Paper Menu >>
Journal Menu >>
 Advances in Bioscience and Biotechnology, 2011, 2, 20-26 ABB doi:10.4236/abb.2011.21004 Published Online February 2011 (http://www.SciRP.org/journal/abb/). Published Online February 2011 in SciRes. http://www.scirp.org/journal/ABB Enzyme electrophoresis method in analysis of active components of haemostasis system Ludmila Ostapchenko, Oleksiy Savchuk, Nataliia Burlova-Vasilieva Educational and Scientific Centre “Institute of Biology” of National Taras Shevchenko University of Kyiv, Kyiv, Ukraine. Email: burlova@mail.ru Received 9 December 2010; revised 16 January 2011; accepted 20 January 2011 ABSTRACT The novel modifications of substrate-containing so- dium dodecyl sulfate-polyacrylamide gel electropho- resis that can be used for the detection of proteases and its activators are reported. The protease/acti- vator samples were separated on a protein substrate- SDS-polyacrylamide gel. To detect plasminogen acti- vators fibrinogen and Glu-plasminogen were incor- porated into the SDS-PAG followed by 1 h incubation at 37˚C in thrombin solution (1 NIH/ml). After elec- trophoresis the gel was stained according to the standard protocol. To detect fibrin-unspecific plasmi- nogen activators from snake venom incubation in thrombin solution was substituted for 12 h incubation in 50 mM Tris-HCl (pH 7.4). To detect fibrinogen- degrading enzymes fibrinogen-containing gel was used. Activity of protease/activator was visualized in the gel as clear bands against the dark background. These new techniques offer several advantages in- cluding determination of the quantity and activity of t-PA and urokinase, however cannot be recommended for precise quantification of activators; the total pro- cedure is quite quick and simple; method is conven- ient tool for detection of novel protein-protein inter- actions in haemostasis system; the sensitivity of the method is ≤0.01 IU per track. Keywords: Substrate-Containing Electrophoresis; Enzyme Electrophoresis; Haemostasis; Proteins Activity; Proteins Identification 1. INTRODUCTION Protein electrophoresis is a convenient approach to characterize sample composition, protein interactions, purity, molecular weight, isoelectric point, and to purify small amounts of protein for further analysis. Different modifications of this widely used method have been developed to suit a variety of purposes [1]. Erickson [2] and Brunner [3] offered fibrin autogra- phy to detect protease activators/inhibitors previously fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After separation the proteins and the substrate were transferred electropho- retically into the fibrin indicator gel. The positions of activators/inhibitors were revealed by the formation of contrast fibrinolytic or lysis-resistant zones. Hanspal et al. [4] described a technique to detect the activity of protease inhibitors present in enzyme sub- strate-containing sodium dodecyl sulfate polyacrylamide gel (SDS-PAG). The method involved 1) incorporation of substrate (gelatin or casein) into the SDS-PAG at the time of casting; 2) electrophoresis of the protease in- hibitors in the presence of SDS; 3) removal of SDS by washing the gel in 2.5% (w/v) Triton X-100; 4) incuba- tion of the gels in a solution containing the proteolytic enzyme at 37 degrees C for 16 h; and 5) staining undi- gested substrate with amido black. Sensitive methods for detecting proteases/protease in- hibitors by using fluorescent protease substrates in gels are reported [5,6]. Wilkesman and Schroder [7] used 2-D zymography, a technique that combines IEF (isoelectric focusing) and zymography. Procedures including high- molecular-mass substrates within the gel, such as starch for identification of amylase