Paper Menu >>
Journal Menu >>
 Vol.2, No.1, 37-48 (2011) Journal of Biophysical Chemistry doi:10.4236/jbpc.2011.21006 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ Peptoids with aliphatic sidechains as helical structures without hydrogen bonds and collagen/ inverse-collagen type structures Fateh S. Nandel, Avneet Saini Department of Biophysics, Panjab University, Chandigarh, India; fateh_nandel@yahoo.com Received 29 September 2010; revised 30 October 2010; accepted 12 November 2010. ABSTRACT Aliphatic homo-polypeptoids of NAla, NVal, NIle and NLeu both in the presence and absence of protecting groups adopt helical structures without hydrogen bonds with Φ, Ψ values of ~ 0, ± 90° with trans amide bonds. These structures are stabilized by carbonyl-carbonyl interactions and characterized by ~ 3.16 residues per turn with a pitch of ~ 6.13 Å. It has been shown that like polyvaline and polyleucine peptides, poly- peptoids can also be exploited for the con- struction of potential surfactant like molecules by incorporating charged amino acid residues at the N terminal. A single-handed template with Φ, Ψ values of ~ 0, 90˚ can be attained by in- corporating L-leu or L-val at the C-terminal of poly-NIle. Analysis of the simulation results in water as a function of time reveals that the opening of helical structures without hydrogen bonds takes place at sub-picosecond time scale starting from the N-terminal. This leads to the formation of collagen or inverse-collagen type structures (Φ, Ψ ~ -60, 145˚ and 60, -145˚ re- spectively) stabilized by interactions of water molecules with the backbone carbonyl groups. Keywords: Peptoids; Conformation; Hel i cal Structure without Hydrogen Bonds; Collagen and Inverse-Co llagen Type Structures 1. INTRODUCTION Design of polymers and oligomers that mimic com- plex structures and biological properties of proteins is an important endeavor with both fundamental and practical implications as polypeptides themselves are generally poor drugs, due to their in vivo rapid degradation by proteases and their immunogenic character [1,2]. To ad- dress multiple design criteria for applications ranging from medicinal chemistry to material science, focus is to identify non-natural chemical scaffolds that recapitulate the desirable attributes of polypeptides i.e. peptidomi- metics. These should have good solubility in aqueous solution, access to facile sequence specific assembly of monomers containing chemically diverse side chains and capacity to form stable structures. ‘Peptoids’ offer attractive peptidomimetics [2] as their backbone is similar to that of peptides. Shifting of the side chain to the main chain nitrogen atom, shown in Figure 1, renders the alpha carbon achiral. Peptoid mo- nomers are linked through polyimide bonds and lack the amide hydrogen; precluding the formation of hydrogen bond networks that stabilize peptide secondary structures. Their conformational behavior can be related to both proline and glycine residues. These modifications be- stow protease resistance [3], while allowing suitable mimicry of the spacing between the critical chemical functionalities of bioactive peptides. Thus, it remains to be an area of active research for conformational investi- gations, analysis of interactions and hence, designing of molecules. In addition, the efficient sub-monomer solid phase synthesis gave a major breakthrough in peptoid research, due to the access to a large number of diverse primary amines that can be added and that too at low costs [4]. Consequently, peptoids have found application as: i) Figure 1. Primary structure of peptoid.  F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 38 attractive targets for the drug discovery process [5], ii) motif for combinatorial strategies [5-10], and iii) to de- velop peptide mimics for biomedical applications [11-17]. They have also been exploited for their cell pe- netrating properties [18] and antifouling action on sur- faces [19,20]. New approaches to direct the peptoid backbone towards formation of specific secondary structures such as helices (a structure in which residues rotate and rise in a repeating manner along an axis, but of what type?) are being explored for the discovery of bioactive peptoid modules [21-26]. In spite of their various applications limited system- atic structural/conformational data is available on pep- toids [27-29]. Peptoids comprising of 100% achiral, aromatic NPhe side chains displayed no net CD. It is not clear whether the no net CD signal is a result of degen- erate conformations of opposite handedness or other structures adopted by the peptoid. It may be mentioned that even systems having no chiral centers can adopt well defined helical structures. Peptoids containing chiral aromatic side chain i.e. NSpe is strongly reminis- cent of the alpha-helical signal on the basis of CD signa- tures that are almost similar to that of α-helices in pep- tides [30,31]. On the other hand NMR and CD studies on peptoids containing α-chiral aliphatic side chains have shown features similar to those of polyproline type heli- ces [28]. On the basis of CD spectroscopic results it is even proposed that α-chiral aliphatic and α-chiral aro- matic sidechains form helices of essentially the same type. Thus, the conformational behavior of peptoids and the secondary structure adopted by them remains an active area of research. In this study we report the conforma- tional preferences of both homo- and hetero-polypepto- ids of the type; Ac-(NXaa)n-NMe2 of varying chain length where ‘NXaa’ is a peptoid residue with the amino acid side chain attached to the amide nitrogen atom by keeping the amide bond geometry both as trans or cis. The conformational results thus obtained, shall aid in the construction and design of peptoid based biomimetics. 