 Vol.2, No.1, 10-18 (2011) doi:10.4236/jbpc.2011.21002 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ Journal of Biophysical Chemistry Involvement of mitochondrial swelling in cytochrome c release from mitochondria treated with calcium and Alloxan Takuya Ichimura, Mika Ito, Kiyoshi Takahashi, Kyohei Oyama and Koichi Sakurai* Division of Biochemistry, Department of Life science, Hokkaido Pharmaceutical University School of Pharmacy, Otaru, Japan; ks51@hokuyakudai.ac.jp Received 29 October 2010; revised 18 November 2010; accepted 25 November 2010. ABSTRACT An early event in the induction of apoptosis is cytochrome c (Cyt c) release from mitochondria. We investigated the involvement of mitochon- drial permeability transition (MPT) and mito- chondrial swelling in Cyt c release from mito- chondria treated with alloxan and/or calcium (Ca2+). When mitochondria were treated with a high concentration of Ca2+ alone or Ca2+ with alloxan (alloxan-Ca2+), the MPT was accompa- nied by mitochondrial swelling and the release of Cyt c. Cyclosporin A prevented the induction of MPT but only slightly decreased the release of Cyt c. High molecular weight polyethylene glycol almost completely inhibited MPT-dependent osmotic mitochondrial swelling and Cyt c re- lease. However, MPT-independent mitochondrial swelling and Cyt c release induced by exoge- nous K+ were inhibited by the high molecular weight polyethylene glycol. Ruthenium red strongly decreased the amount of Cyt c released. These results suggest that mitochondrial swell- ing but not MPT is necessary for Cyt c release induced by Ca2+ alone or alloxan and Ca2+. Keywords: Mitochondrial Swelling; Cytochrome C Release; Calcium; Alloxan; Mitochondri al Permeability Transition 1. INTRODUCTION Mitochondria have a central role in energy metabo- lism and Ca2+ homeostasis in cells [1-3]. The release of mitochondrial proteins, such as cytochrome c (Cyt c), Smac/DIABLO, and apoptosis inducing factor (AIF), into the cytosol is likely involved in the early events in cell death [4,5]. Several triggers of mitochondrial per- meability transition (MPT) result in apoptotic cell death [6-8]. From these findings, it is proposed that the MPT is involved in Cyt c release from mitochondria and apop- tosis [9,10]. MPT is associated with an increase in the permeability of the mitochondrial inner membrane, al- lowing the transmission of a solute with a molecular mass of up to approximately 1.5 k Da. MPT is also asso- ciated with mitochondrial swelling, followed by the re- lease of mitochondrial proteins including Cyt c [10]. Cytochrome c with a molecular weight of approximately 12 k Da is loosely bound to phospholipids of the outer surface of the inner mitochondrial membrane, primarily cardiolipin, and functions to transmit electrons from complex III to complex IV of the electron transport chain in mitochondria [11,12]. There are two distinct processes in the Cyt c release from the intermembrane space of mitochondria into the cytosol. One process is the dissociation of Cyt c from the inner membrane, and the other process is the transfer of the dissociated Cyt c through the outer membrane. Several mechanisms for the release of Cyt c from mi- tochondria have been proposed. Petrosillo et al. demon- strated that Cyt c is dissociated from the inner mito- chondrial membrane response to cardiolipin peroxida- tion by reactive oxygen species (ROS) prior to the re- lease into the cytosol [12]. Several researchers have proposed that the release of Cyt c occurs due to the rup- ture of the mitochondrial outer membrane [10,13-17]. Apoptotic Bcl-2 family proteins, such as Bax and tBid, cause Cyt c release from mitochondria by an increase in outer membrane permeability [18-21]. The induction of MPT is due to the opening of the permeability transition pore (PTP), which consists of the voltage-dependent anion channel (VDAC), adenine nucleotide translocator (ANT), cyclophilin D (CyD), and other proteins, such as hexokinase [10]. Because the physiological function of the PTP is to allow high molecular weight substances to pass through the mitochondrial outer membrane, whereas low molecular weight materials such as water,  T. Ichimura et al. / Journal of Biophysical Chemistry 2 (2011) 10-18 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 11 oxygen, and carbon dioxide [9] can pass freely through the mitochondrial inner membrane, it has been suggested that