activity, and protein sub- strates, such as gelatin, casein, and collagen, for reveal- ing protease/protease inhibitors activity, have been de- scribed [8-10]. There are several features of enzyme activity deter- mination including protection of protein functional ac- tivity. This provides the possibility of enzyme identifica- tion after all biochemical manipulations. Although, it is not possible to save 100% of functional activity, how- ever most of researchers succeeded to save nativity of the protein (including its activity) at the level which is sufficient authentic and adequate identification. In the present study we report 1D SDS-PAG enzyme electrophoresis method. The technique is optimized for identification of proteases/protease activators of haemo- stasis system in the blood plasma or other tissue samples.  Ostapchenko L. I. et al. / Advances in Bioscience and Biotechnology 2 (2011) 20-26 Copyright © 2011 SciRes. ABB 21 The major advantage of the method is the possibility to detect active plasminogen activators – tissue-type plas- minogen activator (t-PA) and urokinase which simplifies analytical work [11-13]. Enzyme electrophoresis can be used as a rapid diagnostic method as gives information not only about the amount and MW of proteins but also reveals active forms of t-PA and urokinase. Fibrinogen or (fibrinogen and plasminogen) was in- corporated into the gel as a substrate for proteases. The convertion of fibrinogen to fibrin under thrombin treat- ment provides conditions for fibrin-dependent activators to generate plasmin causing the background substrate degradation [14]. 2. MATERIALS AND METHODS Chemicals and proteins. Tris, glycine, SDS, acrylamide, bisacrylamide, ammonium persulfate (APS), N,N,N’,N’- tetramethylethylenediamine (TEMED), sucrose, all of analytical grade, were purchased from Amersham Bio- sciences AB (Sweden). t-PA, urokinase and elastase were obtained from Sigma, Germany. Streptokinase was supplied by Kabi Pharmacia AG, Sweden. Sample preparation. Mouse blood plasma from Lewis carcinoma line С57В1/6 was received from Kavetskiy Institute of experimental pathology, oncology and radio- biology of the National Academy of Siences of Ukraine. Subretinal fluid isolated by surgical operation from patients with regmatogenic retina exfoliation was re- ceived from Filatov Institute of eye diseases and tissue therapy of the Academy of Medical Sciences of Ukraine. The crystalline snake venom of Agkistrodon halys halys was received from serpentarium of Tripolskiy biochemical plant, Ukraine. All samples were mixed with treatment buffer in the ratio 1:1 (v/v) and stored before electrophoresis at 4˚C. The treatment buffer was made ready according to Amersham Biosciences protocol [15] with modifications: 1) glycerine was replaced by sucrose to the final concentration 5% and 2) DTT was not added to prevent the loss of enzyme activity. The obligatory condition for enzyme electrophoresis samples preparation was non- thermal treatment of the samples before separation to avoid the loss of enzyme activity. 3. ENZYME ELECTROPHORESIS We have developed a technique on the basis of the method described by Heusen C. and Dowdle E., [16] with following modifications: fibrinogen (1 mg/mL) or (fibrinogen (1 mg/mL) and Glu-plasminogen (10 mkg/ mL) was incorporated into separating PAG. Fibrinogen was used to detect proteases capable of fibrinogen cleavage (in this study plasminogen and mini plasmino- gen, see Example 4, Figures 4(a) and 5). Fibrinogen and plasminogen were incorporated into SDS-PAG for plasminogen activators detection. After separation the gel was incubated in thrombin solution (1 NIH/mL) for 1 h at 37˚C. Fibrin formation was required for develop- ment of