, Stephanie F. Williams4, Olga Frankfurt4, Jessica K. Altman4, Joanne Monreal4, Leo I. Gordon4# 1Division of Hematology-Oncology, University of California, San Francisco, USA 2Division of Hematology-Oncology, The University of Massachusetts Medical School, Worcester, USA 3Department of Preventive Medicine, Northwestern University, Chicago, USA 4Division of Hematology-Oncology, Northwestern University, Chicago, USA; #Corresponding Author: l-gordon@northwestern.edu Received 10 September 2013; revised 15 October 2013; accepted 21 October 2013 Copyright © 2013 Neelima Vidula et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The optimal stem cell transplantation (SCT) con- ditioning therapy for relapsed/refractory non- Hodgkin lymphoma (NHL) is not clearly defined. In a retrospective analysis, we examined 25 pa- tients with “high risk” relapsed/refractory NHL who received busulfan, cyclophosphamide, and etoposide (Bu/Cy/VP16) conditioning with auto- logous or allogeneic SCT. The majority of pa- tients had aggressive histology and 52% had primary refractory NHL. Furthermore, 48% of pa- tient s had chem otherapy-re sist ant disease at the time of SCT. Fifty-six percent of patients under- went allogeneic SCT, while 44% had autologous SCT. The median engraftment time for neutro- phils and platelets was 13.5 and 14 days, re- spectively. The 100-day treatment-related mor- tality (TRM) was 16%, while the 2-year non-re- lapse mortality (NRM) rate was also 16%. At a median follow-up of 15 months, the estimated 2- year disease-free survival (DFS) rate was 64% (95% confidence inter val (CI): 36 % - 82%) and the estimated 2-year overall survival (OS) was 69% (9 5% CI: 40% - 86%). Furthermore, the 2- year dis- ease-specific survival (DSS) rate was 73% (95% CI: 40% - 90%). Using Cox proportional hazard modeling, the International Prognostic Index at time of relapse predicted DFS and OS. Altoge- th er, Bu/Cy/VP16 was associated w ith ear ly TRM; however, lat e toxicities (including NRM) w ere un- common resulting in relatively good survival rates in a high-risk relapsed/refractory NHL population. Keywords: Stem Cell Transplantation; Busulfan; Cyclophosphamide; Etoposide; Non-Hodgk in Lymphoma 1. INTRODUCTION Stem cell transplantation (SCT) is an effective thera- peutic modality for patients with relapsed or refractory non-Hodgkin lymphoma (NHL) [1-5]. The timing and type of SCT (i.e., autologous or allogeneic) depends in part on disease histology, disease status (i.e., relapsed vs. refractory), patient physical condition, and disease-spe- cific prognostic and other risk factors. A number of SCT chemotherapy conditioning regimens have been studied in relapsed/refractory NHL; however, the optimal condi- tioning regimen is not clearly defined. Busulfan, cyclophosphamide, and etoposide (Bu/Cy/ VP16) have been investigated as a SCT conditioning regimen [6-12]. The outcomes of prior studies, however, have been quite variable with 3-year progression-free survival (PFS) rates ranging from 39% to 70% and 3-year overall survival (OS) rates ranging from 43% to 72% [8,10]. Furthermore, there are few Bu/Cy/VP16 studies that have been reported in the past 5 - 10 years as supportive care treatments, and understanding of SCT has improved over the past decade. Also, an improved *Authors contributed equally. Copyright © 2013 SciRes. OPEN A CCESS  N. Vidula et al. / Modern Chemotherapy 2 (2013) 57-65 58 understanding of the complications associated with Bu/ Cy/VP-16 is needed, especially as the early treatment- related mortality (TRM) with this regimen may be as high as 46% [13]. We analyzed the Northwestern experience with utili- zing Bu/Cy/VP16 conditioning therapy for patients with relapsed/refractory NHL. Notably, this conditioning regi- men was reserved at our institution for patients with high-risk disease (e.g., short initial remission, high IPI at relapse, chemotherapy-resistant disease at SCT, etc.). We examined detailed patient and disease characteristics, patient outcomes, and analyzed potential prognostic fac- tors that predicted survival with Bu/Cy/VP16 SCT. 2. MATERIALS AND METHODS 2.1. Study Design We performed a retrospective analysis of all relapsed/ refractory NHL patients who received stem cell trans- plantation with Bu/Cy/VP16 conditioning