PTP has a primary role in the matter transport into mitochondria. Mitochondria are one of the Ca2+ store sites in cells and contribute to the maintenance of Ca2+ density in the cytoplasm [3]. Excessive Ca2+ accumulation in cells in- duces MPT [1,22,23]. We have previously demonstrated that the diabetogenic compound, alloxan, causes MPT in the presence of Ca2+. Quinone compounds that have a similar structure to alloxan induce MPT in the presence of Ca2+ [8,24-26]. Mangiferin quinoid compounds trig- gers MPT via interaction with mitochondrial protein thiol groups [27]. The MPT was inhibited by several chemicals, such as inhibitors of the cyclophilin family, mitochondrial Ca2+ uniporter and mitochondrial respira- tory chain [28-30], suggesting that the release of Cyt c is induced through several different pathways. However, the mechanism by which Cyt c is released from mito- chondria treated with Ca2+ remains largely unknown. The present study demonstrates the involvements of MPT and mitochondrial swelling in Cyt c release from mitochondria treated with various concentrations of Ca2+ alone or Ca2+ with alloxan (alloxan-Ca2+). The MPT in- hibitors significantly inhibited the MPT induced by Ca2+ alone or alloxan-Ca2+ but did not inhibit Cyt c release. High-molecular weight polyethylene glycol (PEG) al- most completely inhibited MPT-dependent osmotic mi- tochondrial swelling and Cyt c release caused by the large amplitude MPT induced by K+ or other inducers. These results suggest that mitochondrial swelling but not MPT relates to Cyt c release from mitochondria treated with alloxan-Ca2+ or high concentrations of Ca2+ alone. 2. MATERIALS AND METHODS 2.1. Materials Cyt c (from horse heart), rotenone, ethyleneglycol- bis-(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), stigmatellin (STG), and carbonyl cyanide m-chlorophenyl- hydrazone (CCCP) were purchased from Sigma Chemi- cal Co. (St. Louis, MO). Calcium chloride (CaCl2), so- dium succinate, potassium chloride (KCl), potassium cyanide (KCN), alloxan, cyclosporin A (CsA), and PEG 4000 (molecular weight of 3 kDa) were from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Ruthenium red (RuR) was from Kanto Chemical Co. (Tokyo, Japan). The polyclonal anti-Cyt c antibody and the goat anti- rabbit IgG HRP were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The ECL plus Western blotting detection system was from GE Healthcare UK Ltd. (Buckinghamshire, England). The BCA protein assay kit was from Pierce Biotechnology Inc. (Rockford, IL). The other chemicals used in this study were of analytical grade from commercial suppliers. 2.2. Preparation of Rat Liver Mitochondria Male Wistar rats that were approximately 200 g were used for the experiments after an overnight fast. Mito- chondria were isolated in Chelex-100 treated medium containing 0.25 M sucrose, 10 mM Tris-HCl (pH 7.4) and 1 mM EGTA by differential centrifugation of liver homogenates as previously described [31]. Mitochon- drial protein concentrations were determined by a BCA protein assay kit (Pierce, USA) according to the manu- facturer’s instruction with bovine serum albumin used as a standard. All rats were treated in accordance with the guiding principles for care and use of experimental ani- mals in Hokkaido Pharmaceutical University School of Pharmacy. 2.3. Measurement of Mitochondrial Swelling Mitochondria (1 mg of protein/ml) were equilibrated in Chelex-100 treated medium consisting of 10 mM Tris-HCl (pH 7.4), 0.25 M sucrose and 0.1 M rotenone for 5 min at 37˚C. After mitochondria were energized by the addition of 5 mM succinate to the mitochondrial suspension for 5 min, mitochondrial swelling was then initiated by adding various MPT inducers (Ca2+ and al- loxan-Ca2+) for 10 min. Mitochondrial swelling induced by K+ was initiated by the addition of 80 mM KCl to mitochondria suspended in Chelex-100 treated medium containing 0.25 M sucrose, 10 mM Tris-HCl (pH 7.4) and 1 mM EGTA. Mitochondrial swelling was measured by recording the decrease in absorbance at 540 nm with a spectrophotometer (Hitachi U-2,000, Japan). 