the fibrin-dependent plasminogen activators (t- PA and urokinase) activity [17]. t-PA or urokinase ap- peared as the clear bands, corresponding to the area where plasmin has degraded fibrin. The separation gel concentration can vary from 11% to 15% to prevent mi- gration of incorporated proteins during electrophoresis. The technique involved: 1) incorporation of fibrino- gen or (fibrinogen and plasminogen) into the SDS-PAG of required concentration; 2) electrophoretic separation under usual conditions [15]; 3) gel washing in 2.5% Tri- ton Х-100 with shaking for 1 h at 25˚C for SDS removal; 4) for (fibrinogen and plasminogen)-incorporated gel —treatment with thrombin solution with shaking for 1 h at 37˚C; 5) proteins visualization according to standard protocol [15]. For electoforesis performing and gel staining proce- dures Hoefer Mighty Small system and Hoefer Processor Plus (Amersham Biosciences AB, Sweden) were used. The sensitivity of the method was ≤0.01 IU of activa- tor or protease per track. 4. WESTERN BLOTTING Procedure was performed according to the protocol [17]. Proteins were transferred to a nitrocellulose membrane for 1 h at 4˚C and 60 MА in 15 mМ Tris-HCl, рН 8.4 with 120 mМ glycine and 20% methanol. The mem- brane was stained with 0.1% Ponso “Sigma” in 5% ace- tic acid with shaking for 30 min followed by overnight incubation in 5% BSA at 4˚C. Proteins were probed using monoclonal antibody (MAb) directed against plasminogen (Merck KGaA, Germany) in dilution 1:1000 and secondary antibody (1:3000) labeled with alkaline phosphatase. Each procedure was followed by rinsing step with 3 buffer substitutions. The washing buffer consisted of 50 mM Tris-HCl with 0.1% Twin-20. The blot was developed using 5-bromo-4-chloro-3- indolyl phosphate. 5. PROTEINS PURIFICATION Human Glu-plasminogen was purified from citrate- anticoagulated blood plasma by affinity chromatography on Lys-Sepharose [18]. The citrate-anticoagulated blood plasma was diluted 1:1 with 50 mM sodium-phosphate, pH 7.4 containing aprotinin (1000 IU/L), filtered and loaded onto Lys-Sepharose column previously equili- brated in the same buffer lacking aprotinin. After loading the column was washed with 50 mM sodium-phosphate, pH 7.4 with 200 mМ NaCl, and eluted with 150 mМ 6-aminohexacapronic acid in the same buffer. By adding 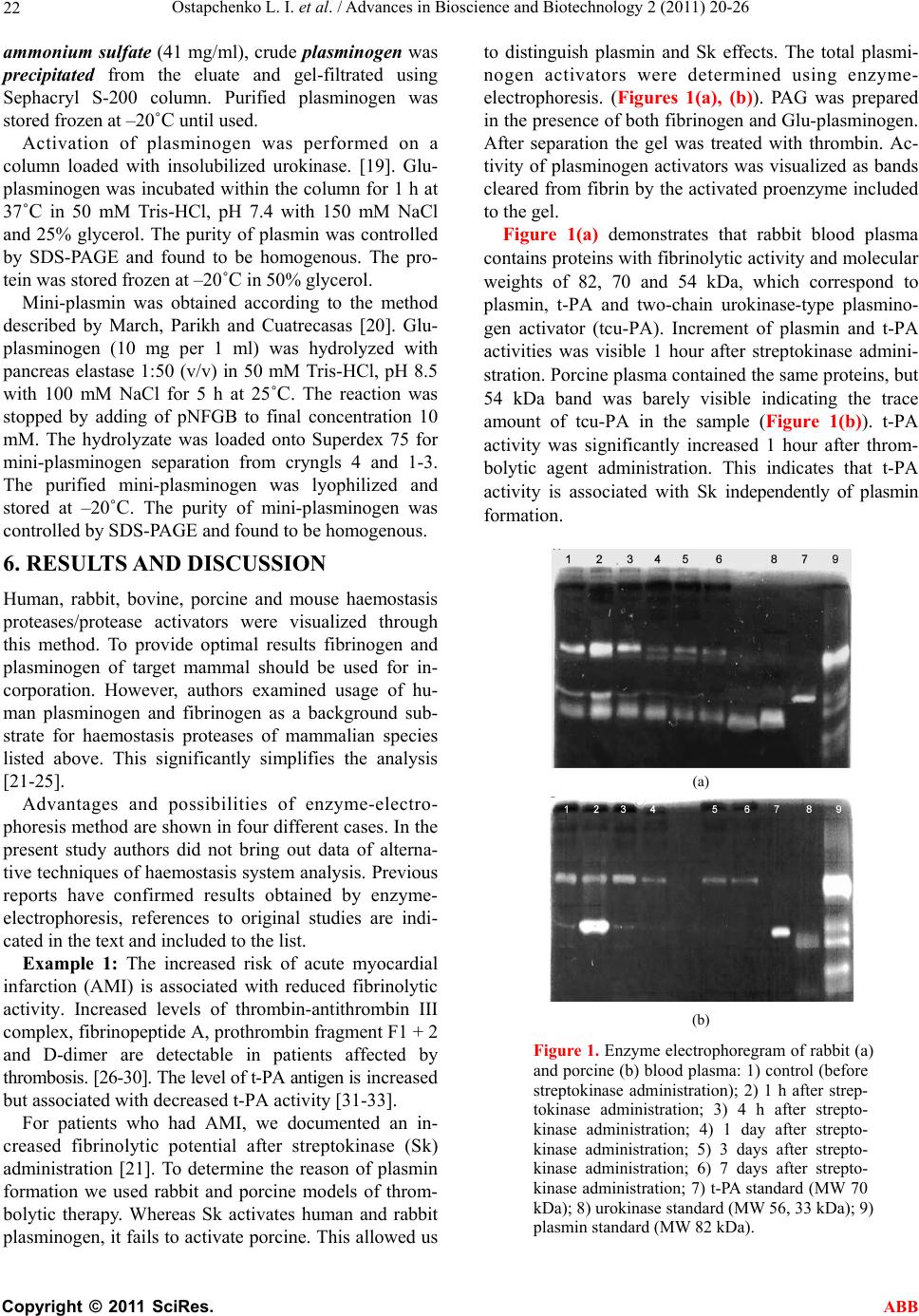 Ostapchenko L. I. et al. / Advances in Bioscience and Biotechnology 2 (2011) 20-26 Copyright © 2011 SciRes. ABB 22 ammonium sulfate (41 mg/ml), crude plasminogen was precipitated from the eluate and gel-filtrated using Sephacryl S-200 column. Purified plasminogen was stored frozen at –20˚C until used. Activation of plasminogen was performed on a column loaded with insolubilized urokinase. [19]. Glu- plasminogen was incubated within the column for 1 h at 37˚C in 50 mМ Tris-НCl, pH 7.4 with 150 mM NaCl and 25% glycerol. The purity of plasmin was controlled by SDS-PAGE and found to be homogenous. The pro- tein was stored frozen at –20˚C in 50% glycerol. Mini-plasmin was obtained according to the method described by March, Parikh and Cuatrecasas [20]. Glu- plasminogen (10 mg per 1 ml) was hydrolyzed with pancreas elastase 1:50 (v/v) in 50 mМ Tris-HCl, pH 8.5 with 100 mМ NaCl for 5 h at 25˚C. The reaction was stopped by adding of pNFGB to final concentration 10 mМ. The hydrolyzate was loaded onto Superdex 75 for mini-plasminogen separation from cryngls 4 and 1-3. The purified mini-plasminogen was lyophilized and stored at –20˚C. The purity of mini-plasminogen was controlled by SDS-PAGE and found to be homogenous. 