chemotherapy at the Northwestern University Robert H. Lurie Com- prehensive Cancer Center from 3/2003 to 6/2011. This study was approved by the Northwestern University In- stitutional Review Board. Relapsed disease was defined as disease that recurred following a response to initial therapy (lasting >6 months), while refractory disease was defined as disease that did not respond to therapy or re- lapsed within 6 months of remission. Patients who had received prior SCT were excluded from the analysis. Detailed patient characteristics were collected including patient age, gender, co-morbidities, baseline pulmonary (diffusing capacity for carbon monoxide (DLCO) and presence of obstructive or restrictive defects on pulmo- nary function testing), liver (liver enzymes), and cardiac function (ejection fraction and diastolic function on echocardiographic evaluation), and performance status. Furthermore, detailed disease characteristics at the time of relapse prior to SCT were examined including disease histology, chemotherapy sensitivity, number of extran- odal sites, disease stage, number of prior chemotherapy regimens, history of prior radiation, LDH immediately before transplant after salvage therapy (pre-SCT LDH), presence of bulk disease (>10 cm), International Prog- nostic Index (IPI), and disease status at time of SCT. 2.2. Preparative Regimen Busulfan was dosed at 3.2 mg/kg intravenous (IV) daily for 4 days on days -8 to -5, etoposide was dosed at 20 - 40 mg/kg (most commonly 30 mg/kg) IV for one day on day -4, and cyclophosphamide was dosed at 50 mg/kg IV daily for 2 days on days -3 and -2 (all based on ideal body weight). Granulocyte colony stimulating fac- tor (G-CSF), dosed at 5 µg/kg, was started on day 5 for autologous SCTs. Dilantin seizure prophylaxis was given with chemotherapy infusion and all patients received ciprofloxacin for prophylaxis prior to conditioning thera- py, and bactrim, acyclovir, and diflucan prophylaxis on discharge after transplant. Patients who received allo- geneic SCT received graft versus host disease (GVHD) prophylaxis with a calcineurin inhibitor and metho- trexate. 2.3. Statistical Analysis Engraftment time was defined as the time to an abso- lute neutrophil count (ANC) > 500/µL for 3 consecutive days and a platelet count > 20,000/µL post-SCT without requiring a transfusion. Toxicity post transplant was graded according to the National Cancer Institute Com- mon Terminology Criteria (NCI CTC) scale. Early toxic- ity was defined as adverse events occurring less than 100 days post-transplant, whereas late toxicity was defined as adverse events occurring ≥ 100 days post-transplant. Al- logeneic SCT patients were also evaluated for the pres- ence of GVHD in the acute (<100 days) and chronic (≥100 days) post-SCT period. Overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS) curves were con- structed using the Kaplan-Meier method. OS was defined as survival after transplant. DFS was defined as survival in complete remission (complete clinical and radiogra- phic resolution of tumor). DSS was defined as survival from disease post SCT. Univariate proportional hazard modeling was performed to determine which patient and disease characteristics as described above might be asso- ciated with survival outcomes. Logrank tests were used to compare survival rates and the Wald chi-square test was used to determine the significance of model coeffi- cients from the Cox regression, which was in turn used to determine the effect of various factors on survival. Fi- sher’s exact test was used to examine the association between toxicity outcomes and categorical patient and disease characteristics, while the Wilcoxon rank-sum test was used to examine the association between toxicity out- comes and continuous patient and disease characteristics. For all analyses, p < 0.05 was considered statistically significant. 