2. METHODOLOGY Knowledge about the global, local and low energy minima for the peptoid models Ac-NXaa-NMe2 was obtained from the Φ, Ψ maps and potential energy curves that were constructed using standard bond lengths and bond angles. Input for peptoids was given in terms of internal co-ordinates i.e. bond lengths, torsion angles and connectivity of the atoms. Energy calculations were carried out using the semi-empirical quantum mechani- cal method PCILO (Pertubative Configuration Interac- tion using Localised molecular Orbitals) [32] and mini- mization was done by the variation of torsion angles. The various conformational states of all constructs were generated based on the global, local and low energy mi- nima in the Φ, Ψ maps and i, j curves/maps of model peptoids and their energies computed. Minimization was further refined by varying Φ, Ψ, ω and values in the neighborhood of the minima in steps of 5/2 degrees. Minima obtained by PCILO calculations are also the minima at the ab initio level for the usual amino acids [33] and for dehydroamino acids [34-36]. In addition the PCILO results [37,38] for the peptides containing usual and unusual amino acids are in conformity with ab initio results [39,40] and knowledge based crystallographic data [41,42]. The charges on various atoms in different conformations of peptoid models were computed using the GRINDOL method [43]. Molecular Dynamic (MD) simulations provide great deal of information regarding the stability of peptoids in water and to the mobility of the peptoid residues. The results obtained by quantum mechanics calculations were used as the starting geometries for simulation stud- ies using GROMACS 3.3.1 MD software package [44]. The Dundee-PRODRG2 [45] server was used to obtain the GROMACS topology and coordinate files. Interac- tion parameters within the design sequence were taken from GROMOS-96 force field G43a1 [46]. It is worth mentioning that the simulation results obtained by GROMOS force field are in good agreement with the experimental results [29,47 and 48]. Energy of the sys- tem was minimized by the steepest descent method, us- ing the convergence criteria of 50 kJ mol-1 followed by conjugate gradient method with a force constant of 20 kJ mol-1. Next, the MD run was carried out in vacuum for 20 ns, with a time step of 2 fs using the Leap Frog Algo- rithm. The temperature was controlled through weak coupling to a bath of constant temperature [49], using a coupling time; τp of 0.1ps and a reference temperature; T0 of 300 K. LINCS algorithm [50] was used to restrict all bonds to their equilibrium lengths and the center of mass motion of the system was removed every step to maintain the effective simulation temperature at 300 K. For the evaluation of coulomb interactions and Van der Waals interaction a cut off of 0.9 and 1.0 nm respec- tively was applied. Long range forces were updated every 10 fs during generation of the neighbor list. The Long Range Electrostatic Interactions were calculated using a Particle Mesh Ewald Summation. Initial veloci- ties of all atoms were taken from a Maxwellian distribu- tion at the desired initial temperature. After the vacuum MD simulation a simple cubic periodic box was set up using the Simple Point Charge (SPC) Water Model [51]. In order to allow equilibration of solvent around the model sequence, position of all peptoid residues was restrained for 20 ps. Finally, MD simulation for 1ns at 300 K, without any restrictions was carried out. The  F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 39 Table 1. Conformational results* of the various dipeptoid models. Φ, ψ, ω ΔE Φ, ψ, ω ΔE Φ, ψ, ω ΔE Φ, ψ, ω ΔE χi, χj (deg) kcal/mol χi, χj (deg) kcal/mol χi, χj (deg) kcal/mol χi, χj (deg) kcal/mol Ac-NAla-NMe2 Ac-NVal-NMe2 –2, 92, 180 0 –175, 85, 0 1.8 0, –85, 174 0 100, 135, –3 –1 175 75 –5, –80, 180 0 180, –95, 0 3.7 0, 90, 174 0.4 –70, 170, –10 0.7 175 120 180, –90, 180 1.8 –95, –170, 175 1.5 135 180, 90, 180 1.9 85, 175, 170 2.1 110 120, 180, 180 2 –120, 180, 180 2.4 Ac-NLeu-NMe2 Ac-NIle-NMe2 15, –105, 180 0 –125, –170, 03.2 5, 85, 180 0 –95, –155, 0 2.5 130, 175 –65, 175 145, 170 155, 180 15, 70, 178 1.5 120, –175, 03.5 0, –90, 180 0.3 90, 180, 0 5.2 130, 175 65, 70 145, 170 155, 180 120, 180, 180 2.6 –60, 175, –54 –90, 180, 180 2.9 –110, 55 115, 180 145, 170 –120, 180, 178 2.9 55, –160, 8 4.2 90, 180, 180 4 –5, –60 –115, 60 145, 170 * Φ, ψ values are in bold, ω in italics and χi, χj in normal text. pressure was controlled using weak coupling with a time constant of 0.5 ps and a reference pressure of 1 Bar. 3. RESULTS AND DISCUSSION Conformational results in terms of Φ, Ψ, ω and χ val- ues of the various model dipeptoids in both cis and trans amide bond geometries are summarized in Table 1. In general, there is not much difference in the energy of the various conformers and hence, with a change in experi- mental conditions different states may be populated. Results in Table 1 also reveal that NAla can be popu- lated in several conformations due to its simple and small side chain with no branching. Thus, incorporation of NAla in peptides may lead to disruption of helices as the energy difference between the states is small and different states may be populated depending on the en- vironment. This observation is consistent with the ex- perimental finding that incorporation of NAla in the oth- erwise helical antimicrobial peptides decreases the heli- cal content [52]. In higher mers of all peptoid models it is apparent from the results in Table 2 that degenerate helical struc- tures without hydrogen bonds with Φ, Ψ values of ~ 0, + 90˚ are most stable with trans amide bond geometry. These structures characterized by n = 3.16, with a pitch of 6.13 Å have also been reported in poly-dehydro ami- no acids [36]. A molecular view of the models Ac-NVal7- NMe2 and Ac-NIle7-NMe2 shown in Figure 2 with trans amide bonds for the conformational states with Φ, Ψ values ~ 0, -90˚ and 0, 90˚ respectively clearly shows pore formation with an average diameter of 4.6 Å with hydrophobic side