2.4. Release of Cyt C Released Cyt c was detected by Western blot analysis. After the mitochondrial suspension was treated with various compounds, the suspension was centrifuged at 10,000 × g for 30 min at 4˚C. The collected supernatant was mixed with loading buffer, boiled for 5 min and subjected to 15% SDS-polyacrylamide gel (Atto, Japan) for 40 min at 200 V followed by electroblotting to nitro- cellulose membranes for 1.5 h at 2 mA/cm2. Membranes were blocked for 1 h with 5% skim milk in TBS-T at room temperature, rinsed and subsequently probed with a polyclonal anti-Cyt c antibody (1:1,000 dilution) for 1 h at room temperature. The membranes were rinsed and incubated with a horseradish peroxidase-labeled anti- rabbit antibody (1:20,000 dilution) for 1 h at room tem- perature. After incubation with the secondary antibody, the membranes were rinsed, and the Cyt c blots were visualized by enhanced chemiluminescence using the 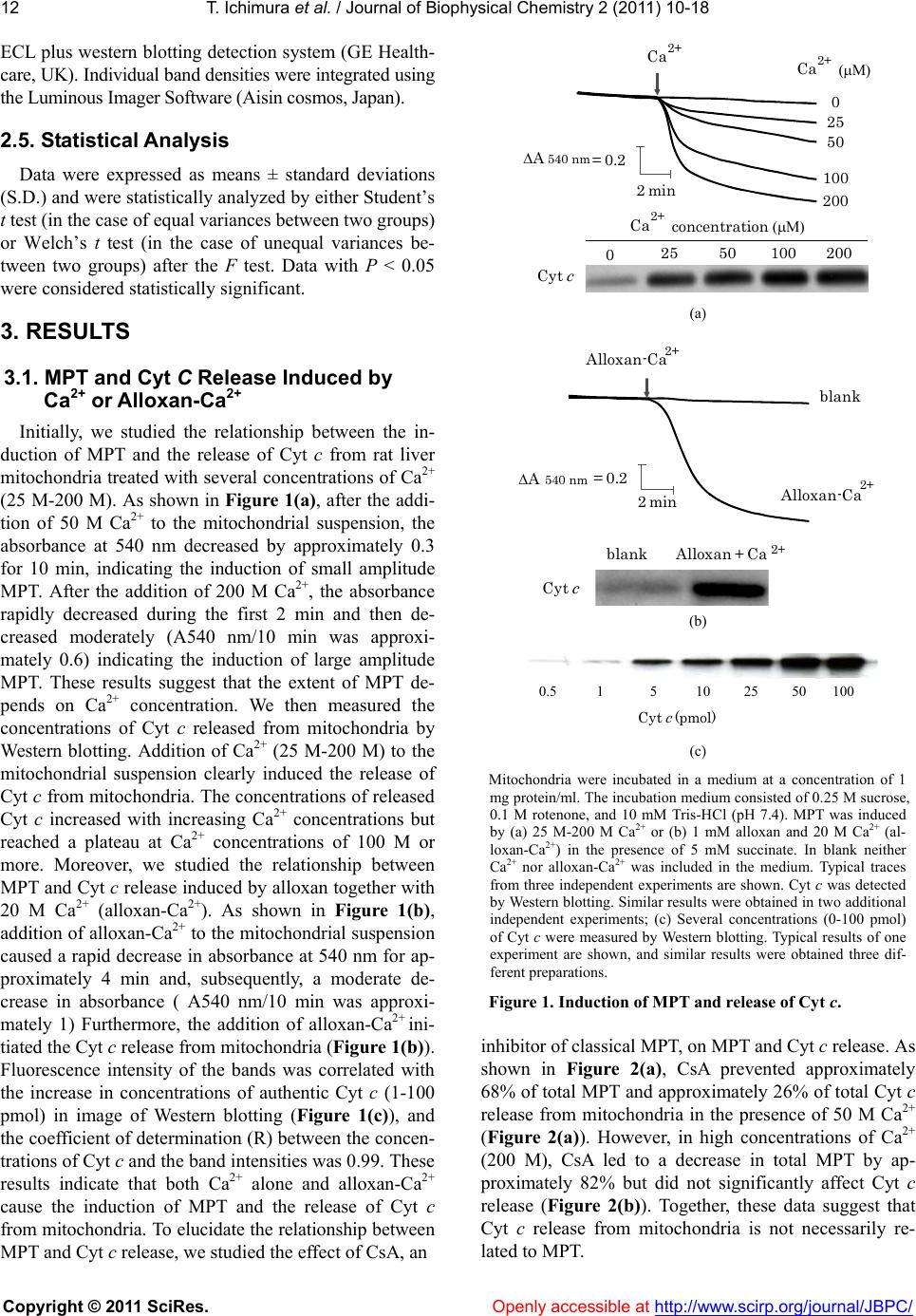 T. Ichimura et al. / Journal of Biophysical Chemistry 2 (2011) 10-18 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 12 ECL plus western blotting detection system (GE Health- care, UK). Individual band densities were integrated using the Luminous Imager Software (Aisin cosmos, Japan). 2.5. Statistical Analysis Data were expressed as means ± standard deviations (S.D.) and were statistically analyzed by either Student’s t test (in the case of equal variances between two groups) or Welch’s t test (in the case of unequal variances be- tween two groups) after the F test. Data with P < 0.05 were considered statistically significant. 3. RESULTS 3.1. MPT and Cyt C Release Induced by Ca2+ or Alloxan-Ca2+ Initially, we studied the relationship between the in- duction of MPT and the release of Cyt c from rat liver mitochondria treated with several concentrations of Ca2+ (25 M-200 M). As shown in Figure 1(a), after the addi- tion of 50 M Ca2+ to the mitochondrial suspension, the absorbance at 540 nm decreased by approximately 0.3 for 10 min, indicating the induction of small amplitude MPT. After the addition of 200 M Ca2+, the absorbance rapidly decreased during the first 2 min and then de- creased moderately (A540 nm/10 min was approxi- mately 0.6) indicating the induction of large amplitude MPT. These results suggest that the extent of MPT de- pends on Ca2+ concentration. We then measured the concentrations of Cyt c released from mitochondria by Western blotting. Addition of Ca2+ (25 M-200 M) to the mitochondrial suspension clearly induced the release of Cyt c from mitochondria. The concentrations of released Cyt c increased with increasing Ca2+ concentrations but reached a plateau at Ca2+ concentrations of 100 M or more. Moreover, we studied the relationship between MPT and Cyt c release induced by alloxan together with 20 M Ca2+ (alloxan-Ca2+). As shown in Figure 1(b), addition of alloxan-Ca2+ to the mitochondrial suspension caused a rapid decrease in absorbance at 540 nm for ap- proximately 4 min and, subsequently, a moderate de- crease in absorbance ( A540 nm/10 min was approxi- mately 1) Furthermore, the addition of alloxan-Ca2+ ini- tiated the Cyt c release from mitochondria (Figure 1(b)). Fluorescence intensity of the bands was correlated with the increase in concentrations of authentic Cyt c (1-100 pmol) in image of Western blotting (Figure 1(c)), and the coefficient of determination (R) between the concen- trations of Cyt c and the band intensities was 0.99. These results indicate that both Ca2+ alone and alloxan-Ca2+ cause the induction of MPT and the release of Cyt c from mitochondria. To elucidate the relationship between MPT and Cyt c release, we studied the effect of CsA, an 0 25 50 100 200 2min Ca 2+ (μM) 02550 100 200 Cyt c ΔA 540 nm =0.2 Ca 2+ concentration (μM) Ca 2+ (a) Cyt c Alloxan + Ca 2+ ΔA 540 nm =0.2 blank blank Alloxan-Ca 2+ Alloxan-Ca 2+ 2 min (b) Cyt c (pmol) 0.5 15 102550 100 (c) Mitochondria were incubated in a medium at a concentration of 1 mg protein/ml. The incubation medium consisted of 0.25 M sucrose, 0.1 M rotenone, and 10 mM Tris-HCl (pH 7.4). MPT was induced by (a) 25 M-200 M Ca2+ or (b) 1 mM alloxan and 20 M Ca2+ (al- loxan-Ca2+) in the presence of 5 mM succinate. In blank neither Ca2+ nor alloxan-Ca2+ was included in the medium. Typical traces from three independent experiments are shown. Cyt c was detected by Western blotting. Similar results were obtained in two additional independent experiments; (c) Several concentrations (0-100 pmol) of Cyt c were measured by Western blotting. Typical results of one experiment are shown, and similar results were obtained three dif- ferent preparations. Figure 1. Induction of MPT and release of Cyt c. inhibitor of classical MPT, on MPT and Cyt c release. As shown in Figure 2(a), CsA prevented approximately 68% of total MPT and approximately 26% of total Cyt c release from mitochondria in the presence of 50 M Ca2+ (Figure 2(a)). However, in high concentrations of Ca2+ (200 M), CsA led to a decrease in total MPT by ap- proximately 82% but did not significantly affect Cyt c release (Figure 2(b)). Together, these data suggest that Cyt c release from mitochondria is not necessarily re- lated to MPT. 