3. RESULTS 3.1. Patient Demographics Twenty-five patients with relapsed or refractory NHL underwent SCT with Bu/Cy/VP16 conditioning from 3/ 2003 to 6/2011. The demographics of the study popula- tion and the associated disease characteristics are sum- marized in Table 1. The majority of patients (76%) had aggressive NHL histology. All patients had relapsed or refractory disease. Copyright © 2013 SciRes. OPEN A CCESS 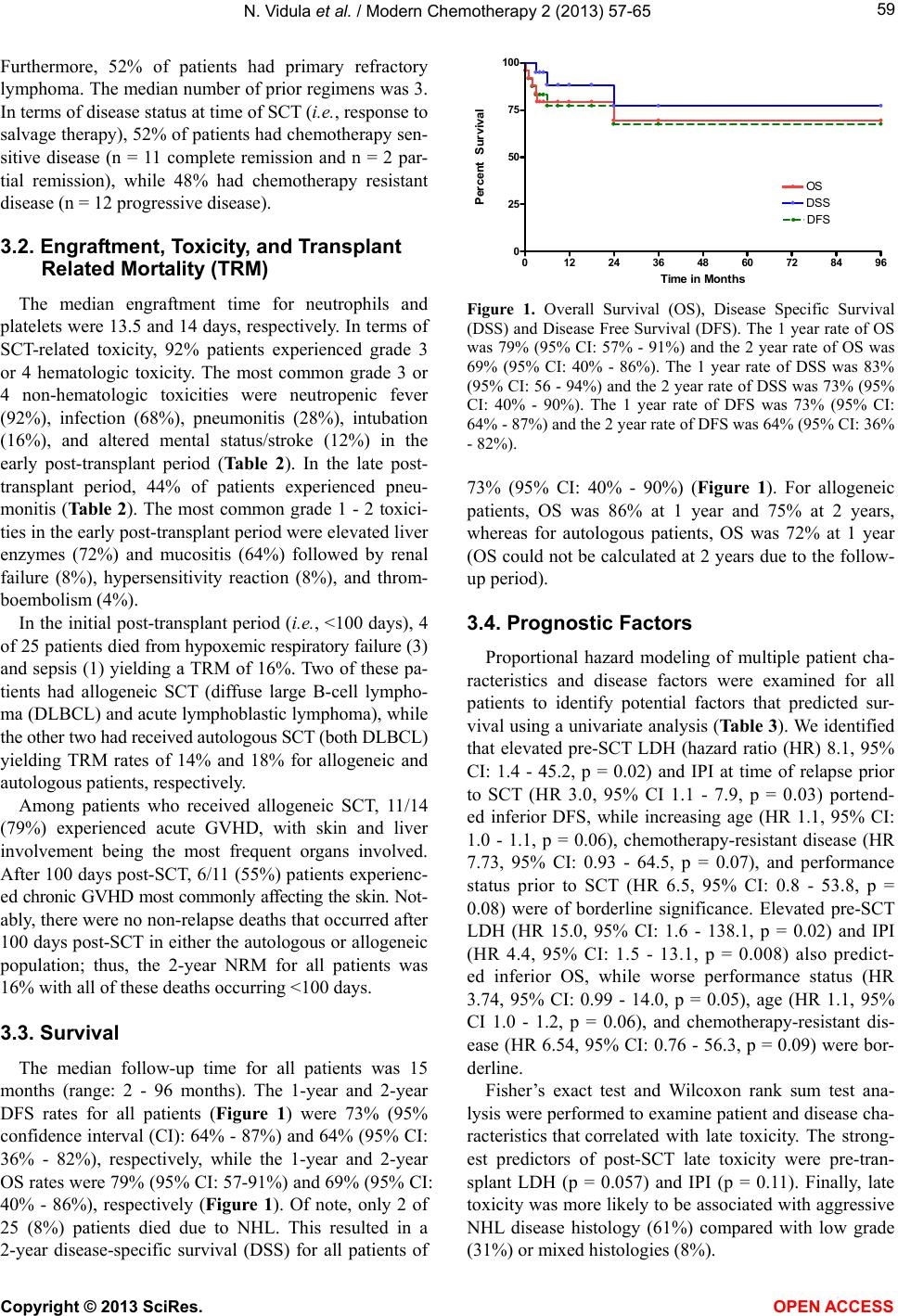 N. Vidula et al. / Modern Chemotherapy 2 (2013) 57-65 59 Furthermore, 52% of patients had primary refractory lymphoma. The median number of prior regimens was 3. In terms of disease status at time of SCT (i.e., response to salvage therapy), 52% of patients had chemotherapy sen- sitive disease (n = 11 complete remission and n = 2 par- tial remission), while 48% had chemotherapy resistant disease (n = 12 progressive disease). 3.2. Engraftment, Toxicity, and Transplant Related Mortality (TRM) The median engraftment time for neutrophils and platelets were 13.5 and 14 days, respectively. In terms of SCT-related toxicity, 92% patients experienced grade 3 or 4 hematologic toxicity. The most common grade 3 or 4 non-hematologic toxicities were neutropenic fever (92%), infection (68%), pneumonitis (28%), intubation (16%), and altered mental status/stroke (12%) in the early post-transplant period (Table 2). In the late post- transplant period, 44% of patients experienced pneu- monitis (Tabl e 2). The most common grade 1 - 2 toxici- ties in the early post-transplant period were elevated liver enzymes (72%) and mucositis (64%) followed by renal failure (8%), hypersensitivity reaction (8%), and throm- boembolism (4%). In the initial post-transplant period (i.e., <100 days), 4 of 25 patients died from hypoxemic respiratory failure (3) and sepsis (1) yielding a TRM of 16%. Two of these pa- tients had allogeneic SCT (diffuse large B-cell lympho- ma (DLBCL) and acute lymphoblastic lymphoma), while the other two had received autologous SCT (both DLBCL) yielding TRM rates of 14% and 18% for allogeneic and autologous patients, respectively. Among patients who received allogeneic SCT, 11/14 (79%) experienced acute GVHD, with skin and liver involvement being the most frequent organs involved. After 100 days post-SCT, 6/11 (55%) patients experienc- ed chronic GVHD most commonly affecting the skin. Not- ably, there were no non-relapse deaths that occurred after 100 days post-SCT in either the autologous or allogeneic population; thus, the 2-year NRM for all patients was 16% with all of these deaths occurring <100 days. 3.3. Survival The median follow-up time for all patients was 15 months (range: 2 - 96 months). The 1-year and 2-year DFS rates for all patients (Figure 1) were 73% (95% confidence interval (CI): 64% - 87%) and 64% (95% CI: 36% - 82%), respectively, while the 1-year and 2-year OS rates were 79% (95% CI: 57-91%) and 69% (95% CI: 40% - 86%), respectively (Figure 1). Of note, only 2 of 25 (8%) patients died due to NHL. This resulted in a 2-year disease-specific survival (DSS) for all patients of 012 24 36 48 60 72 84 96 0 25 50 75 100 DFS OS DSS Time in Months Percent Survival Figure 1. Overall Survival (OS), Disease Specific Survival (DSS) and Disease Free Survival (DFS). The 1 year rate of OS was 79% (95% CI: 57% - 91%) and the 2 year rate of OS was 69% (95% CI: 40% - 86%). The 1 year rate of DSS was 83% (95% CI: 56 - 94%) and the 2 year rate of DSS was 73% (95% CI: 40% - 90%). The 1 year rate of DFS was 73% (95% CI: 64% - 87%) and the 2 year rate of DFS was 64% (95% CI: 36% - 82%). 73% (95% CI: 40% - 90%) (Figure 1). For allogeneic patients, OS was 86% at 1 year and 75% at 2 years, whereas for autologous patients, OS was 72% at 1 year (OS could not be calculated at 2 years due to the follow- up period). 3.4. Prognostic Factors Proportional hazard modeling of multiple patient cha- racteristics and disease factors were examined for all patients to identify potential factors that predicted sur- vival using a univariate analysis (Table 3). We identified that elevated pre-SCT LDH (hazard ratio (HR) 8.1, 95% CI: 1.4 - 45.2, p = 0.02) and IPI at time of relapse prior to SCT (HR 3.0, 95% CI 1.1 - 7.9, p = 0.03) portend- ed inferior DFS, while increasing age (HR 1.1, 95% CI: 1.0 - 1.1, p = 0.06), chemotherapy-resistant disease (HR 7.73, 95% CI: 0.93 - 64.5, p = 0.07), and performance status prior to SCT (HR 6.5, 95% CI: 0.8 - 53.8, p = 0.08) were of borderline significance. Elevated pre-SCT LDH (HR 15.0, 95% CI: 1.6 - 138.1, p = 0.02) and IPI (HR 4.4, 95% CI: 1.5 - 13.1, p = 0.008) also predict- ed inferior OS, while worse performance status (HR 3.74, 95% CI: 0.99 - 14.0, p = 0.05), age (HR 1.1, 95% CI 