chains protruding outwards. Such structures are maximally stable in the hydrophobic en- vironment masking the carbonyl oxygens that point in- wards towards the central cavity. Negative charge on carbonyl oxygen (~ -0.66) may provide a negative po- tential gradient along the pore facilitating the cation to 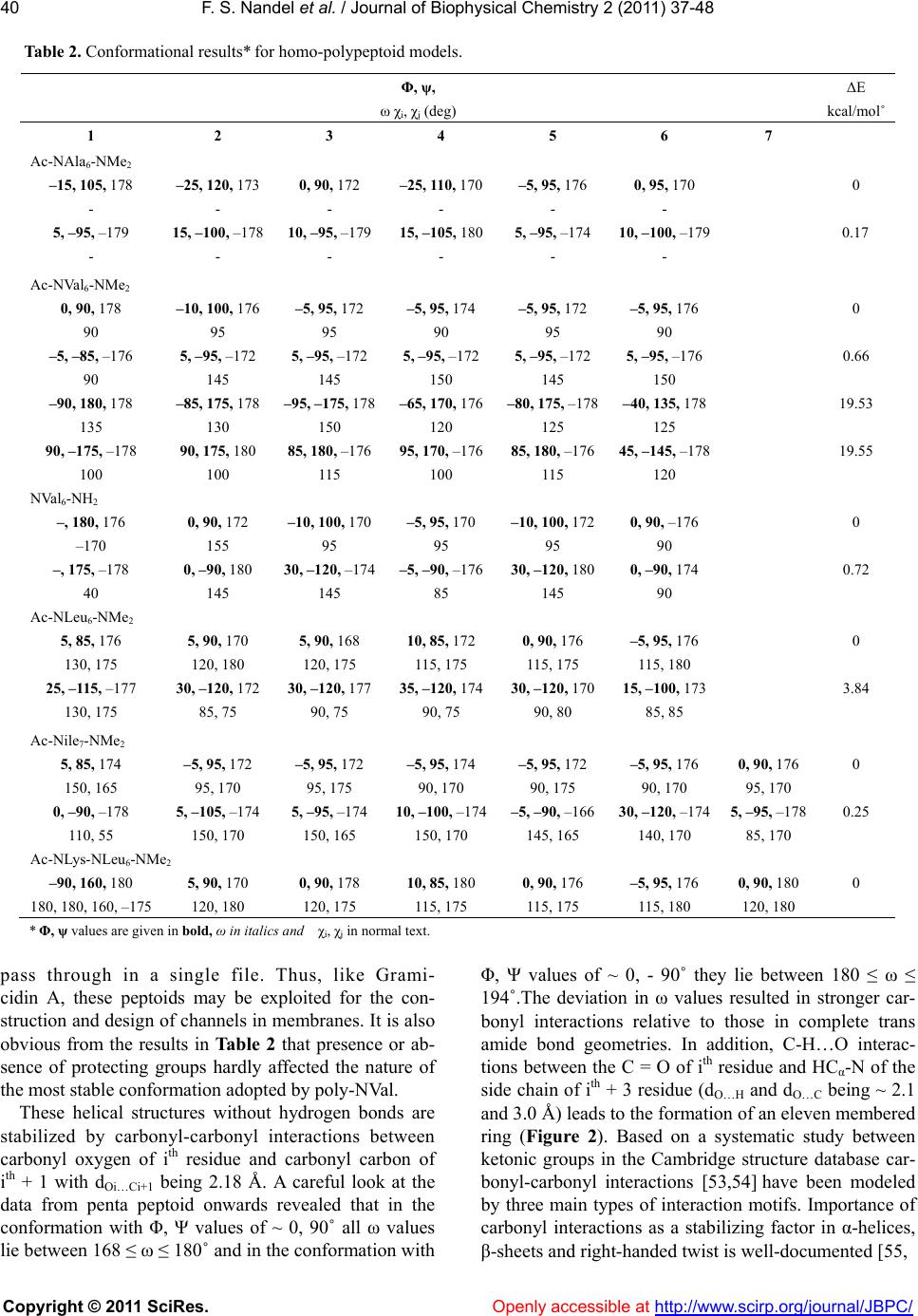 F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 40 Table 2. Conformational results* for homo-polypeptoid models. ΔE Φ, ψ, ω χi, χj (deg) kcal/mol˚ 1 2 3 4 5 6 7 Ac-NAla6-NMe2 –15, 105, 178 –25, 120, 173 0, 90, 172 –25, 110, 170 –5, 95, 176 0, 95, 170 0 - - - - - - 5, –95, –179 15, –100, –178 10, –95, –179 15, –105, 180 5, –95, –174 10, –100, –179 0.17 - - - - - - Ac-NVal6-NMe2 0, 90, 178 –10, 100, 176 –5, 95, 172 –5, 95, 174 –5, 95, 172 –5, 95, 176 0 90 95 95 90 95 90 –5, –85, –176 5, –95, –172 5, –95, –172 5, –95, –172 5, –95, –172 5, –95, –176 0.66 90 145 145 150 145 150 –90, 180, 178 –85, 175, 178 –95, –175, 178 –65, 170, 176 –80, 175, –178 –40, 135, 178 19.53 135 130 150 120 125 125 90, –175, –178 90, 175, 180 85, 180, –176 95, 170, –176 85, 180, –176 45, –145, –178 19.55 100 100 115 100 115 120 NVal6-NH2 –, 180, 176 0, 90, 172 –10, 100, 170–5, 95, 170 –10, 100, 172 0, 90, –176 0 –170 155 95 95 95 90 –, 175, –178 0, –90, 180 30, –120, –174–5, –90, –17630, –120, 1800, –90, 174 0.72 40 145 145 85 145 90 Ac-NLeu6-NMe2 5, 85, 176 5, 90, 170 5, 90, 168 10, 85, 172 0, 90, 176 –5, 95, 176 0 130, 175 120, 180 120, 175 115, 175 115, 175 115, 180 25, –115, –177 30, –120, 172 30, –120, 17735, –120, 174 30, –120, 170 15, –100, 173 3.84 130, 175 85, 75 90, 75 90, 75 90, 80 85, 85 Ac-Nile7-NMe2 5, 85, 174 –5, 95, 172 –5, 95, 172 –5, 95, 174 –5, 95, 172 –5, 95, 176 0, 90, 176 0 150, 165 95, 170 95, 175 90, 170 90, 175 90, 170 95, 170 0, –90, –178 5, –105, –174 5, –95, –174 10, –100, –174 –5, –90, –16630, –120, –174 5, –95, –178 0.25 110, 55 150, 170 150, 165 150, 170 145, 165 140, 170 85, 170 Ac-NLys-NLeu6-NMe2 –90, 160, 180 5, 90, 170 0, 90, 178 10, 85, 180 0, 90, 176 –5, 95, 176 0, 90, 180 0 180, 180, 160, –175 120, 180 120, 175 115, 175 115, 175 115, 180 120, 180 * Φ, ψ values are given in bold, ω in italics and χi, χj in normal text. pass through in a single file. Thus, like Grami- cidin A, these peptoids may be exploited for the con- struction and design of channels in membranes. It is also obvious from the results in Table 2 that presence or ab- sence of protecting groups hardly affected the nature of the most stable conformation adopted by poly-NVal. These helical structures without hydrogen bonds are stabilized by carbonyl-carbonyl interactions between carbonyl oxygen of ith residue and carbonyl carbon of ith + 1 with dOi…Ci+1 being 2.18 Å. A careful look at the data from penta peptoid onwards revealed that in the conformation with Φ, Ψ values of ~ 0, 90˚ all ω values lie between 168 ≤ ω ≤ 180˚ and in the conformation with Φ, Ψ values of ~ 0, - 90˚ they lie between 180 ≤ ω ≤ 194˚.The deviation in ω values resulted in stronger car- bonyl interactions relative to those in complete trans amide bond geometries. In addition, C-H…O interac- tions between the C = O of ith residue and HCα-N of the side chain of ith + 3 residue (dO…H and dO…C being ~ 2.1 and 3.0 Å) leads to the formation of an eleven membered ring (Figure 2). Based on a systematic study between ketonic groups in the Cambridge structure database car- bonyl-carbonyl interactions [53,54] have been modeled by three main types of interaction motifs. Importance of carbonyl interactions as a stabilizing factor in α-helices, β-sheets and right-handed twist is well-documented [55,  F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 41 Figure 2. Molecular view of (a) Ac-NVal7-NMe2 in the con- formation state with Φ, Ψ, ω values of ~ 0, -90, 180˚depicting carbonyl-carbonyl interactions and formation of an eleven member ring due to C-H…O interactions between the ith and ith + 3 residue; (b) Ac-NIle7-NMe2 with Φ, Ψ , ω values of ~ 0, 90, 180˚showing the formation of a hydrophilic pore with the masking of the carbonyl groups by the hydrophobic side chains. 56]. They also stabilize the partially allowed Ramachandran conformations of aspartic acid and asparagines [55] and helical structures without hydrogen bonds in peptides con- structed from achiral and unusual amino acids [36,58-59]. Degenerate helical structures without hydrogen bonds with opposite handedness observed in polypeptoid mod- els of NVal/NIle having branching at the N-Cα position implies population of both states to the same extent and hence, very well explains the very weak/no signal in CD spectroscopy for aliphatic peptoids [28]. Interestingly, for poly-NLeu only one state with Φ, Ψ values of ~ 0, 90 ° is predicted; possibly due to the side chain branching at the N-Cβ position. Thus, the difference in the branching pattern changes the local environment and may be re- sponsible for the difference in the conformational be- havior of peptoid models. Φ, Ψ values of 0, 90˚ appear to be unusual even in peptides, but this region has been predicted a minima for some amino acids [36]. Also, for the dipeptides of glycine & alanine [60] a stationary point near Φ = 0˚ , Ψ = 90˚ at the HF/3.21G and HF/6.31+G levels has been reported. Ramachandran plots based on; i) PDB- 40 dataset [61] corresponding to X-ray protein structures with the resolu- tion of 2.5 Å or better for 470 proteins, 95778 total resi- dues plotted, proline and glycine excluded and (ii) NMR derived structure for 113 proteins, 84719 total residues plotted, (proline and glycine excluded) show appreciable density between the left-handed helical region and the collagen-type structural region [62,63] and between the right-handed helical region and the inverse-collagen type structural region [60,61]. Though, the dataset for the NMR derived structures is small compared to the X-ray protein structures, yet in the mentioned regions the data point density is more for NMR derived structures i.e. in the solution phase. Thus, the conformational states with Φ, Ψ values of ~ 0, 90˚ are not an over-estimation and may be realized in solvents with low polarity. 