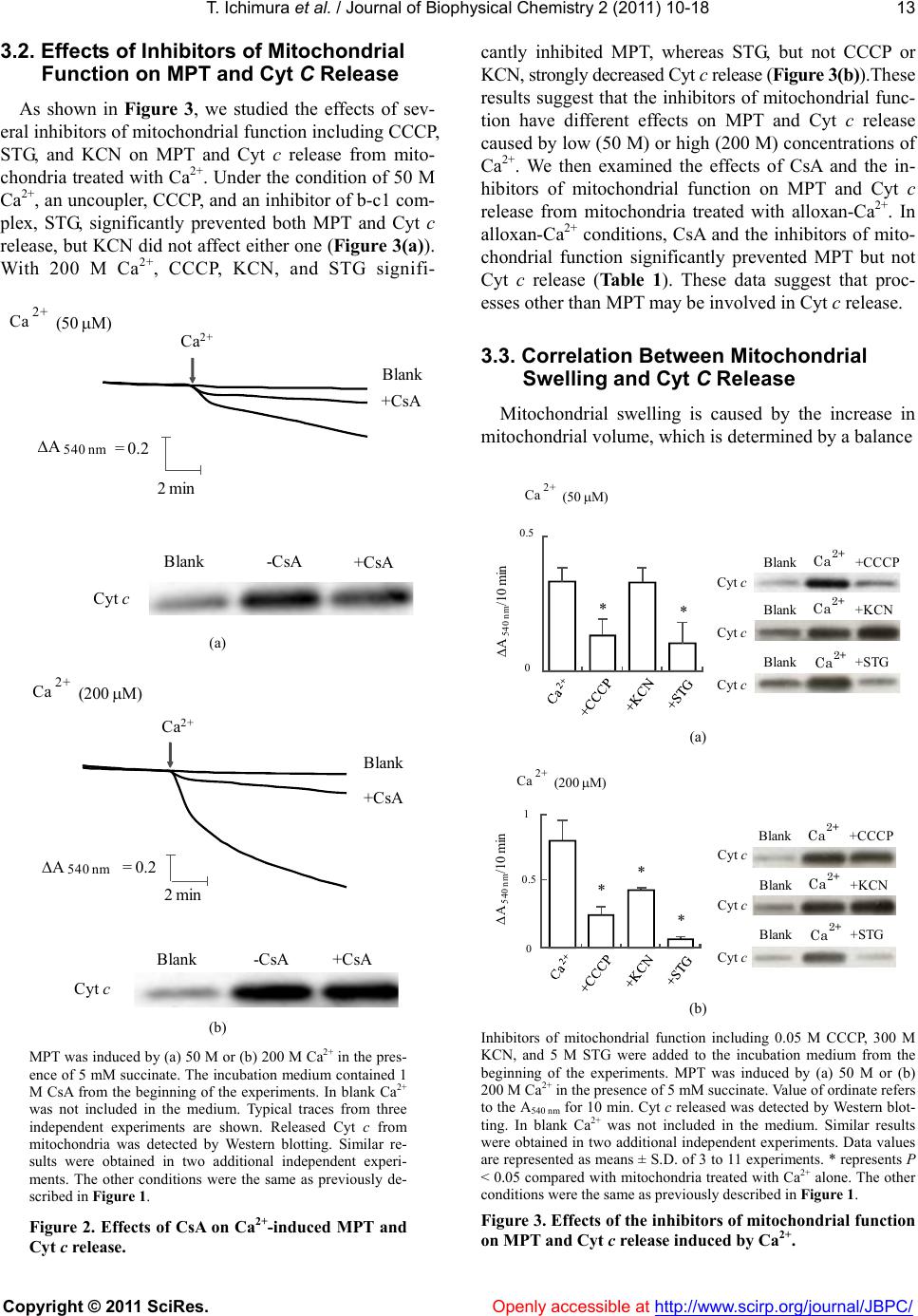 T. Ichimura et al. / Journal of Biophysical Chemistry 2 (2011) 10-18 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 13 3.2. Effects of Inhibitors of Mitochondrial Function on MPT and Cyt C Release As shown in Figure 3, we studied the effects of sev- eral inhibitors of mitochondrial function including CCCP, STG, and KCN on MPT and Cyt c release from mito- chondria treated with Ca2+. Under the condition of 50 M Ca2+, an uncoupler, CCCP, and an inhibitor of b-c1 com- plex, STG, significantly prevented both MPT and Cyt c release, but KCN did not affect either one (Figu re 3(a) ). With 200 M Ca2+, CCCP, KCN, and STG signifi- Blank-CsA +CsA +CsA Blank 2min Cytc Ca 2+ (50 μM) ΔA 540nm =0.2 Ca 2+ (a) 2min +CsA Blank Cyt c Blank-CsA +CsA Ca 2 (200 μM) ΔA 540 nm =0.2 Ca2+ (b) MPT was induced by (a) 50 M or (b) 200 M Ca2+ in the pres- ence of 5 mM succinate. The incubation medium contained 1 M CsA from the beginning of the experiments. In blank Ca2+ was not included in the medium. Typical traces from three independent experiments are shown. Released Cyt c from mitochondria was detected by Western blotting. Similar re- sults were obtained in two additional independent experi- ments. The other conditions were the same as previously de- scribed in Figure 1. Figure 2. Effects of CsA on Ca2+-induced MPT and Cyt c release. cantly inhibited MPT, whereas STG, but not CCCP or KCN, strongly decreased Cyt c release (Figure 3(b)).These results suggest that the inhibitors of mitochondrial func- tion have different effects on MPT and Cyt c release caused by low (50 M) or high (200 M) concentrations of Ca2+. We then examined the effects of CsA and the in- hibitors of mitochondrial function on MPT and Cyt c release from mitochondria treated with alloxan-Ca2+. In alloxan-Ca2+ conditions, CsA and the inhibitors of mito- chondrial function significantly prevented MPT but not Cyt c release (Table 1). These data suggest that proc- esses other than MPT may be involved in Cyt c release. 3.3. Correlation Between Mitochondrial Swelling and Cyt C Release Mitochondrial swelling is caused by the increase in mitochondrial volume, which is determined by a balance Blank +CCCP Cyt c 0 0.5 ** ΔA 540 nm /10 min Ca 2+ (50 μM) Blank +KCN Blank +STG Cyt c Cyt c Ca 2+ Ca 2+ Ca 2+ (a) * * * 0 1 ΔA 540nm /10 min 0.5 Cyt c Cyt c Cyt c Ca 2+ (200μM) Blank +CCCP Ca 2+ Blank+KCN Ca 2+ Blank+STG Ca 2+ (b) Inhibitors of mitochondrial function including 0.05 M CCCP, 300 M KCN, and 5 M STG were added to the incubation medium from the beginning of the experiments. MPT was induced by (a) 50 M or (b) 200 M Ca2+ in the presence of 5 mM succinate. Value of ordinate refers to the A540 nm for 10 min. Cyt c released was detected by Western blot- ting. In blank Ca2+ was not included in the medium. Similar results were obtained in two additional independent experiments. Data values are represented as means ± S.D. of 3 to 11 experiments. * represents P < 0.05 compared with mitochondria treated with Ca2+ alone. The other conditions were the same as previously described in Figure 1. Figure 3. Effects of the inhibitors of mitochondrial function on MPT and Cyt c release induced by Ca2+.  T. Ichimura et al. / Journal of Biophysical Chemistry 2 (2011) 10-18 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 14 Table 1 .Effect of CsA and mitochondrial function inhibi- tors on swelling and Cyt c release induced by alloxan-Ca2+. Conditions ΔA540 nm Cyt c release (a.u.) Alloxan-Ca2+ 1.008 ± 0.079 2.8 ± 0.6 + CsA 0.482 ± 0.155* 2.9 ± 0.5 + CCCP 0.647 ± 0.068* 2.2 ± 0.8 + KCN 0.428 ± 0.193* 2.9 ± 0.6 + STG 0.073 ± 0.031* 2.2 ± 0.6 Mitochondrial swelling was induced by 1 mM alloxan and 20 mM Ca2+ (alloxan-Ca2+) in the presence of 5 mM succinate. Swelling data refer to the DA540 nm for 10 min. The amount of released Cyt c was analyzed by West- ern blotting and quantified by densitometry, and is relatively represented as compared to that of