6. RESULTS AND DISCUSSION Human, rabbit, bovine, porcine and mouse haemostasis proteases/protease activators were visualized through this method. To provide optimal results fibrinogen and plasminogen of target mammal should be used for in- corporation. However, authors examined usage of hu- man plasminogen and fibrinogen as a background sub- strate for haemostasis proteases of mammalian species listed above. This significantly simplifies the analysis [21-25]. Advantages and possibilities of enzyme-electro- phoresis method are shown in four different cases. In the present study authors did not bring out data of alterna- tive techniques of haemostasis system analysis. Previous reports have confirmed results obtained by enzyme- electrophoresis, references to original studies are indi- cated in the text and included to the list. Example 1: The increased risk of acute myocardial infarction (AMI) is associated with reduced fibrinolytic activity. Increased levels of thrombin-antithrombin III complex, fibrinopeptide A, prothrombin fragment F1 + 2 and D-dimer are detectable in patients affected by thrombosis. [26-30]. The level of t-PA antigen is increased but associated with decreased t-PA activity [31-33]. For patients who had AMI, we documented an in- creased fibrinolytic potential after streptokinase (Sk) administration [21]. To determine the reason of plasmin formation we used rabbit and porcine models of throm- bolytic therapy. Whereas Sk activates human and rabbit plasminogen, it fails to activate porcine. This allowed us to distinguish plasmin and Sk effects. The total plasmi- nogen activators were determined using enzyme- electrophoresis. (Figures 1(a), (b)). PAG was prepared in the presence of both fibrinogen and Glu-plasminogen. After separation the gel was treated with thrombin. Ac- tivity of plasminogen activators was visualized as bands cleared from fibrin by the activated proenzyme included to the gel. Figure 1(a) demonstrates that rabbit blood plasma contains proteins with fibrinolytic activity and molecular weights of 82, 70 and 54 kDa, which correspond to plasmin, t-PA and two-chain urokinase-type plasmino- gen activator (tcu-PA). Increment of plasmin and t-PA activities was visible 1 hour after streptokinase admini- stration. Porcine plasma contained the same proteins, but 54 kDa band was barely visible indicating the trace amount of tcu-PA in the sample (Figure 1(b)). t-PA activity was significantly increased 1 hour after throm- bolytic agent administration. This indicates that t-PA activity is associated with Sk independently of plasmin formation. (a) (b) Figure 1. Enzyme electrophoregram of rabbit (a) and porcine (b) blood plasma: 1) control (before streptokinase administration); 2) 1 h after strep- tokinase administration; 3) 4 h after strepto- kinase administration; 4) 1 day after strepto- kinase administration; 5) 3 days after strepto- kinase administration; 6) 7 days after strepto- kinase administration; 7) t-PA standard (MW 70 kDa); 8) urokinase standard (MW 56, 33 kDa); 9) plasmin standard (MW 82 kDa).  Ostapchenko L. I. et al. / Advances in Bioscience and Biotechnology 2 (2011) 20-26 Copyright © 2011 SciRes. ABB 23 Example 2: Snake venoms are complex mixtures containing many different biologically active proteins and peptides. A number of these proteins affect the blood coagulation pathway, endothelial cells, and plate- lets [34]. Several venom enzymes have been used as anticoagulants and other are under examination of their possible therapeutic potential. We used enzyme- electrophoresis to detect potential plasminogen activator in Agkistrodon halys halys venom. For this purpose fi- brinogen and plasminogen were incorporated into the PAGE. The mixture of previously purified activator and plasminogen 1:1 (q/q) was used as a sample. The gel was not treated with thrombin but after SDS on