1.0 - 1.2, p = 0.06), and chemotherapy-resistant dis- ease (HR 6.54, 95% CI: 0.76 - 56.3, p = 0.09) were bor- derline. Fisher’s exact test and Wilcoxon rank sum test ana- lysis were performed to examine patient and disease cha- racteristics that correlated with late toxicity. The strong- est predictors of post-SCT late toxicity were pre-tran- splant LDH (p = 0.057) and IPI (p = 0.11). Finally, late toxicity was more likely to be associated with aggressive NHL disease histology (61%) compared with low grade (31%) or mixed histologies (8%). Copyright © 2013 SciRes. OPEN A CCESS 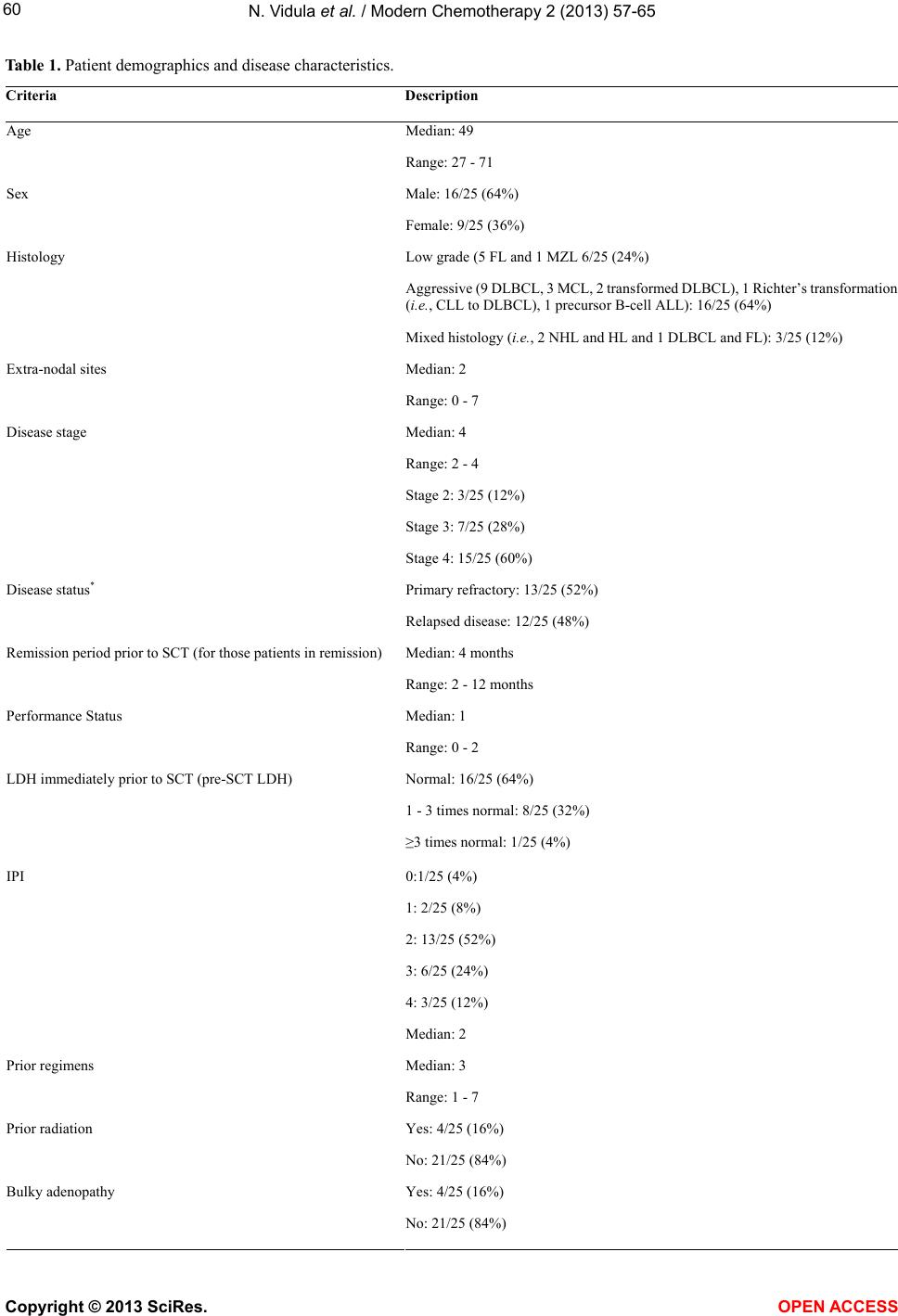 N. Vidula et al. / Modern Chemotherapy 2 (2013) 57-65 Copyright © 2013 SciRes. OPEN A CCESS 60 Table 1. Patient demographics and disease characteristics. Criteria Description Median: 49 Age Range: 27 - 71 Male: 16/25 (64%) Sex Female: 9/25 (36%) Low grade (5 FL and 1 MZL 6/25 (24%) Aggressive (9 DLBCL, 3 MCL, 2 transformed DLBCL), 1 Richter’s transformation (i.e., CLL to DLBCL), 1 precursor B-cell ALL): 16/25 (64%) Histology Mixed histology (i.e., 2 NHL and HL and 1 DLBCL and FL): 3/25 (12%) Median: 2 Extra-nodal sites Range: 0 - 7 Median: 4 Range: 2 - 4 Stage 2: 3/25 (12%) Stage 3: 7/25 (28%) Disease stage Stage 4: 15/25 (60%) Primary refractory: 13/25 (52%) Disease status* Relapsed disease: 12/25 (48%) Median: 4 months Remission period prior to SCT (for those patients in remission) Range: 2 - 12 months Median: 1 Performance Status Range: 0 - 2 Normal: 16/25 (64%) 1 - 3 times normal: 8/25 (32%) LDH immediately prior to SCT (pre-SCT LDH) ≥3 times normal: 1/25 (4%) 0:1/25 (4%) 1: 2/25 (8%) 2: 13/25 (52%) 3: 6/25 (24%) 4: 3/25 (12%) IPI Median: 2 Median: 3 Prior regimens Range: 1 - 7 Yes: 4/25 (16%) Prior radiation No: 21/25 (84%) Yes: 4/25 (16%) Bulky adenopathy No: 21/25 (84%) 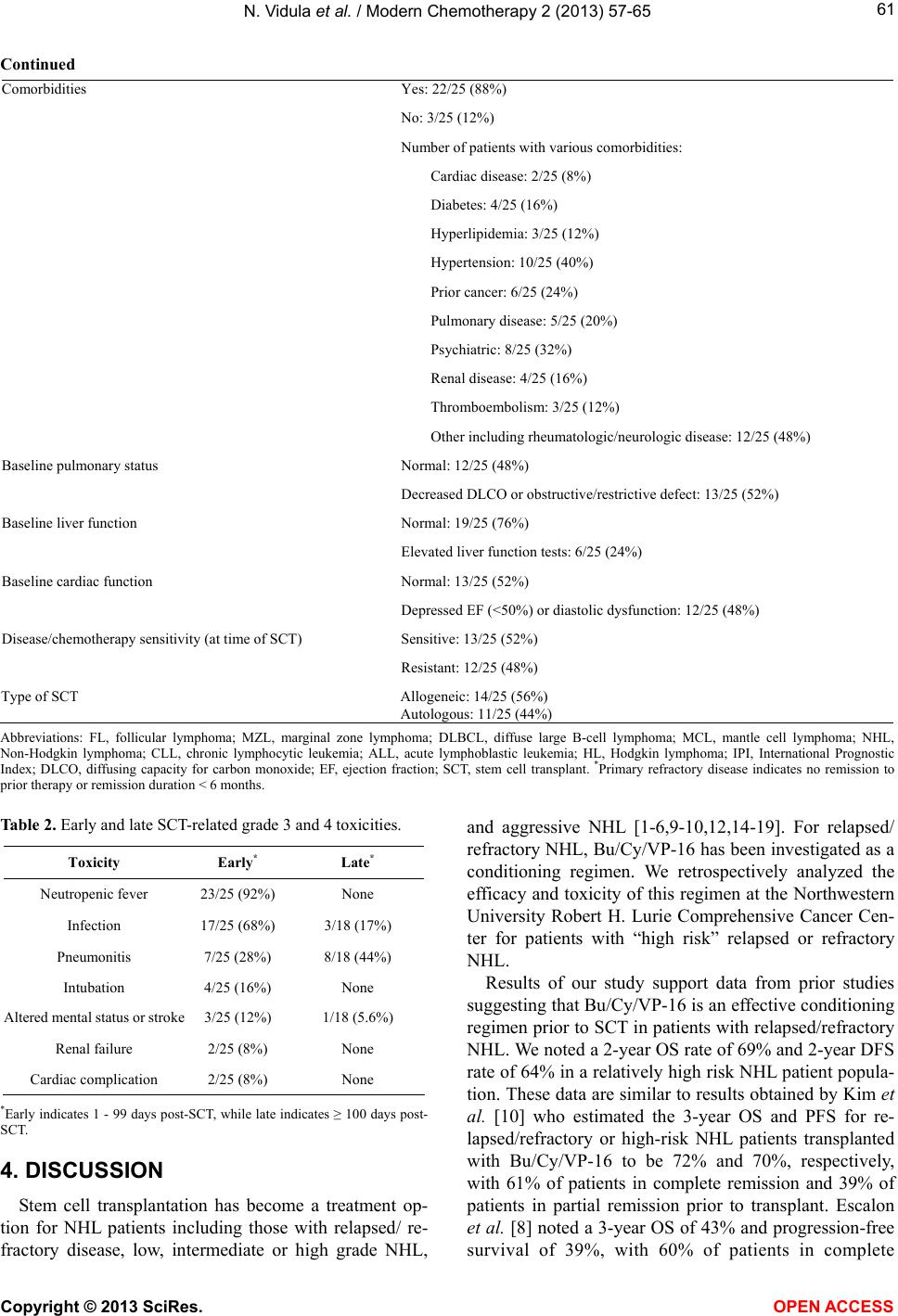 N. Vidula et al. / Modern Chemotherapy 2 (2013) 57-65 61 Continued Yes: 22/25 (88%) No: 3/25 (12%) Number of patients with various comorbidities: Cardiac disease: 2/25 (8%) Diabetes: 4/25 (16%) Hyperlipidemia: 3/25 (12%) Hypertension: 10/25 (40%) Prior cancer: 6/25 (24%) Pulmonary disease: 5/25 (20%) Psychiatric: 8/25 (32%) Renal disease: 4/25 (16%) Thromboembolism: 3/25 (12%) Comorbidities Other including rheumatologic/neurologic disease: 12/25 (48%) Normal: 12/25 (48%) Baseline pulmonary status Decreased DLCO or obstructive/restrictive defect: 13/25 (52%) Normal: 19/25 (76%) Baseline liver function Elevated liver function tests: 6/25 (24%) Normal: 13/25 (52%) Baseline cardiac function Depressed EF (<50%) or diastolic dysfunction: 12/25 (48%) Sensitive: 13/25 (52%) Disease/chemotherapy sensitivity (at time of SCT) Resistant: 12/25 (48%) Type of SCT Allogeneic: 14/25 (56%) Autologous: 11/25 (44%) Abbreviations: FL, follicular lymphoma; MZL, marginal zone lymphoma; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; NHL, Non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; ALL, acute lymphoblastic leukemia; HL, Hodgkin lymphoma; IPI, International Prognostic Index; DLCO, diffusing capacity for carbon monoxide; EF, ejection fraction; SCT, stem cell transplant. *Primary refractory disease indicates no remission to prior therapy or remission duration < 6 months. Table 2. Early and late SCT-related grade 3 and 4 toxicities. Toxicity Early* Late* Neutropenic fever 23/25 (92%) None Infection 17/25 (68%) 3/18 (17%) Pneumonitis 7/25 (28%) 8/18 (44%) Intubation 4/25 (16%) None Altered mental status or stroke3/25 (12%) 1/18 (5.6%) Renal failure 2/25 (8%) None Cardiac complication 2/25 (8%) None *Early indicates 1 - 99 days post-SCT, while late indicates ≥ 100 days post- SCT. 4. DISCUSSION Stem cell transplantation has become a treatment op- tion for NHL patients including those with relapsed/ re- fractory disease, low, intermediate or high grade NHL, and aggressive NHL [1-6,9-10,12,14-19]. For relapsed/ refractory NHL, Bu/Cy/VP-16 has been investigated as a conditioning regimen. We retrospectively analyzed the efficacy and toxicity of this regimen at the Northwestern University Robert H. Lurie Comprehensive Cancer Cen- ter for patients with “high risk” relapsed or refractory NHL. Results of our study support data from prior studies suggesting that Bu/Cy/VP-16 is an effective conditioning regimen prior to