mitochondria treated with neither alloxan-Ca2+ nor the inhibitors. Data values are represented as a mean ± S.D. of three to eight experiments. * represents P < 0.05 when compared to alloxan-Ca2+. The other conditions were the same as previously described in Figure 1 and Figure 3. Blank 2 min +PEG Blank +PEGCa 2+ Cytc ΔA 540 nm =0.2 Ca 2+ (a) k 2 min +PEG Blank ΔA=0.2 Cytc Blank +PEGAlloxan-C a 2+ Alloxan-C a 2+ 540 nm (b) Mitochondrial swelling was induced by (a) 200 M Ca2+ (b) or 1 mM alloxan and 20 M Ca2+ in the presence of 5 mM succinate. Forty M of PEG 4000 was included in the in- cubation medium from the beginning of the experiments. Cyt c was detected by Western blotting. In blank neither Ca2+ nor alloxan-Ca2+ was included in the medium. Simi- lar results were obtained in two additional independent experiments. The other conditions were the same as pre- viously described in Figure 1. Figure 4. Effects of PEG on MPT and Cyt c re- lease induced by Ca2+ and alloxan-Ca2+. of osmotic pressure across the inner mitochondrial membrane. To examine the correlation between mito- chondrial swelling and Cyt c release from mitochondrial intermembrane space, we examined the effect of PEG on mitochondrial swelling and Cyt c release from mito- chondria induced by high concentrations of Ca2+ alone or alloxan-Ca2+. PEG strongly inhibited mitochondrial swelling induced by Ca2+ and completely inhibited Cyt c release from mitochondria (Figure 4(a)). In addition, PEG significantly prevented mitochondrial swelling and Cyt c release from mitochondria treated with al- loxan-Ca2+ (Figure 4(b)). These results indicate that mitochondrial swelling associated with MPT is con- nected with the release of Cyt c from mitochondria. We next investigated the regulation of Cyt c release from mitochondria with mitochondrial swelling induced by another mechanism that is not mediated by MPT: hypotonic shock triggered by low K+ concentrations [32]. As shown in Figure 5, 80 mM K+ significantly induced mitochondrial swelling and Cyt c release from mito- chondria when compared to a blank which neither K+ nor CsA is included. CsA did not inhibit K+-induced mitochondrial swelling and Cyt c release, indicating that Cyt c release from mitochondria may be due to mito- chondrial swelling regardless of functional MPT. CsA Blank Blank +CsA 0 0.2 K + * * ΔA 540nm /10 min 0.1 K + +CsA Cyt c Mitochondria were incubated in a medium at a concentration of 1 mg protein/ml. The incubation medium consisted of 0.25 M sucrose, 0.1 M rotenone, 5 mM succinate, 0.1 mM EGTA, and 10 mM Tris-HCl (pH 7.4). Mitochondrial swelling was induced by 80 mM K+. The incubation medium contained 1 M CsA from the beginning of the experiments. Value of ordinate refers to the A540 nm for 10 min. Data values are represented as means ± S.D. of three separate experiments. Cyt c was de- tected by Western blotting. In blank neither Ca2+ nor K+ was included in the medium. Similar results were obtained in two additional independent experiments. * represents P < 0.05 when compared to the blank. Figure 5. K+-induced mitochondrial swelling and Cyt c release.  T. Ichimura et al. / Journal of Biophysical Chemistry 2 (2011) 10-18 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 15 alone did not cause MPT or Cyt c release from mito- chondria (data not shown). These results suggest that mitochondrial swelling may act as a major regulator of Cyt c release from mitochondria treated with K+. 3.4. VDAC Opening May Be Involved in Cyt C Release To investigate the involvement of the passage process through the mitochondrial outer membrane in Cyt c re- lease, we studied the effect of RuR that inhibits both the mitochondrial Ca2+ uniporter and the VDAC opening. As shown in Figure 6(a), RuR significantly inhibited MPT caused by high concentrations of Ca2+ or alloxan-Ca2+. The degree of MPT inhibition by RuR was almost equal to the CsA inhibition (Figure 2). In addition, RuR strongly inhibited the Cyt c release caused by the induc- 0 0.5 1 Blank +RuR * ** +RuR ΔA 540 nm /10 min Ca 2+ A llo x a n - C a 2+ (a) 4 2 0 Cyt crelease (a.u.) * ** Blank +RuR+RuRCa2+ Alloxan-Ca2+ (b) The incubation medium contained 25 M RuR from the beginning of the experiments. (a) MPT and (b) release of Cyt c were induced by 200 M Ca2+ or 1 mM alloxan and 20 M Ca2+ in the presence of 5 mM suc- cinate. In blank neither Ca2+, alloxan-Ca2+ nor RuR was included in the medium. Data are represented as means ± S.D. of 3 to 11 experiments. * represents P < 0.05 compared with mitochondria treated with Ca2+ alone. ** represents P < 0.05 compared with mitochondria treated with alloxan-Ca2+. The other conditions were the same as previously de- scribed in Figure 1. Figure 6. Effects of RuR on MPT and Cyt c release. ers (Figure 6(b)), but CsA did not (Figure 2). These results indicate that RuR significantly inhibits mito- chondrial swelling and Cyt c release in mitochondria treated with high concentrations of Ca2+ or alloxan-Ca2+, and these data suggest that VDAC opening may be in- volved in Cyt c release from mitochondria. 