4. CONSTRUCTION AND DESIGN OF NANO-STRUCTURES Single Handed Template: Alipihatic polypeptoids were realized in degenerate helical structures without hydrogen bonds with rise & rotation per residue of 1.94 Å and 114˚ respectively. To construct a template with a given handedness from these peptoid residues, either L/D-Leu or L/D-Val residue was incorporated either at the N-terminal or the C-terminal of the poly Nile/Nval peptoid sequences of required length. Incorporation of such chiral amino acids has been shown to control the screw sense of helical peptides constructed from achiral residues [36,58,62-63]. Therefore, these amino acids (L/D-Val or L/D-Leu having branching in the side chain) were incorporated in achiral peptoids to control the screw sense of helical structures. The conformational results thus obtained are given in Table 3 and it is obvious that in poly-Nile peptoid models the degeneracy was lifted and only one conformation with Φ, Ψ values of ~ 0, 90˚ was realized by incorporating L-leu or L-val at the C-terminal. Incorporation of L or D-leu either at the N or C-terminal of poly-NVal sequence models did not lift the degeneracy. This may be attributed to the local environ- ment around N-Cα, which is asymmetrical in NIle resi- dues and symmetrical in NVal residues. Thus, NIle with its asymmetrical side chain plays a vital role in lifting the degeneracy and hence, construction of a single handed template. Peptoids as Surfactants: The helical structures adopted by homo-polypeptoid models (NVal, NLeu, NIle) appear similar (although with different Φ, Ψ values) to the corresponding helical structures in polypeptides of leucine, valine, isoleucine and norleucine with the non-polar side chains projecting outwards forming well-defined hydrophobic structures with a hydrophilic pore. Poly-leucine, poly-valine, poly-isoleucine and poly-norleucine like peptides have shown surface activ- ity that helped in accelerating the surface spreading at the air-water interface and thus, exhibited improved dy- namic activity. Poly-leucine peptides or peptides rich in leucine have been exploited for their surfactant like properties especially in lung surfactant proteins SP-B  F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 42 Table 3. Construction of a single handed template for Ac-L-leu-(NIle)6-NMe2. Φ, ψ, ω χi, χj (deg) ΔE kcal/mol 1 2 3 4 5 6 7 Ac-L-leu-(NIle)6-NMe2 –100, 130, –92 10, 80, 176 0, 95, 170 –5, 95, 172 –5, 100, 172 –5, 95, 172 0, 90, 174 0 –55, 170 155, 170 90, 175 95, 175 95, 175 95, 170 90, 175 –155, 150, 90 0, –90, –178 –5, –90, –172 5, –95, –176 25, –120, –174 0, –90, –176 0, –90, –178 1.99 55, 150 150, 170 155, 160 150, 170 150, 170 90, 170 150, 170 Ac-(NIle)6-L-leu-NMe2 15, 90, 90 0, 95, 170 0, 95, 170 –15, 100, 1760, 90, 172 15, 70, 178 –20, 115, 174 0.68 140, 170 100, 170 95, 170 95, 165 90, 170 90, 175 –65, 170 –10, –80, –86 10, –100, –178 0, –95, –170 25, –120, –1760, –90, –176 –5, –75, 176 180, 160, –178 7.00 130, 50 145, 175 150, 165 140, 175 90, 170 155, 170 60, 140 Ac-(NIle)6-L-val- NMe2 5, 85, 178 –5, 100, 170 5, 90, 166 –15, 100, 176 0, 90, 172 5, 75, –176 –10, 105, 174 0 145, 170 90, 170 100, 165 90, 160 90, 170 90, 170 180 –5, –85, –178 15, –105, –176 0, –95, –166 0, –95, –170 0, –90, –172 –5, –75, 176 –30, 115, –170 3.56 115, 60 145, 170 150, 170 150, 160 150, 170 155, 170 50 and SP-C [13,14]. On similar lines the corresponding peptoid mimics are constructed to design surfactant like molecules by incorporation of NLys residue at the N-terminal in poly-NLeu and the conformational results are summarized in Table 2. A graphical view of the peptoid in the most stable state as shown in Figure 3 clearly depicts that NLys residue resembles the polar head group and the NLeu residues formed the hydro- phobic tail like structure similar to that of lipids. 5. SIMULATION STUDIES Simulation studies on aliphatic peptoids reveals almost Figure 3. A graphical view of the surfactant peptoid design ‘Ac-NLys-(NLeu)6-NMe2’. similar conformational results irrespective of the initial geometry taken for simulations (i.e. with Φ, Ψ = 0, 90˚ ; 60, 180˚ ; or 120, 180˚ ) and the side chain. This means shifting of the side chain from the carbon alpha to the amide nitrogen makes the peptoid backbone less flexible and this observation is consistent with the experimental and computational results [29,64-65]. A molecular view of the model Ac-NVal7-NMe2 after 1ns simulation in water (with starting geometry having Φ, Ψ values of ~ 0, 90˚ and ~ 0, - 90˚ ) is shown in Fig- ure 4. It is apparent from the results in Table 4 and the molecular graphics shown in Figures 4 & 5; that the interactions of water molecules with the carbonyl oxy- gen of the backbone lead to the population of structures with average Φ, Ψ values around -60, 145˚ and 60, -145˚. The conformation with Φ, Ψ values of ~ -60, 145˚ is called collagen type structure [66] and the structure with inverse Φ, Ψ values of collagen type structure i.e. 60, -145˚ is named as inverse-collagen type structure. Col- lagen type structures are also well documented in globu- lar proteins [67,41]. The distance between the carbonyl oxygen of the peptoid backbone and hydrogen atoms of water molecules are found to lie between 1.5 to 1.9 Å. The angle/OHO(C) are observed to lie in the range from 150 to 180˚. This observation is consistent with the ex- perimental finding that the hydrogen bond angle in bio- logical systems is never 180˚ but less [68]. In QM cal- culations both collagen and inverse-collagen type struc- tures are predicted to be higher in energy (Table 4). Helical structures with Φ = ± 79.2˚, Ψ = ± 174.6˚ with  F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 43 Table 4. Torsion angles