SCT in patients with relapsed/refractory NHL. We noted a 2-year OS rate of 69% and 2-year DFS rate of 64% in a relatively high risk NHL patient popula- tion. These data are similar to results obtained by Kim et al. [10] who estimated the 3-year OS and PFS for re- lapsed/refractory or high-risk NHL patients transplanted with Bu/Cy/VP-16 to be 72% and 70%, respectively, with 61% of patients in complete remission and 39% of patients in partial remission prior to transplant. Escalon et al. [8] noted a 3-year OS of 43% and progression-free survival of 39%, with 60% of patients in complete Copyright © 2013 SciRes. OPEN A CCESS  N. Vidula et al. / Modern Chemotherapy 2 (2013) 57-65 62 Table 3. Proportional hazard modeling of prognostic factors affecting survival. OS DFS Prognostic Factors HR 95% CI P HR 95% CI P Allogeneic vs. autologous 0.52 0.09 - 3.11 0.47 0.66 0.13 - 3.36 0.62 Stage III vs. II 0.3 0.02 - 4.92 0.4 0.3 0.02 - 4.96 0.4 IV vs. II 0.64 0.07 - 5.97 0.69 0.75 0.09 - 6.69 0.8 III/IV vs. II 0.52 0.06 - 4.67 0.56 0.6 0.07 - 5.20 0.65 Chemotherapy-resistant disease 6.54 0.76 - 56.3 0.09 7.73 0.93 - 64.5 0.07 IPI (continuous) 4.39 1.48 - 13.1 0.008 3.01 1.15 - 7.92 0.03 Bulky disease 0.97 0.11 - 8.34 0.98 0.77 0.09 - 6.45 0.81 Age (continuous) 1.07 1.00 - 1.15 0.06 1.06 1.00 - 1.13 0.06 Gender: Male vs. Female 1.08 0.20 - 5.92 0.93 1.3 0.25 - 6.72 0.75 Number of extra nodal sites >3 2.47 0.45 - 13.57 0.3 2.03 0.39 - 10.51 0.4 Number of prior regimens >3 0.51 0.09 - 2.84 0.45 0.41 0.08 - 2.13 0.29 Performance status 2 - 4 3.74 0.99 - 14.0 0.05 6.5 0.8 - 53.8 0.08 Baseline liver function* 1.55 0.28 - 8.62 0.62 2.29 0.50 - 10.34 0.29 Baseline cardiac function* 0.55 0.10 - 2.99 0.49 0.47 0.09 - 2.41 0.36 Baseline pulmonary function* 1.42 0.28 - 7.24 0.67 1.05 0.23 - 4.79 0.95 Pre-SCT LDH 1 - 3 normal 15.03 1.64 - 138.08 0.02 8.05 1.44 - 45.18 0.02 >3 normal 114.45 4.00 - 3277.54 0.01 69.22 3.23 - 1480.45 0.01 Abbreviations: OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; P, p-value; IPI, International Prognostic Index; Pre-SCT LDH, LDH immediately prior to transplant. *Analysis involved comparing abnormal versus normal status. remission/complete remission unconfirmed and 30% of patients with partial remission prior to transplant, while Copelan et al. [7] noted a 3-year progression free sur- vival of 47%, with 41% of patients in sensitive-first re- lapse. The majority of our patients in the current series had aggressive disease, which was noted by Copelan et al . [7] to be an adverse prognostic factor. Weaver et al. [20] showed that disease stage may be an important determi- nant of survival outcomes. While disease stage alone did not have a statistically significant impact on survival outcomes in our study, it was factored into the calculated IPI, which we identified as a predictive factor for sur- vival. In addition, several studies have shown that dis- ease status at time of SCT is a critical factor that predicts survival [21,22]. Stiff et al. [22] found that the 3-year OS and PFS of patients with chemotherapy-resistant disease was statistically inferior (p = 0.009) at 29% and 22%, re- spectively, in comparison with chemo-sensitive disease patients whose 3-year OS and PFS were 55% and 42%, respectively. Gulati et al. [21] found that the DFS of pa- tients who were in complete remission prior to transplant was 80% in comparison with 11% for those patients who had progressive disease. Nearly 50% of patients in our series had chemotherapy-resistant disease at time of SCT. Despite these high-risk features of patients herein, Bu/ Cy/VP-16 appeared to mitigate this adverse prognostic factor. Our study was limited by sample size and follow-up. Other investigators have estimated the 4 - 5 years survival of patients with NHL to be approximately 50% [6,15,23], although Zhang et al. [24] noted a 5-year survival rate of 64%, so further longitudinal analysis of our population is needed to determine whether the pre-transplant charac- teristics we have identified (lower baseline IPI and nor- mal pre-transplant LDH) continue to confer a survival advantage in the long-term. This will be critical not only for observation of potential disease relapse, but also re- Copyright © 2013 SciRes. OPEN A CCESS  N. Vidula et al. / Modern Chemotherapy 