4. DISCUSSION The present study shows that the addition of MPT in- ducers, such as high concentrations of Ca2+ alone or al- loxan-Ca2+, to mitochondrial suspension causes mito- chondrial swelling and then Cyt c release from mito- chondria isolated from rat livers. The release of Cyt c, which is bound to the outer surface of the inner mito- chondrial membrane, into the cytosol has an important role in an early event in apoptosis [4,5,7]. CsA, a repre- sentative MPT inhibitor, significantly attenuated the ex- tent of MPT induced by Ca2+ alone or alloxan-Ca2+, whereas Cyt c release from mitochondria was still ob- served (Figure 2 and Table 1). When the osmotic pressure in the matrix and inter- membranous space of mitochondria was equilibrated with PEG that cannot pass the mitochondrial inner membrane, mitochondrial swelling and Cyt c release induced by Ca2+ alone or alloxan-Ca2+ were almost completely prevented (Figure 4). Brustovetsky and Dubinsky demonstrated that high molecular compounds of PEG inhibit mitochondrial swelling induced by high concentrations of Ca2+ but do not inhibit MPT or the collapse of the mitochondrial membrane potential [28]. From these results, we propose that MPT is not required to release Cyt c from mitochondria. K+ causes mito- chondrial swelling without the induction of MPT in mi- tochondria isolated from adult rabbit hearts [33]. In our study, the addition of K+ to the mitochondrial suspension resulted in the release of Cyt c, which was not inhibited by CsA (Figure 5). These results suggest that mitochon- drial swelling that is induced through a process other than MPT is involved in the release of Cyt c from mito- chondria treated with alloxan- Ca2+ or high concentra- tions of Ca2+ alone. Halestrap et al. showed that swelling was slightly induced by the addition of 20 mM KCl [32]. Crouser et al. demonstrated that the swelling and the release of Cyt c were induced at ionic strengths that var- ied from 5 to 150 mM of KCl with 0.1 mol CaCl2, but the activity of an intermembrane space marker adenylate kinase was not released during the swelling at interme- diate values of ionic strength [34], suggesting that the rupture of mitochondrial outer membrane might not de- velop in mitochondria treated with intermediate strength of KCl. Further studies are required to elucidate the mechanism by which Cyt c is released from mitochon- dria incubated with various ionic strengths of KCl.  T. Ichimura et al. / Journal of Biophysical Chemistry 2 (2011) 10-18 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 16 Accumulating evidence indicates that there are mechanisms for passage through the outer mitochondrial membrane including the rupture of the outer membrane or VDAC opening for the release of Cyt c [10,13-17]. Normally, VDAC monomers form an aqueous pore (2.5 to 3 nm in diameter) that allows uncharged polymers, such as dextran and PEG with a molecular mass of ap- proximately 5 kDa, to pass the membranes [35,36]. Cyt c has a molecular mass of 12 kDa and should be unable to pass through the mitochondrial outer membrane by VDAC opening alone. Alternatively, oligomeric forms of VDAC create a large pore, such as the Bax-VDAC pore, with conductance levels 4-fold greater than the levels of VDAC monomers allowing Cyt c translocation across the outer membrane [19,37]. Ca2+ binding site(s) of VDAC is involved in the regulation of VDAC opening [38]. RuR, an inhibitor of MPT and of VDAC opening, strongly inhibited the Cyt c release (Figure 6). RuR binds to Ca2+ binding site(s) of VDAC and completely closes VDAC [38,39]. These data suggested that RuR may inhibit the opening of VDAC and oligomerization with MPT induction in mitochondria treated with high concentrations of Ca2+ alone or alloxan- Ca2+. However, another VDAC