for the peptoids in trans amide bond geometry after 1ns simulation with different starting geometries having 1) Φ, Ψ values of ~ 0˚, - 90˚and 2) Φ, Ψ values of ~ 0˚, 90˚. Φ ψ ω χi, χj (deg) Φ ψ ω χi, χj (deg) Ac-NAla7-NMe2 Ac-NVal7-NMe2 1 72.3 –159.2 178.2 1 56.3 ﹣131.9–163.0 108.1 107.7 –153.3 175.2 61.0 ﹣143.0–170.8 121.6 70.5 –113.8 157.7 79.6 ﹣137.7 158.4 138.0 55.9 –120.1 175.3 63.4 ﹣122.6–174.7 126.7 57.7 –128.4 177.2 64.9 ﹣125.6 177.1 122.3 92.4 –137.1 159.9 77.5 ﹣151.2–175.1 122.7 72.9 –138.5 179.3 –61.4 99.0 –172.5 113.3 2 –63.8 148.8 –162.6 2 –49.7 113.9 178.3 –104.2 –79.4 134.9 –174.4 –59.4 127.7 –174.0 –118.8 –117.7 148.1 –173.6 –66.4 153.8 166.6 –123.4 –83.5 –146.0 163.5 –58.5 128.7 168.8 –116.0 –90.5 –164.7 163.8 –75.4 125.9 –179.3 –120.9 –104.3 –156.7 160.2 –44.9 124.1 –171.8 –121.4 70.4 –117.5 157.2 –76.1 145.4 175.1 –104.6 Ac-NLeu7-NMe2 Ac-NIle7-NMe2 1 79.8 166.6 –169.6 95.5, –88.4 1 65.3 –154.6 –176.1 103.6, 69.4 46.8 –117.7 –176.2 –90.5, 148.2 57.8 –127.4 –162.7 112.6, 71.6 65.6 –162.9 –165.7 –145.6, –157.4 78.6 –155.4 –175.3 103.0, 110.6 81.1 –107.2 168.0 105.1, –79.0 64.3 –142.8 –189.6 124.8, 87.6 61.4 –108.2 179.6 –110.7, –162.9 65.9 –135.4 177.8 97.3, 87.4 55.3 –136.2 172.4 –97.3, 78.7 89.4 169.3 –168.7 102.9, 143.5 94.1 –152.8 163.2 131.6, –84.2 66.8 –120.6 –175.0 108.0, 76.5 2 –68.2 133.9 –170.5 113.5, –62.1 2 –87.8 –169.7 167.9 109.8, 87.9 –67.9 145.6 179.5 118.1, –83.6 –61.6 110.4 177.6 131.1, 152.9 –57.3 123.4 –176.0 119.7, –85.9 –75.1 146.8 –176.1 85.9, 153.6 –72.4 129.6 –160.9 85.1, –117.3 –61.8 152.9 174.1 123.7, –174.6 –64.6 142.5 –170.3 107.9, –70.7 –64.5 145.7 174.3 109.4, 95.6 –76.3 157.2 179.4 –118.9, –98.8 –50.8 135.3 –174.1 110.6, 76.9 –52.5 152.8 –174.9 115.5, –84.1 –67.0 137.1 176.5 104.9, 95.7 Table 5. Torsion angles for the model Ac-NVal7-NMe2 at different simulation time in water with starting conformation of Φ, Ψ, ω ~ 0˚, -90˚, 180˚. Φ, Ψ Residue number → Time/ ps ↓ 1 2 3 4 5 6 7 0.5 57.7, –149.1 68.8, –134.7 23.2, –88.7 12.8, –100.5 36.6, –112.7 17.4, –110.4 –20.9, –67.1 1.0 70.0, –152.6 62.5, –141.5 53.5, –128.9 43.2, –103.7 20.2, –96.2 68.4, –134.6 –27.7, –70.9 5.0 51.6, –137.2 70.8, 178.9 67.6, –125.4 66.5, –119.5 37.3, –100.6 62.9, –126.4 –30.2, –73.7 10.0 49.9, –123.8 73.2, 178.0 63.1, –140.2 46.8, –120.1 48.1, –133.7 67.0, –129.7 18.7, –100.6 1000.0 58.3, –131.9 61.0, –143.0 79.6, –137.7 63.4, –122.6 64.9, –125.6 77.5, –151.2 –61.4, 99.0 trans amide bond geometry for sarcosine have also been reported by ab initio calculations using dielectric con- stants to replicate an aqueous environment [69] but not in explicit solvent. Our results are also consistent with the peptoid backbone conformational landscape de- scribed by Butterfoss et al. using ab initio Gaussian03 package [29]. Opening of Helical Structure without hydrogen bonds: To gain insight into the opening of the helical structures, whether opening starts from the N or C-terminal 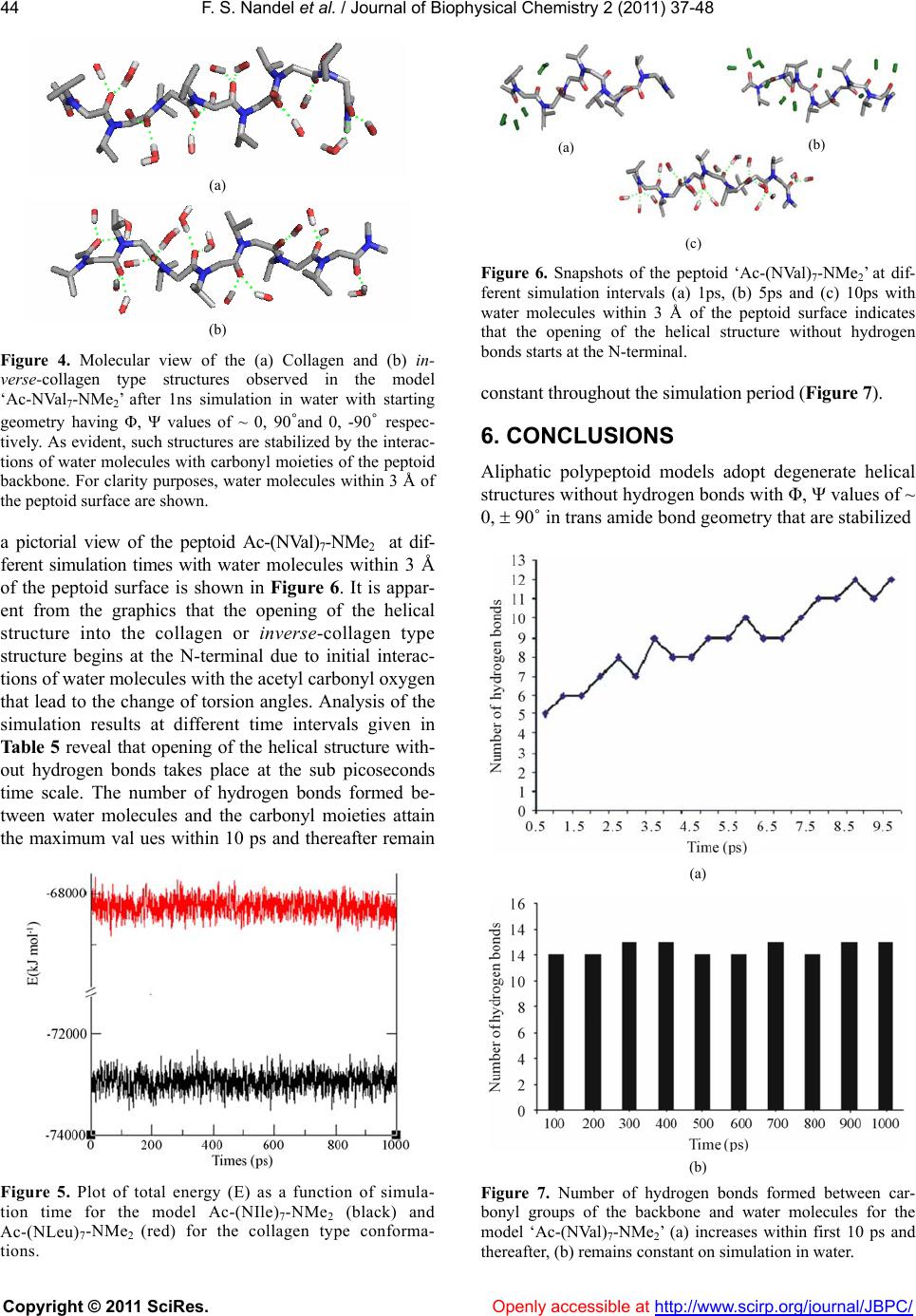 F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 44 (a) (b) Figure 4. Molecular view of the (a) Collagen and (b) in- verse-collagen type structures observed in the model ‘Ac-NVal7-NMe2’ after 1ns simulation in water with starting geometry having Φ, Ψ values of ~ 0, 90˚and 0, -90˚ respec- tively. As evident, such structures are stabilized by the interac- tions of water molecules with carbonyl moieties of the peptoid backbone. For clarity purposes, water molecules within 3 Å of the peptoid surface are shown. a pictorial view of the peptoid Ac-(NVal)7-NMe2 at dif- ferent simulation times with water molecules within 3 Å of the peptoid surface is shown in Figure 6. It is appar- ent from the graphics that the opening of the helical structure into the collagen or inverse-collagen type structure begins at the N-terminal due to initial interac- tions of water molecules with the acetyl carbonyl oxygen that lead to the change of torsion angles. Analysis of the simulation results at different time intervals given in Table 5 reveal that opening of the helical structure with- out hydrogen bonds takes place at the sub picoseconds time scale. The number of hydrogen bonds formed be- tween water molecules and the carbonyl moieties attain the maximum val ues within 10 ps and thereafter remain Figure 5. Plot of total energy (E) as a function of simula- tion time for the model Ac-(NIle)7-NMe2 (black) and Ac-(NLeu)7-NMe2 (red) for the collagen type conforma- tions. ( a ) ( c ) (b) Figure 6. Snapshots of the peptoid ‘Ac-(NVal)7-NMe2’ at dif- ferent simulation intervals (a) 1ps, (b) 5ps and (c) 10ps with water molecules within 3 Å of the peptoid surface indicates that the opening of the helical structure without hydrogen bonds starts at the N-terminal. constant throughout the simulation period (Figure 7). 6. CONCLUSIONS Aliphatic polypeptoid models adopt degenerate helical structures without hydrogen bonds with Φ, Ψ values of ~ 0, 90˚ in trans amide bond geometry that are stabilized (a) (b) Figure 7. Number of hydrogen bonds formed between car- bonyl groups of the backbone and water molecules for the model ‘Ac-(NVal)7-NMe2’ (a) increases within first 10 ps and thereafter, (b) remains constant on simulation in water.  F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 45 by carbonyl interactions. The population of poly-NLeu in only one state with Φ, Ψ values of ~ 0, 90˚ is attrib- uted to the difference in the side chain branching pat- terns of these residues. A single handed template was realized by incorporating L-leu or L-val at the C-termi- nal of poly-NIle sequences. Such templates provide pat- terns and find utility in generating a comple- mentary molecule. In peptoids, helical structures without hydro- gen bonds can also be exploited for the design of surfac- tant molecules by incorporating charged residues at the terminal positions. Simulation studies reveal that inter- actions of water molecules with the carbonyl moieties of peptoid backbone act as the primary driving force behind the opening of helical structures without hydrogen bonds resulting in the population of collagen or in- verse-collagen type structures. 