Triton X-100 substitution was incubated for 12 hours in 50 mM Tris-HCl, рН 7.4 for development of plasmin activity due to action of potential activator. In the analyzed sam- ple (Figure 2) two bands were found. One of them cor- responded to 82 kDa plasmin, which had been formed under activator action. The second band corresponded to MW of activator itself (32 kDa), its appearance was pro- vided by plasminogen incorporation. These results sug- gest that induction of plasmin formation by the snake venom activator was specific and involved a bond cleavage at a specific site in the plasminogen molecule without formation of active fragments. These results were completely confirmed using spe- cific chromogenic substrate for plasmin [22]. Example 3: To detect components of plasminogen ac- tivation system in the subretinal fluid of patients with regmatogenic retina exfoliation enzyme-electrophoresis was performed. This substance is accumulated in the Figure 2. Enzyme electrophoregram of plasminogen and plasminogen ac- tivator from snake venom mixture: 1) plasminogen and plasminogen acti- vator mixture; 2) plasmin standard (MW 82 kDa); 3) plasminogen stan- dard used for incubation and incorpo- ration. cavity formed by pathologic retina exfoliation. It was shown that subretinal fluid possessed fibrinolytic activity and included an activator capable of plasminogen trans formation. To determine the nature of this activator (or activators) fibrinogen and plasminogen-incorporated gel was used. After separation and SDS removal the gel was incubated in thrombin for fibrin formation. This step was necessary due to fibrin-dependent nature of t-PA and urokinase activities. The 54 kDa band was revealed at the electrophoregram (Figure 3(a)) and confirmed the existence of tcu-PA in the sample. Previous reports have confirmed results obtained by substrate-incorporated electrophoresis [23,35]. Example 4: Production of elastase is significantly enhanced in tumor cells leading to formation of plasmi- nogen internal fragments (angiostatine that consists of kringles 1 to 4 and approximately 85% of kringle 5) [36- 39]. Unbound plasminogen/plasmin cringles are biologi- cally active molecules that act on distant sites affecting fibrinolytic efficiency [39-42]. Using enzyme-electrophoresis method we analyzed proteases and plasminogen activators composition in the blood plasma of Lewis carcinoma mice (Figure 4(a)). The analysis of haemostasis system during Lewis carci- noma growth is reflected in the study [24]. Fibrinogen and Glu-plasminogen were incorporated into the PAG. After separation of the samples and SDS removal the gel was incubated in thrombin solution for fibrinogen transformation. Appearance of active bands revealed plasminogen activators and proteases capable of fibrin cleavage. As migration pattern of the size (Mr) standards elastase (27, 29 and 31 kDa), urokinase (33 and 56 kDa), tPA (70 kDa) and mini-plasmin (36 kDa) were used. The resulting electropherogram for this case Figure 3. Enzyme electropho- regram of subretinal fluid: 1) subretinal fluid; 2) t-PA stan- dard (MW 70 kDa); 3) uroki- nase standard (MW 56, 33 kDa). 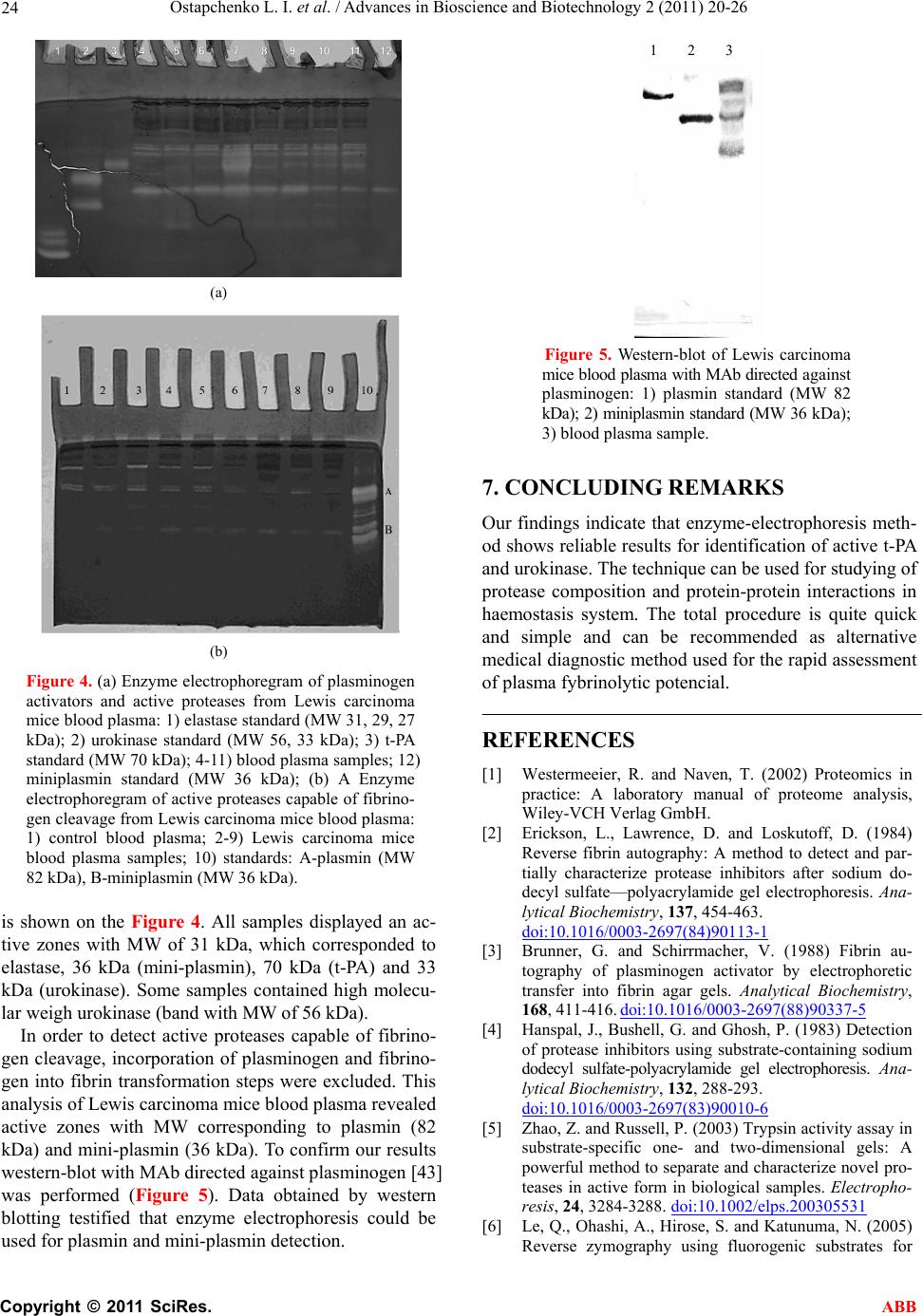 Ostapchenko L. I. et al. / Advances in Bioscience and Biotechnology 2 (2011) 20-26 Copyright © 2011 SciRes. ABB 24 (a) (b) Figure 4. (a) Enzyme electrophoregram of plasminogen activators and active proteases from Lewis carcinoma mice blood plasma: 1) elastase standard (MW 31, 29, 27 kDa); 2) urokinase standard (MW 56, 33 kDa); 3) t-PA standard (MW 70 kDa); 4-11) blood plasma samples; 12) miniplasmin standard (MW 36 kDa); (b) A Enzyme electrophoregram of active proteases capable of fibrino- gen cleavage from Lewis carcinoma mice