inhibitor, DIDS [12], did not decrease Cyt c release with high concentrations of Ca2+ (date not shown). G3139 caused a closure of VDAC and increased the sensitivity of MPT induction to high concentrations of Ca2+ (200 M) [40]. Additional studies are needed to elucidate the mechanism by which Cyt c travels through the outer mitochondrial membrane to the cytosol. Alloxan has cytotoxicity against pancreatic-cells and, thus, causes type 1 (insulin-dependent) diabetes mellitus. Although detailed mechanisms of the cytotoxicity are not yet clearly understood, alloxan causes apoptotic cell death with the release of Cyt c [7]. We previously dem- onstrated that in mitochondria, which were previously incubated with alloxan, enhanced MPT was clearly ob- served after addition of succinate [8]. The characteristics of MPT in the present study agreed with our previous report [8]. The inhibitors of mitochondrial function, such as CCCP, STG, and KCN, significantly inhibited MPT but did not inhibit the release of Cyt c (Figure 3(b) and Table 1). These findings suggest that the release of Cyt c from mitochondria is not parallel with MPT induced by alloxan-Ca2+ and may be initiated independently of en- ergy status. The present study demonstrates that mito- chondrial swelling directly correlates with Cyt c release from mitochondria treated with high concentrations of Ca2+ alone or alloxan-Ca2+. There are several lines of evidence to support that overexpression of calmodulin in mice results in insulin secretion defects, loss of pancre- atic-cells and diabetes [41,42]. The elevation of cytoso- lic Ca2+ concentrations by free fatty acid induces-cells apoptosis [43]. Several quinones, which have similar structures to alloxan, reduce the threshold of Ca2+ con- centrations to induce mitochondrial swelling [26]. These findings lead us to speculate that alloxan may reduce the threshold of Ca2+ concentration to induce MPT accom- panied by swelling and that the subsequent mitochon- drial dysfunction resulting from Cyt c release may be a cause of pancreatic-cell death. REFERENCES [1] Brookes, P.S., Yoon, Y., Robotham, J.L., Anders, M.W. and Sheu, S.S. (2004) Calcium, ATP, and ROS: A mito- chondrial love-hate triangle. American Journal of Physi- ology. Cell Physiology, 287(4), c817-c833. doi:10.1152/ajpcell.00139.2004 [2] Passarella, S., Atlante, A., Valenti, D. and Bari, L.D. (2003) The role of mitochondrial transport in energy metabolism. Mitochondrion, 2(5), 319-343. doi:10.1016/S1567-7249(03)00008-4 [3] Gunter, T. E., Yule, D. I., Gunter, K. K., Eliseev, R. A. and Salter, J. D., (2004) Calcium and mitochondria. FEBS letters, 567(1), 96-102. doi:10.1016/j.febslet.2004.03.071 [4] Danial, N.N. and Korsmeyer, S.J., (2004) Cell death: Critical control points. Cell, 116(2), 205-219. [5] Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S.M., Ahmad, M., Alnemri, E.S. and Wang, X., (1997) Cyto- chrome c and dATP-dependent formation of Apaf-1/ caspase-9 complex initiates an apoptotic protease cascade. Cell, 91(4 ), 479-489. [6] Sparagna, G.C., Hickson-Bick, D.L., Buja, LM. and McMillin, J.B. (2000) A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Ameri- can Journal of Physiology. Heart and Circulatory Physi- ology, 279(5), H2124-H2132. [7] Sakurai, K., Katoh, M., Someno, K. and Fujimoto, Y. (2001) Apoptosis and mitochondrial damage in INS-1 cells treated with alloxan. Biological & Pharmaceutical Bulletin, 24(8), 876-882. doi:10.1248/bpb.24.876 [8] Sakurai, K., Katoh, M. and Fujimoto, Y. (2001) Al- loxan-induced mitochondrial permeability transition triggered by calcium, thiol oxidation, and matrix ATP. The Journal of Biological Chemistry, 276(29), 26942- 26946. doi:10.1074/jbc.M102029200 [9] Kumarswamy, R. and Chandna, S. (2009) Putative part- ners in Bax mediated cytochrome-c release: ANT, CypD, VDAC or none of them? Mitochondrion, 9(1), 1-8. doi:10.1016/j.mito.2008.10.003 [10] Tsujimoto, Y., Nakagawa, T. and Shimizu, S. (2006) Mi- tochondrial membrane permeability transition and cell death. Biochimica et Biophysica Acta, 1757(9-10), 1297- 1300. doi:10.1016/j.bbabio.2006.03.017 [11] Ott, M., Robertson, J.D., Gogvadze, V., Zhivotovsky, B. and Orrenius, S. (2002) Cytochrome c release from mi- tochondria proceeds by a two-step process. Proceedings of the National Academy of Sciences of the United States  T. Ichimura et al. / Journal of Biophysical Chemistry 2 (2011) 10-18 