7. ACKNOWLEDGMENTS We gratefully acknowledge the funding for this project by the De- partment of Biotechnology, India. REFERENCES [1] Latham, P.W. (1999) Therapeutic Peptides Revisited. Nature Boitechnology, 17, 755-757. doi:10.1038/11686 [2] Patch, J.A. and Barron, A.E. (2002) Mimicry of bioactive peptides via non-natural, sequence-specific peptidomi- metic oligomers. Current Opinion in Chemical Biology, 6(6), 872-877. doi:10.1016/S1367-5931(02)00385-X [3] Miller, S.M., Simon, R.J., Ng, S., Zuckermann, R.N., Kerr, J.M. and Moos, W.H. (1995) Comparison of the proteolytic susceptibilities of homologous L-amino acid, D-amino acid and N-substituted glycine peptides and peptoid oligomers. Drug Developement Research, 35(1), 20-32. doi:10.1002/ddr.430350105 [4] Zuckermann, R.N., Kerr, J.M., Kent, S.B.H. and Moos, W.H. (1992) Efficient method for the preparation of pep- toids [oligo (N-substituted) glycines] by submonomer solid-phase synthesis. Journal of American Chemical So- ciety, 114(26), 10646-10647. doi:10.1021/ja00052a076 [5] Zuckermann, R.N., Martin, E.J., Spellmeyer, D.C., Stau- ber, G.B., Shoemaker, K.R., Kerr, J.M., Flgliozzi, G.M., Goff, D.A., Siani, M.A., Simon, R.J., Banville, S.C., Brown, E.G., Wang, L., Richter, L.S. and Moos, W.H. (1994) Discovery of nanomolar ligands for 7-transmembrane G-Protein coupled receptors from a diverse (N-substituted) glycine peptoid library. Journal of Medicinal Chemistry, 37(17), 2678-2685. doi:10.1021/jm00043a007 [6] Gibbons, J.A., Hancock, A.A., Vitt, C.R., Knepper, S., Buckner, S.A., Brune, M.E., Milicic, I., Kerwin, J.F., Richter, L.S., Taylor, E.W., Spear, K.L., Zuckermann, R.N., Spellmeyer, D.C., Braeckman, R.A. and Moos, W.H. (1996) Pharmacologic characterization of CHIR 2279, an N-substituted glycine peptoid with high-affinity binding for alpha 1-adrenoceptors. Journal of Pharma- cology and Experimental Therapeutics, 277(2), 885-899. [7] Ng, S., Goodson, B., Ehrhardt, A., Moos, W.H., Siani, M. and Winter, J. (1999) Combinatorial discovery process yields antimicrobial peptoid. Bioorganic and Medicinal Chemistry, 7(9), 1781-1785. doi:10.1016/S0968-0896(99)00132-7 [8] Garcia-Martinez, C., Humet, M., Planells-Cases, R., Gomis, A., Caprini, M., Viana, F., Pena, E.D, San- chez-Baeza, F., Carbonell, T., Felipe, C.D., Perez-Paya, E., Belmonte, C., Messeguer, A. and Ferre-Montiel, A. (2002) Attenuation of thermal nociception and hyperal- gesia by VR1 blockers. Proceedings of the National Academy of Sciences USA., 99(4), 2374-2379. doi:10.1073/pnas.022285899 [9] Heizmann, G., Hildebrand, P., Tanner, H., Ketterer, S., Panksy, A., Froidevaux, S., Beglinger, C. and Eberle, A.N. (1999) A combinatorial peptoid library for the iden- tification of novel MSH and GRP/Bombesin receptor li- gands. Journal of Receptors and Signal Transduction Research, 19(1-4), 449-466. doi:10.3109/10799899909036664 [10] Thompson, D.A., Chai, B.X., Rood, H.L.E., Siani, M.A., Douglas, N.R., Grantiz, I. and Millhauser, G.L. (2003) Peptoid mimetics of agouti related protein. Bioorganic and Medicinal Chemistry Letters, 13(8), 1409-1413. doi:10.1016/S0960-894X(03)00164-1 [11] Statz, A.R., Park, J.P., Chongsiriwatana, N.P. , Barron, A.E. and Messersmith, P.B. (2008) Surface-immobilized antimicrobial peptoids. Biofouling, 24(6), 439-448. doi:10.1080/08927010802331829 [12] Brown, N.J., Wu, C.W., Seurynck-Servoss, S.L. and Barron, A.E. (2008) Effects of hydrophobic helix length and side chain chemistry on biomimicry in peptoid ana- logues of SP-C. Biochemistry, 47(6), 1808-1818. doi:10.1021/bi7021975 [13] Seurynck-Servoss, S.L., Brown, N.J., Dohm, M.T., Wu, C.W. and Barron, A.E. (2007) Lipid composition greatly affects the in vitro surface activity of lung surfactant protein mimics. Colloids & Surfaces B: Biointerfaces, 57(1), 37-55. doi:10.1016/j.colsurfb.2007.01.001 [14] Seurynck-Servoss, S.L., Dohm, M.T. and Barron, A.E. (2006) Effects of including an N-terminal insertion re- gion and arginine-mimetic side chains in helical peptoid analogues of lung surfactant protein B. Biochemistry, 45(39), 11809-11811. doi:10.1021/bi060617e [15] Rouhi, A.M. (2001) A peptoid promise: Synthetic lung surfactant mimics may widen access to treatment of res- piratory disease. Chemical and Engineering News, 79, 50-51. doi:10.1021/cen-v079n038.p050 [16] Seurynck, S.L., Patch, J.A. and Barron, A.E. (2004) Sim- ple, helical peptoid analogs of lung surfactant protein B. Chemistry and Biology, 12(1), 77-88. doi:10.1016/j.chembiol.2004.10.014 [17] Wu, C.W., Seurynck, S.L., Lee, K.Y.C. and Barron, A.E. (2003) Helical peptoid mimics of lung surfactant protein C. Chemistry and Biology, 10(11), 1057-1063. doi:10.1016/j.chembiol.2003.10.008  F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 46 [18] T.M. Rossa, R.N. Zuckermann, C.R. William and H. Frey, “Intranasal administration delivers peptoids to the rat central nervous system”, Neuroscience Letters, Vol. 439, No. 1, 2008, pp. 30-33. doi:10.1016/j.neulet.2008.04.097 [19] A.R. Statz, R.J. Meagher, A.E. Barron and P.B. Mess- ersmith, “New peptidomimetic polymers for antifouling surfaces”, Journal of the American Chemical Society, Vol. 127, No. 22, 2005, pp. 7972-7973. doi:10.1021/ja0522534 [20] R. Statz, A.E. Barron and P.B. Messersmith, “Protein, cell and bacterial fouling resistance of polypep- toid-modified surfaces: Effect of side-chain chemistry”, Soft Matter, Vol. 4, 2008, pp. 131-139. doi:10.1039/b711944e [21] B. Yoo and K. Kirshenbaum, “Peptoid architectures: Elaboration, actuation and application”, Current Opinion in Chemical Biology, Vol. 12, No. 6, 2008, pp. 714-21. doi:10.1016/j.cbpa.2008.08.015 [22] T.S. Ryge, N. Frimodt-Møller and P.R. Hansen, ” Antim- icrobial Activities of Twenty Lysine-Peptoid Hybrids against Clinically