2 (2013) 57-65 63 garding the development of late effects, in particular the second malignancies/leukemia. The early TRM with Bu/Cy/VP-16 in our study was 16%. This overall result compares favorably with the 46% TRM noted by Vaughan et al. [13]. The majority of patients in our series with TRM died due to sepsis and/or hypoxemic respiratory failure, which has been described [8,23]. Mucositis also occurred frequently in the early post-transplant period, which has been noted by other authors [7-9,25]. Hepatic toxicity was commonly seen in the initial pre-transplant period, which was also noted by Kim et al. [10] and Copelan et al. [7]. However, veno- occlusive disease was rare in our population (n = 1); al- though more cases have been noted in the literature [10, 13]. This may be due to intravenous administration of etoposide as opposed to oral dosing, as suggested by Kayshap et al. [26]. Pneumonitis was a common late toxicity, which has previously been described with this regimen [9,27]. Crilley et al. [28] noted significant pulmonary toxicity with this regimen in patients who had received prior ra- diation, but Spitzer et al. [29] found no difference in pulmonary toxicity between patients receiving total body irradiation and busulfan in combination with etoposide; the pulmonary toxicity noted in our population may therefore be a consequence of busulfan [30]. Given the prevalence of this toxicity, alternative regimens may be considered in those patients with poor baseline pulmo- nary function, given the risk of long-term disabling pul- monary consequences. Nevertheless, despite these tox- icities, there were no late non-relapse fatal events noted (i.e. >100 days). Additionally, there were no secondary malignancies or myelodysplasia seen in contrast with Vaughan et al. [13]. This may be due in part to our fol- low-up time period, and further longitudinal study of our patient population is needed. Altogether, we conclude that Bu/Cy/VP-16 may serve as an effective conditioning regimen for patients with relapsed/refractory NHL. This includes patients with chemotherapy-resistant NHL, including active disease at time of SCT. However, additional analysis and continued refinement of this conditioning regimen are warranted in order to decrease the transplant-related mortality associ- ated with this therapy. 5. ACKNOWLEDGEMENTS The authors would like to acknowledge the Northwestern University Feinberg School of Medicine and the Robert H. Lurie Comprehensive Cancer Center. REFERENCES [1] Haioun, C., Lepage, E., Gisselbrecht, C., Salles, G., Coif- fier, B., Brice, P., et al. (2000) Survival benefit of high- dose therapy in poor-risk aggressive non-Hodgkin’s lym- phoma: Final analysis of the prospective LNH87-2 pro- tocol—a groupe d’Etude des lymphomes de l’Adulte stu- dy. Journal of Clinical Oncology, 18, 3025-3030. PMid:10944137. [2] Milpied, N., Deconinck, E., Gaillard, F., Delwail, V., Foussard, C., Berthou, C., et al. (2004) Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. New England Journal of Medici ne, 350, 1287-1295. http://dx.doi.org/10.1056/NEJMoa031770 [3] Pettengell, R., Radford, J.A., Morgenstern, G.R., Scarffe, J.H., Harris, M., Woll, P.J., et al. (1996) Survival benefit from high-dose therapy with autologous blood progeni- tor-cell transplantation in poor-prognosis non-Hodgkin’s lymphoma. Journal of Clinical Oncology, 14, 586-592. PMid:8636775. [4] Philip, T., Guglielmi, C., Hagenbeek, A., Somers, R., Van der Lelie, H., Bron, D., et al. (1995) Autologous bone marrow transplantation as compared with salvage chemo- therapy in relapses of chemotherapy-sensitive non-Hodg- kin’s lymphoma. New England Journal of Medicine, 333, 1540-1545. http://dx.doi.org/10.1056/NEJM199512073332305 [5] Vose, J.M., Armitage, J.O., Bierman, P.J., Weisenburger, D.D., Hutchins, M., Dowling, M.D., et al. (1989) Salvage therapy for relapsed or refractory non-Hodgkin’s lympho- ma utilizing autologous bone marrow transplantation. The American Journal of Medicine, 87, 285-288. http://dx.doi.org/10.1016/S0002-9343(89)80152-4 [6] Aggarwal, C., Gupta, S., Vaughan, W.P., Saylors, G.B., Salzman, D.E., Katz, R.O., et al. (2006) Improved out- comes in intermediate- and high-risk aggressive non- Hodgkin lymphoma after autologous hematopoietic stem cell