blood plasma: 1) control blood plasma; 2-9) Lewis carcinoma mice blood plasma samples; 10) standards: A-plasmin (MW 82 kDa), B-miniplasmin (MW 36 kDa). is shown on the Figure 4. All samples displayed an ac- tive zones with MW of 31 kDa, which corresponded to elastase, 36 kDa (mini-plasmin), 70 kDa (t-PA) and 33 kDa (urokinase). Some samples contained high molecu- lar weigh urokinase (band with MW of 56 kDa). In order to detect active proteases capable of fibrino- gen cleavage, incorporation of plasminogen and fibrino- gen into fibrin transformation steps were excluded. This analysis of Lewis carcinoma mice blood plasma revealed active zones with MW corresponding to plasmin (82 kDa) and mini-plasmin (36 kDa). To confirm our results western-blot with MAb directed against plasminogen [43] was performed (Figure 5). Data obtained by western blotting testified that enzyme electrophoresis could be used for plasmin and mini-plasmin detection. Figure 5. Western-blot of Lewis carcinoma mice blood plasma with MAb directed against plasminogen: 1) plasmin standard (MW 82 kDa); 2) miniplasmin standard (MW 36 kDa); 3) blood plasma sample. 7. CONCLUDING REMARKS Our findings indicate that enzyme-electrophoresis meth- od shows reliable results for identification of active t-PA and urokinase. The technique can be used for studying of protease composition and protein-protein interactions in haemostasis system. The total procedure is quite quick and simple and can be recommended as alternative medical diagnostic method used for the rapid assessment of plasma fybrinolytic potencial. REFERENCES [1] Westermeeier, R. and Naven, T. (2002) Proteomics in practice: A laboratory manual of proteome analysis, Wiley-VCH Verlag GmbH. [2] Erickson, L., Lawrence, D. and Loskutoff, D. (1984) Reverse fibrin autography: A method to detect and par- tially characterize protease inhibitors after sodium do- decyl sulfate—polyacrylamide gel electrophoresis. Ana- lytical Biochemistry, 137, 454-463. doi:10.1016/0003-2697(84)90113-1 [3] Brunner, G. and Schirrmacher, V. (1988) Fibrin au- tography of plasminogen activator by electrophoretic transfer into fibrin agar gels. Analytical Biochemistry, 168, 411-416. doi:10.1016/0003-2697(88)90337-5 [4] Hanspal, J., Bushell, G. and Ghosh, P. (1983) Detection of protease inhibitors using substrate-containing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Ana- lytical Biochemistry, 132, 288-293. doi:10.1016/0003-2697(83)90010-6 [5] Zhao, Z. and Russell, P. (2003) Trypsin activity assay in substrate-specific one- and two-dimensional gels: A powerful method to separate and characterize novel pro- teases in active form in biological samples. Electropho- resis, 24, 3284-3288. doi:10.1002/elps.200305531 [6] Le, Q., Ohashi, A., Hirose, S. and Katunuma, N. (2005) Reverse zymography using fluorogenic substrates for 1 2 3  Ostapchenko L. I. et al. / Advances in Bioscience and Biotechnology 2 (2011) 20-26 Copyright © 2011 SciRes. ABB 25 protease inhibitor detection. Electrophoresis, 26, 1038- 1045. doi:10.1002/elps.200306142 [7] Wilkesman, J. and Schroder, H. (2007) Analysis of ser- ine proteases from marine sponges by 2-D zymography. Electrophoresis, 28, 429-436. doi:10.1002/elps.200600332 [8] Le, Q. and Katunuma, N. (2004) Detection of protease inhibitors by a reverse zymography method, performed in a tris(hydroxymethyl)aminomethane-Tricine buffer system. Analytical Biochemistry, 324, 237-240. doi:10.1016/j.ab.2003.09.033 [9] Martinez, M., Newbold, C., Wallace, R. and Movano, F. (2002) Effects of high-molecular-mass substrates on protein migration during sodium dodecyl sulfatepoly- acrylamide gel electrophoresis. Electrophoresis, 23, 1-7. [10] Vazquez, D. and Peyronel, A.C. (1995) A