Relevant Bacteria and Fungi”, Che- motherapy, Vol 54, No. 2, 2008, pp. 152-156. doi:10.1159/000119707 [23] D. Daelemans, D. Schols, M. Witvrouw, C. Pannecouque, S. Hatse, S.V. Dooren, F. Hamy, T. Klimkait, E. Clercq and A.M. Vandamme, “A second target for the peptoid Tat/transactivation response element inhibitor CGP64222: Inhibition of human immunodeficiency virus replication by blocking CXC-chemokine receptor 4-mediated virus entry”, Molecular Pharmacology, Vol. 57, No. 1, 2000, pp. 116-124. [24] F. Hamy, E.R. Felder, G. Heizmann, J. Lazdins, F. Ab- oul-Ela, G. Varani, J. Karn and J. Klimkait, “An inhibitor of the Tat/TAR interaction that effectively suppresses HIV-1 replication”, Proceedings of the National Academy of Sciences USA, Vol. 94, No. 8, 1997, pp. 3548-3553. doi:10.1073/pnas.94.8.3548 [25] J.A. Patch and A.E. Barron, “Helical peptoid Mimics of Magainin-2 amide”, Journal of American Chemical So- ciety, Vol. 125, No. 40, 2003, pp. 12092-12093. doi:10.1021/ja037320d [26] N.P. Chongsiriwatana, J.A. Patch, A.M. Czyzewski, M.T. Dohm, A. Ivankin, D. Gidalevitz, R.N. Zuckermann and A.E. Barron, “Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides”, Pro- ceedings of the National Academy of Sciences USA, Vol. 105, No. 8, 2008, pp. 2794-2799. doi:10.1073/pnas.0708254105 [27] S.A. Fowler, R. Luechapanichkul and H.E. Blackwell, “Structure-function relationships in peptoids: Recent ad- vances toward deciphering the structural requirements for biological function”, Journal of Organic Chemistry, Vol. 74, No. 4, 2009, pp. 1440-1449. doi:10.1021/jo8023363 [28] C.W. Wu, K. Kirshenbaum, T.J. Sanborn, K. Huang, R.N. Zuckermann, A.E. Barron, J.A. Patch and K.A. Dill, “Structural and spectroscopic studies of peptoid oli- gomers with -chiral, aliphatic side chains”, Journal of American Chemical Society, Vol. 125, No. 44, 2003, pp. 13525-13530. doi:10.1021/ja037540r [29] G.L. Butterfoss, P.D. Renfren, B. Kuhlman and K.A. Kirshenbaum, “Preliminary survey of the peptoid folding landscape”, Journal of American Chemical Society, Vol. 131, No. 46, 2009, pp. 16798-16807. doi:10.1021/ja905267k [30] C.W. Wu, T.J. Sanborn, R.N. Zuckermann and A.E. Bar- ron, “Peptoid oligomers with α-chiral, aromatic side chains: effect of chain length on secondary structure”, Journal of American Chemical Society, Vol. 123, No.13, 2001, pp. 2958-2963. doi:10.1021/ja003153v [31] W. Wu, T.J. Sanborn, K. Huang, R.N. Zuckermann and A.E. Barron, “Peptoid oligomers with -chiral, aromatic side chains: Sequence requirements for the formation of stable peptoid helices”, Journal of American Chemical Society, Vol. 123, No. 28, 2001, pp. 6778-6784. doi:10.1021/ja003154n [32] B. Pullman and A. Pullman, “Molecular orbital calcula- tions on the conformations of amino acid residues of proteins”, Advances in Protein Chemistry, Vol. 28, 1974, pp. 347-526. doi:10.1016/S0065-3233(08)60233-8 [33] R.P. Lawrence and C. Thompson, “The boron analogue of glycine: A theoretical investigation of structure and properties”, Journal of Molecular Structure: Theochem, Vol. 88, No. 1-2, 1982, pp. 37-43. doi:10.1016/0166-1280(82)80105-X [34] C. Aleman and J. Casanovas, “Ab initio SCF and force-field calculations on low-energy conformers of 2-acetylamino-2,N-dimethylpropanamide”, Journal of the Chemical Society of Perkin Transactions, Vol. 2, 1994, pp. 563-568. doi:10.1039/p29940000563 [35] C. Aleman and J. Casanovas, “Molecular conformational analyses of dehydroalanine analogues”, Biopolymers, Vol. 36, No. 1, 1995, pp. 71-82. doi:10.1002/bip.360360107 [36] F.S. Nandel and B. Khare, “Conformation of peptides constructed from achiral amino acid residues Aib and ZPhe: Computational study of the effect of L/D- Leu at terminal positions”, Biopolymers, Vol. 77, No. 1, 2005, pp. 63-73. doi:10.1002/bip.20128 [37] F.S. Nandel, N. Malik, B. Singh and D.V.S. Jain, “Con- formational structure of peptides containing dehydroa- lanine. Formation of beta bend ribbon structure”, Inter- national Journal of Quantum Chemistry, Vol. 72, No. 1, 1999, pp. 15-23. doi:10.1002/(SICI)1097-461X(1999)72:1<15::AID-QUA 2>3.0.CO;2-2 [38] F.S. Nandel, N. Malik, B. Singh and M. Virdi, “Design- ing of peptides with left handed helical structure by in- corporating the unusual amino acids”, Indian Journal of Biochemistry and Biophysics, Vol. 36, No. 3, 1999, pp. 195-203. [39] S.J. Weiner, P.A. Kollman, D.T. Nguyen and D.A. Case, “An all atom force field for simulations of proteins and nucleic acids”, Journal of Computational Chemistry, Vol. 7, No. 2, 1986, pp. 230-252. doi:10.1002/jcc.540070216 [40] K. Mohle, H.J. Hoffman, “Secondary structure formation in N-substituted peptides”, J. Peptide Research, Vol. 51,  F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 47 No. 1, 1998, pp. 19-28. doi:10.1111/j.1399-3011.1998.tb00412.x [41] A.A. Adzhubei, F. Eisenmenger, V.G. Tumanyan, M. Zinke and N. G. Esipova, “Third type of secondary structure: Noncooperative mobile conformation. Protein Data Bank analysis”, Biochemical and Biophysical Re- search Communications, Vol. 146, No. 3, 1987, pp. 934-938. doi:10.1016/0006-291X(87)90736-4 [42] A.A. Aduzbei and J.E. Sternberg, “Left-handed poly- proline II helices commonly occur in globular proteins”, Journal of Molecular Biology, Vol. 229, No. 2, 1993, pp. 472-493. doi:10.1006/jmbi.1993.1047 [43] J. Lipinski, “Modified all-valence INDO/spd method for ground and excited state properties of isolated molecules and molecular complexes”, International Journal of Quantum Chemistry, Vol. 34, No. 5, 1988, pp. 423-435. doi:10.1002/qua.560340504 [44] D. van der Spoel, E. Lindahl, B. Hess, A.R. van Buuren, E. Apol, P.J. Meulenhoff, D.P. Tieleman, A.L.T.M. Si- jbers, K.A. Feenstra, R. van Drunen and H.J.C. Berend- sen, Gromacs User Manual version 3.3, 2005. [45] A.W. Schuettelkopf and D.M.F. van Aalten, “PRODRG -a tool for high-throughput crystallography of pro- tein-ligand complexes” Acta Crystallographica, Vol. D60, No. 8, 