transplantation substituting intravenous for oral bus- ulfan in a busulfan, cyclophosphamide, and etoposide preparative regimen. Biology of Blood and Marrow Tran- splantation, 12, 770-777. http://dx.doi.org/10.1016/j.bbmt.2006.03.016 [7] Copelan, E.A., Penza, S.L., Pohlman, B., Avalos, B.R., Goormastic, M., Andresen, S.W., et al. (2000) Autotrans- plantation following busulfan, etoposide and cyclophos- phamide in patients with non-Hodgkin’s lymphoma. Bone Marr ow Transplant, 25, 1243-1248. http://dx.doi.org/10.1038/sj.bmt.1702433 [8] Escalón, M.P., Stefanovic, A., Venkatraman, A., Pereira, D., Santos, E.S., Goodman, M., et al. (2009) Autologous transplantation for relapsed non-Hodgkin’s lymphoma using intravenous busulfan and cyclophosphamide as conditioning regimen: A single center experience. Bone Marr ow Transplant, 44, 89-96. http://dx.doi.org/10.1038/bmt.2008.429 [9] Hänel, M., Kröger, N., Sonnenberg, S., Bornhäuser, M., Krüger, W., Kroschinsky, F., et al. (2002) Busulfan, cyclophosphamide, and etoposide as high dose condition- ing regimen in patients with malignant lymphoma. Annals of Hematology, 81, 96-102. http://dx.doi.org/10.1007/s00277-001-0413-8 [10] Kim, J.G ., Sohn, S.K., Chae, Y.S., Yang, D.H., Lee, J.J., Kim, H.J., et al. (2007) Multicenter study of intravenous Copyright © 2013 SciRes. OPEN A CCESS  N. Vidula et al. / Modern Chemotherapy 2 (2013) 57-65 64 busulfan, cyclophosphamide, and etoposide (i.v. Bu/Cy/E) as conditioning regimen for autologous stem cell trans- plantation in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant, 40, 919-924. http://dx.doi.org/10.1038/sj.bmt.1705841 [11] Kim, J.E., Lee, D.H., Yoo, C., Kim, S., Kim, S.W., Lee, J.S., et al. (2011) BEAM or BuCyE high-dose chemo- therapy followed by autologous stem cell transplantation in non-Hodgkin’s lymphoma patients: A single center comparative analysis of efficacy and toxicity. Leukemia Research, 35, 183-187. http://dx.doi.org/10.1016/j.leukres.2010.07.016 [12] Kröger, N., Hoffknecht, M., Dreger, P., Krüger, W., Zeller, W., Krüll, A., et al. (1998) Long-term disease-free survi- val of patients with advanced mantle-cell lymphoma fol- lowing high-dose chemotherapy. Bone Marrow Trans- plant, 21, 55-57. http://dx.doi.org/10.1038/sj.bmt.1701033 [13] Vaughan, W.P., Dennisone, J.D., Reed, E.C., Klassen, L., McGuire, T.R., Sanger, W.G., et al. (1991) Improved re- sults of allogeneic bone marrow transplantation for ad- vanced hematologic malignancy using busulfan, cyclo- phosphamide and etoposide as cytoreductive and immu- nosuppressive therapy. Bone Marrow Transplant, 8, 489- 495. PMid:1790429. [14] Bolwell, B., Kalaycio, M., Andresen, S., Goormastic, M., McBee, M., Kuczkowski, E., et al. (2000) Autologous peripheral blood progenitor cell transplantation for trans- formed diffuse large-cell lymphoma. Clinical Lymphoma, 1, 226-233. http://dx.doi.org/10.3816/CLM.2000.n.019 [15] Kröger, N, Hoffknecht, M., Dreger, P., Krüger, W., Zeller, W., Krüll, A., et al. (1998) Long-term disease-free sur- vival of patients with advanced mantle-cell lymphoma following high-dose chemotherapy. Bone Marrow Trans- plant, 21, 55-57. http://dx.doi.org/10.1038/sj.bmt.1701033 [16] Philip, T., Armitage, J.O., Spitzer, G., Chauvin, F., Jagan- nath, S., Cahn, J., et al. (1987) High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate- grade or high-grade non-Hodgkin’s lymphoma. New Eng- land Journal of Medicine, 316, 1493-1498. http://dx.doi.org/10.1056/NEJM198706113162401 [17] Schouten, H.C., Colombat, P., Verdonck, L.F., Gorin, N.C., Björkstrand, B., Taghipour, G., et al. (1994) Autolo- gous bone marrow transplantation for low-grade non- Hodgkin’s lymphoma: The European Bone Marrow Tran- splant Group experience. EBMT Working Party for Lym- phoma. Annals of Oncology, 5, 147-149. http://dx.doi.org/10.1093/annonc/5.suppl_2.S147 [18] Sweetenham, J.W., Proctor, S.J., Blaise, D., De Laurenzi, A., Pearce, R., Taghipour, G., et al. (1994) High-dose therapy and autologous bone marrow transplantation in first complete remission for adult patients with high- grade non-Hodgkin’s lymphoma: The EBMT experience. Lymphoma Working Party of the European Group for Bone Marrow Transplantation. Annals of Oncology, 5, 155-159. http://dx.doi.org/10.1093/annonc/5.suppl_2.S155 [19] Takvorian, T., Canellos, G.P., Ritz, J., Freedman, A.S., Anderson, K.C., Mauch, P., et al. (1987) Prolonged dis- ease-free survival after autologous bone marrow trans- plantation in patients with non-Hodgkin’s lymphoma with a poor prognosis. New England Journal of Medicine, 316, 1499-1505. http://dx.doi.org/10.1056/NEJM198706113162402 [20] Weaver, C.H., Petersen, F.B., Appelbaum, F.R., Bensinger, W.I., Press, O., Martin, P., et al. (1994) High-dose frac- tionated total body irradiation, etoposide, and cyclophos- phamide followed by autologous stem cell support in pa- tients with malignant lymphoma. Journal of Clinical On- cology, 12, 2559-2566. PMid:7989929. [21] Gulati, S., Yahalom, J., Acaba, L., Reich, L., Motzer, R., Crown, J., et al. (1992) Treatment of patients with relaps- ed and resistant non-Hodgkin’s lymphoma using total body irradiation, etoposide, and cyclophosphamide and autologous bone marrow transplantation. Journal of Cli- nical Oncology, 10, 936-941. [22] Stiff, P.J., Dahlberg, S., Forman, S.J., McCall, A.R., Horn- ing, S.J., Nademanee, A.P., et al. (1998) Autologous bone marrow transplantation for patients with relapsed or re- fractory diffuse aggressive non-Hodgkin’s lymphoma: Value of augmented preparative regimens—A Southwest Oncology Group trial. Journal of Clinical Oncology, 16, 48-55. [23] Santos, E.C., Sessions, J., Hutcherson, D., Flowers, C., Langston, A. and Waller EK. (2007) Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide, cyclophosphamide, and autologous stem cell transplantation—A similar experience. Biology of Blood and Marrow Transplantation, 13, 746-747. http://dx.doi.org/10.1016/j.bbmt.2007.02.006 [24] Zhang, H., Graiser, M., Hutcherson, D.A., Olufemi Dada, M., McMillan, S., Zahir, A., et al. (2012) Pharmacoki- netic-directed high dose busulfan combined with cyclo- phosphamide and etoposide results in predictable drug levels and durable long term survival in lymphoma pa- tients undergoing autologous stem cell transplantation. Biology of Blood and Marrow Transplant, 18, 1287-1294. http://dx.doi.org/10.1016/j.bbmt.2012.02.006 [25] Ritchie, D.S., Szer, J., Roberts, A.W., Shuttleworth, P. and Grigg, A.P. (2002) A phase I dose-escalation study of eto- poside continuous infusion added to busulphan/cyclo- phosphamide as conditioning prior to autologous or al- logeneic stem cell transplantation. Bone Marrow Trans- plantation, 30, 645-650. http://dx.doi.org/10.1038/sj.bmt.1703698 [26] Kashyap, A., Wingard, J., Cagnoni, P., Roy, J., Tarantolo, S., Hu, W., et al. (2002) Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regi- men for allogeneic hematopoietic stem cell transplanta- tion: Decreased incidence of hepatic venoocclusive dis- ease (HVOD), HVOD-related mortality, and overall 100- day mortality. Biology of Blood and Marrow Transplan- tation, 8, 493-500. http://dx.doi.org/10.1053/bbmt.2002.v8.pm12374454 [27] Wadehra, N., Farag, S., Bolwell, B., Elder, P., Penza, S., Kalaycio, M., et al. (2006) Long-term outcome of Hodg- kin disease patients following high-dose busulfan, eto- poside, cyclophosphamide, and autologous stem cell transplantation. Biology of Blood and Marrow Transplan- Copyright © 2013 SciRes. OPEN A CCESS  N. Vidula et al. / Modern Chemotherapy 2 (2013) 57-65 Copyright © 2013 SciRes. OPEN A CCESS 65 tation, 12, 1343-1349. http://dx.doi.org/10.1016/j.bbmt.2006.08.039 [28] Crilley, P., Topolsky, D., Styler, M.J., Bernstein, E., Res- nick K, Mullaney, R., et al. (1995) Extramedullary toxic- ity of a conditioning regimen containing busulphan, cyclophosphamide and etoposide in 84 patients undergo- ing autologous and allogenic bone marrow transplantation. Bone Marrow Transplantation, 15. 361-365. PMid:7599559 [29] Spitzer, T.R., Cottler-Fox, M., Torrisi, J., Cahill, R., Greenspan, A., Lynch, M., et al. (1989) Escalating doses of etoposide with cyclophosphamide and fractionated to- tal body irradiation or busulfan as conditioning for bone marrow transplantation. Bone Marrow Transplantation, 4, 559-565. PMid:2676044. [30] Kalaycio, M., Pohlman, B., Kuczkowski, E., Rybicki, L., Andresen, S., Sobecks, R., et al. (2006) High-dose busul- fan and the risk of pulmonary mortality after autologous stem cell transplant. Clinical Transplantation, 20, 783- 787. http://dx.doi.org/10.1111/j.1399-0012.2006.00581.x
|