simple and rapid technique for postelectrophoretic detection of pro- teases using azocasein. Electrophoresis, 16, 1894-1897. [11] Fareed, J., Hoppensteadt, D. and Leya, F. (1998) Useful laboratory tests for studying thrombogenesis in acute cardiac syndromes. Clinical Chemistry, 44, 1845-1853. [12] Zawilska, K. (1995) Progress in the detection of intravascu- lar activation of fibrinolysis. Acta haematologica polonica, 26, 33-38. [13] Bu, C., Zhang, C. and Li, Z. (2007) Autoantibodies to plasminogen and tissue plasminogen activator in women with recurrent pregnancy loss. Clinical & Experimental Immunology, 149, 31-39. doi:10.1111/j.1365-2249.2007.03382.x [14] Hoylaerts, M., Rijken, D., Lijnen, H. and Collen, D. (1982) Kinetics of the activation of plasminogen by hu- man tissue plasminogen activator. Role of fibrin. Jour- nal of Biological Chemistry, 257, 2912-2919. [15] Protein Electrophoresis. (1999) Technical manual, Am- ersham Biosciences Inc. [16] Heussen, C. and Dowdle, E. (1980) Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymer- ized substrates. Analytical Biochemistry, 102, 196-202. doi:10.1016/0003-2697(80)90338-3 [17] Harlow, E. and Lane, D. (1988) Antibodies. Cold Spring Harbor Laboratory, New York. [18] Chibber, B., Deutsch, D. and Mertz, E. (1974) Affinity chromatography of plasminogen. Methods in Enzymol- ogy, 34, 424-432. [19] Wiman, B. and Wallen, P. (1973) Activation of human plasminogen by an insoluble derivative of urokinase. Structural changes of plasminogen in the course of acti- vation to plasmin and demonstration of a possible inter- mediate compound. European Journal of Biochemistry, 36, 25-31. doi:10.1111/j.1432-1033.1973.tb02880.x [20] March, S., Parikh, I. and Cuatrecasas, P. (1974) A simpli- fied method for cyanogen bromide activation of agarose for affinity chromatography. Analytical Biochemistry, 60, 149-152. doi:10.1016/0003-2697(74)90139-0 [21] Krasnobryzha, E., Savchuk, O. and Volkov, G. (2004) Study of streptokinase influence on the haemostasis sys- tem parameters in model systems in vivo. Ukrainian Biochemical Journal, 76, 56-61. [22] Savchuk, O., Levkin, M., Karbovskyy, V., Gornitskaya, O., Volkov, G. and Tseren, B. (2006) Plasminogen acti- vatof from Agkistrodon halys halys venom. Ukrainian Biochemical Journal, 78, 32-37. [23] Platonova, T., Gornitskaya, O., Metelitsina, I. and Savchuk, O. (2001) Components of blood clotting sys- tem and fibrinolytic system in subretinal fluid at regma- togenic retina exfoliation. Medichna Chimia, 3, 5-8. [24] Petik, A., Platonova, T. and Savchuk, O. (2001) Indexes of haemostasis system during Lewis carcinoma growth. Experimental Oncology, 23, 73-75. [25] Zhukova, A., Krasnobrysha, I. Savchuk, O. and Volkov, G. (2009) The investigation of protein-protein interac- tions in haemostasis system using enzyme electrophore- sis method. XXII Congress of the International Society of Thrombosis and Haemostasis. Journal of Thrombosis and Haemostasis, 7, Boston, USA, 367. [26] Bruhn, H., Conard, J., Mannucci, M., Monteagudo, J., Pelzer, H., Reverter, J., Samama, M., Tripodi, A. and Wagner, C. (1992) Multicentric evaluation of a new as- say for prothrombin fragment F1 + 2 determination. Thromb Haemost, 68, 413-417. [27] Ferlito, S., Gallina, M., Mangiameli, S. and Chiaranda, G. (1995) Thrombotic markers during myocardial in- farctionPanminerva. Panminerva Medica, 37, 133-136. [28] Granger, C., Becker, R., Tracy, R., Califf, R., Topol, E., Pieper, K., Ross, A., Roth, S., Lambrew, C. and Bovill, E. (1998) Thrombin generation, inhibition and clinical outcomes in patients with acute myocardial infarction treated with thrombolytic therapy and heparin: Results from the GUSTO-I Trial. GUSTO-I Hemostasis Substudy Group. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. Journal of the American College of Cardiology, 31, 497-505. doi:10.1016/S0735-1097(97)00539-1 [29] Johns, J., Gold, H., Leinbach, R., Yasuda, T., Gimple, L., Werner, W., Finkelstein, D., Newell, J., Ziskind, A. and Collen, D. (1988) Prevention of coronary artery reocclu- sion and reduction in late coronary artery stenosis after thrombolytic therapy in patients with acute myocardial infarction. A randomized study of maintenance infusion of recombinant human tissue-type plasminogen activator. Circulation, 78, 546-556. [30] Nilsen, D., Goransson, L., Larsen, A., Hetland, O. and Kierulf, P. (1997) Systemic thrombin generation and ac- tivity resistant to low molecular weight heparin admin- istered prior to streptokinase in patients with acute myocardial infarction. Tromb Haemost, 77, 57-61. [31] Chandler, W. and Stratton, J. (1994) Laboratory evalua- tion of fibrinolysis in patients with a history of myocar- dial infarction. American Journal of Clinical Pathology, 102, 248-252. [32] Genser, N., Lechleitner, P., Maier, J., Dienstl, F., Artner-Dworzak, E., Puschendorf, B. and Mair, J. (1998) Rebound increase of plasminogen activator inhibitor type I after cessation of thrombolytic treatment for acute myocardial infarction is independent of type of plasmi- nogen activator used. Clinical Chemistry, 44, 209-214. [33] Yamada, S., Yamada, R., Ishii, A., Ashikawa, K., Kawamitsu, H. and Fujita, K. (1996) Evaluation of tis- sue plasminogen activator and plasminogen activator in- hibitor-I levels in acute myocardial infarction. Journal of Cardiology, 27, 171-178.  Ostapchenko L. I. et al. / Advances in Bioscience and Biotechnology 2 (2011) 20-26 Copyright © 2011 SciRes. ABB 26 [34] Markland, F. (1998) Snake venoms and the hemostatic system. Toxicon, 36, 1749-1800. doi:10.1016/S0041-0101(98)00126-3 [35] Immonen, I., Konttinen, Y., Sorsa, T., Tommila, P. and Sirén, V. (1996) Proteinases in subretinal fluid. Graefes Archive for Clinical and Experimental Ophthalmology, 234, 105-109. doi:10.1007/BF00695249 [36] O’Reilly, M. (1997) Angiostatin: An endogenous in- hibitor of angiogenesis and of tumor growth. EXS, 79, 273-294. [37] Geiger, J. and Cnudde, S. (2004) What the structure of angiostatin may tell us about its mechanism of action. Thromb Haemost, 2, 23-34. doi:10.1111/j.1538-7836.2004.00544.x [38] Chen, Y., Wu, H., Li, C., Huang, Y., Chiang, C., Wu, M. and Wu, L. (2006) Anti-angiogenesis mediated by an- giostatin K1-3, K1-4 and K1-4.5. Involvement of p53, FasL, AKT and mRNA deregulation. Thromb Haemost, 95, 668-677. [39] Wang, H., Doll, J., Jiang, K., Cundiff, D., Czarnecki, J., Wilson, M., Ridge, K. and Soff, G. (2006) Differential binding of plasminogen, plasmin, and angiostatin 4.5 to cell surface beta-actin: Implications for cancer-mediated angiogenesis. Cancer Research, 66, 7211-7215. doi:10.1158/0008-5472.CAN-05-4331 [40] Kastrikina, T., Taran, L. and Kudinov, S. (1986) Kinetic characteristics of fibrinogen and fibrin hydrolysis by plasmin 1 and 2 and miniplasmin. Thromb Research, 41, 681-688. doi:10.1016/0049-3848(86)90365-8 [41] Kolev, K., Komorowicz, E., Owen, W. and Machovich, R. (1996) Quantitative comparison of fibrin degradation with plasmin, miniplasmin, neurophil leukocyte elastase and cathepsin G. Thromb Haemost, 75, 140-146. [42] Kolev, K., Léránt, I., Tenekejiev, K. and Machovich, R. (1994) Regulation of fibrinolytic activity of neutrophil leukocyte elastase, plasmin, and miniplasmin by plasma protease inhibitors. Journal of Biological Chemistry, 269, 17030-17034. [43] Ponting, C., Marshall, J. and Cederholm-Williams, S. (1992) Plasminogen: A structural review. Blood Coagu- lation & Fibrinolysis, 3, 605-614. |