2004, pp. 1355-1363. [46] W.F. van Gunsteren, S.R. Billeter, A.A. Eising, P.H. Hünenberger, P. Krüger, A.E. Mark, W.R.P. Scott and I.G. Tironi, “Biomolecular Simulation: The GROMOS96 manual and user guide. Z¨urich, Switzerland: Hoch- schulverlag AG an der ETH Zürich, 1996. [47] N.H. Shah, G.L. Butterfoss, K. Nguyen, B. Yoo, R. Bon- neau, D.L. Rabenstein and K. Kirshenbaum, “Oligo (N-aryl glycines): A new twist on structural peptoids”, Journal of American Chemical Society, Vol. 130, No. 49, 2008, pp. 16622-16632. doi:10.1021/ja804580n [48] Q. Sui, D. Borchardt and D.L. Rabenstein, “Kinetics and equilibria of cis/trans isomerization of backbone amide bonds in peptoids”, Journal of American Chemical Soci- ety, Vol. 129, No. 39, 2007, pp. 12042-12048. doi:10.1021/ja0740925 [49] H.J.C. Berendsen, J.P.M. Postma, A. DiNola and J.R. Haak, “Molecular dynamics with coupling to an external bath”, Journal of Chemical Physics, Vol. 81, No. 8, 1984, pp. 3684-3690. doi:10.1063/1.448118 [50] B. Hess, H. Bekker, H.J.C. Berendsen and J.G.E.M. Fraaije, “LINCS: A linear constraint solver for molecular simulations”, Journal of Computational Chemistry, Vol. 18, No. 12, 1997, pp. 1463-1472. doi:10.1002/(SICI)1096-987X(199709)18:12<1463::AID -JCC4>3.3.CO;2-L [51] H.J.C. Berendsen, J.P.M. Postma, W.F. van Gunsteren and J. Hermans, “Interaction models for water in relation to protein hydration” In: Intermolecular Forces. Dor- drecht: D Reidel Publishing Company, 1981, pp. 331-342. [52] Y.M. Song, Y.K. Park, S.S. Lim, S.T. Yang, E.R. Woo, I.S. Park, J.S. Lee, J.I. Kim, K.S. Hahm, Y. Kim and S.U. Shin, “Cell selectivity and Mechanism of action of an- timicrobial model peptides containing peptoid residues”, Biochemistry, Vol. 44, No. 36, 2005, pp. 12094-106. doi:10.1021/bi050765p [53] F.H. Allen, C.A. Baalham, J.P.M. Lommerse and P.R. Raithby, “Carbonyl-carbonyl interactions can be com- petitive with hydrogen bonds”, Acta Crystallographica, Vol. B54, No. 3, 1998, pp. 320-329. [54] F.H. Allen, J.E. Dacies, J.J. Galloy, O. Johnson, O. Ken- nard, C.F. Macrae, E.M. Mitchell, G.F. Mitchell, J.M. Smith and D.G. Watson, “The development of version 3 and 4 of the Cambridge Structural Database System”, Journal of Chemical Information Computer Science, Vol. 31, No. 2, 1991, pp. 187-204. doi:10.1021/ci00002a004 [55] P.H. Maccallum, R. Poet and E.J. Milner-White, “Coulombic interactions between partially charged main-chain atoms not hydrogen-bonded to each other in- fluence the conformations of alpha-helices and an- ti-parallel beta-sheet. A new method for analyzing the forces between hydrogen bonding groups in proteins in- cludes all the Coulombic interactions”, Journal of Mo- lecular Biology, Vol. 248, No. 2, 1995, pp. 361-373. doi:10.1016/S0022-2836(95)80056-5 [56] P.H. Maccallum, R. Poet and E.J. Milner-White, “Coulombic attractions between partially charged main-chain atoms stabilize the right-handed twist found in most beta-strands”, Journal of Molecular Biology, Vol. 248, No. 2, 1995, pp. 374-384. doi:10.1016/S0022-2836(95)80057-3 [57] C.M. Deane, F.H. Allen, R. Taylor and T.L. Blundell, “Carbonyl-carbonyl interactions stabilize the partially allowed Ramachandran conformations of asparagine and aspartic acid”, Protein Engineering, Vol. 12, No. 12, 1999, pp. 1025-1028. doi:10.1093/protein/12.12.1025 [58] F.S. Nandel and H. Kaur, “Effect of terminal achiral and chiral residues on the conformational behaviour of poly zPhe and analysis of various interactions”, Indian Journal of Biochemistry and Biophysics, Vol. 40, No. 4, 2003, pp. 265-273. [59] F.S. Nandel, H. Kaur, N. Malik, N. Shankar and D.V.S. Jain, “Conformational study of peptides containing de- hydrophenylalanine: Helical structures without hydrogen bonds”, Indian Journal of Biochemistry and Biophysics, Vol. 38, No. 6, 2001, pp. 417-425. [60] T.M. Head-Gordon, M.J. Frisch, C.L. Brooks III and J.A. Pople, “Theoretical study of blocked glycine and alanine peptide analogs”, Journal of American Chemical Society, Vol. 113, No. 16, 1991, pp. 5989-5997. doi:10.1021/ja00016a010 [61] J. Park, S.A. Teichmann, T. Hubbard and C. Chothia, “Intermediate sequences increase the detection of ho- mology between sequences”, Journal of Molecular Bi- ology, Vol. 273, No. 1, 1997, pp. 349-354. doi:10.1006/jmbi.1997.1288 [62] F.S. Nandel and R. Jaswal, “New type of helix and 27 ribbon structure formation in poly Leu peptides: Con- struction of a single-handed template”, Biomacro- molecules, Vol. 8, No. 10, 2007, pp. 3093-3101. doi:10.1021/bm700504h [63] Y. Demizu, N. Yamagata, Y. Sato, M. Doi, M. Tanaka, H. Okuda and M. Kurihara, “Controlling the helical screw  F. S. Nandel et al. / Journal of Biophys ical Chemistry 2 (2011) 37-48 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 48 sense of peptides with C-terminal L-valine”, Journal of Peptide Science, Vol. 16, No. 3, 2010, pp. 153-158. [64] Z. Liu, K. Chen, A. Ng, Z. Shi, R.W. Woody and N.R. Kallenbach, “Solvent dependence of PII conformation in model alanine peptides”, Journal of American Chemical Society, Vol. 126, No. 46, 2004, pp. 15141-15150. doi:10.1021/ja047594g [65] B.W. Chellgren and T.P. Creamer, “Effects of H2O and D2O on polyproline II helical structure”, Journal of American Chemical Society, Vol. 126, No. 45, 2004, pp. 14734-14735. doi:10.1021/ja045425q [66] D. Voet, J.G. Voet and C.W. Pratt, “Fundamentals of Bi- ochemistry”, John Wiley and Sons, Inc., 2008. [67] F.S. Nandel, A. Ahluwalia and H. Kaur, “A conforma- tional structure of the amphipathic peptide insecticide L-KALA”, International Journal of Quantum Chemistry, Vol. 55, No. 1, 1995, pp. 61-69. doi:10.1002/qua.560550109 [68] G.R. Desiraju and T. Steiner, “The Weak Hydrogen Bond”, In: Structural Chemistry and Biology, Oxford Science Publications, 1999. [69] C. Baldauf, R. Günther and H.J. Hofmann, “Helices in peptoids of α- and β-peptides”, Physical Biology, Vol. 3, No. 1, 2006, pp. S1-S9. doi:10.1088/1478-3975/3/1/S01 |