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ 17 of America, 99(3), 1259-1263. doi:10.1073/pnas.241655498 [12] Petrosillo, G., Ruggiero, F.M. and Paradies, G. (2003) Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. The FASEB Journal, 17(15), 2202-2208. doi:10.1096/fj.03-0012com [13] Tsujimoto, Y. and Shimizu, S. (2000) VDAC regulation by the Bcl-2 family of proteins. Cell Death and Differen- tiation, 7(12), 1174-1181. doi:10.1038/sj.cdd.4400780 [14] Garrido, C., Galluzzi, L., Brunet, M., Puig, P. E., Didelot, C. and Kroemer, G. (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death and Differentia- tion, 13(9), 1423-1433. doi:10.1038/sj.cdd.4401950 [15] Brustovetsky, N., Brustovetsky, T., Jemmerson, R. and Dubinsky, J.M. (2002) Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer mem- brane. Journal of Neurochemistry, 80(2), 207-218. doi:10.1046/j.0022-3042.2001.00671.x [16] Petit, P.X., Goubern, M., Diolez, P., Susin, S.A., Zamzami, N. and Kroemer, G. (1998) Disruption of the outer mito- chondrial membrane as a result of large amplitude swell- ing: the impact of irreversible permeability transition. The FASEB Journal, 426(1), 111-116. [17] Doran, E. and Halestrap, A.P. (2000) Cytochrome c re- lease from isolated rat liver mitochondria can occur in- dependently of outer-membrane rupture: Possible role of contact sites. The Biochemical Journal, 348(2), 343-350. doi:10.1042/0264-6021:3480343 [18] Jürgensmeier, J.M., Xie, Z., Deveraux, Q., Ellerby, L., Bredesen, D. and Reed, J.C. (1998) Bax directly induces release of cytochrome c from isolated mitochondria. Proceedings of the National Academy of Sciences of the United States of America, 95(9), 4997-5002. doi:10.1073/pnas.95.9.4997 [19] Shimizu, S., Ide, T., Yanagida, T. and Tsujimoto, Y. (2000) Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. The Journal of Biological Chemistry, 275(16), 12321-12325. doi:10.1074/jbc.275.16.12321 [20] Kim, T.H., Zhao, Y., Barber, M.J., Kuharsky, D.K. and Yin, X.M. (2000) Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. The Journal of Biological Chemistry, 275(50), 39474-39481. doi:10.1074/jbc.M003370200 [21] Uren, R.T., Dewson, G., Bonzon, C., Lithgow, T., New- meyer, D.D. and Kluck, R.M. (2005) Mitochondrial re- lease of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mito- chondrial membranes. The Journal of Biological Chem- istry, 280(3), 2266-2274. doi:10.1074/jbc.M411106200 [22] Haworth, R.A. and Hunter, D.R. (1979) The Ca2+-in- duced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Archives of Biochemistry and Bio- physics, 195(2), 460-467. doi:10.1016/0003-9861(79)90372-2 [23] Johnston, J.D. and Brand, M.D. (1990) The mechanism of Ca2+ stimulation of citrulline and N-acetylglutamate synthesis by mitochondria. Biochimica et Biophysica Acta, 1033(1), 85-90. [24] Paola, M.D. and Lorusso, M. (2006) Interaction of free fatty acids with mitochondria: Coupling, uncoupling and permeability transition. Biochimica et Biophysica Acta, 1757(9-10), 1330-1337. doi:10.1016/j.bbabio.2006.03.024 [25] Mironova, G.D., Gritsenko, E., Gateau-Roesch, O., Levrat, C., Agafonov, A., Belosludtsev, K., Prigent, A. F., Muntean, D., Dubois, M. and Ovize, M. (2004) Formation of palmitic acid/Ca2+ complexes in the mitochondrial mem- brane: a possible role in the cyclosporin-insensitive per- meability transition. Journal of Bioenergetics and Bio- membranes, 36(2), 171-178. doi:10.1023/B:JOBB.0000023620.42653.b7 [26] Halestrap, A.P., Woodfield, K.Y. and Connern, C.P. (1997) Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transi- tion by affecting nucleotide binding to the adenine nu- cleotide translocase. The Journal of Biological Chemistry, 272(6), 3346-3354. doi:10.1074/jbc.272.6.3346 [27] Andreu, G.L.P., Delgado, R., Velho, J.A., Curti, C. and Vercesi, A.E. (2005) Mangiferin, a natural occurring glucosyl xanthone, increases susceptibility of rat liver mitochondria to calcium-induced permeability transition. Archives of Biochemistry and Biophysics, 439(2), 184-193. doi:10.1016/j.abb.2005.05.015 [28] Brustovetsky, N. and Dubinsky, J.M. (2000) Limitations of cyclosporin A inhibition of the permeability transition in CNS mitochondria. The Journal of Neuroscience, 20(22), 8229-8237. [29] Gunter, T.E., Buntinas, L., Sparagna, G.C. and Gunter, K.K. (1998) The Ca2+ transport mechanisms of mito- chondria and Ca2+ uptake from physiological-type Ca2+ transients. Biochimica et Biophysica Acta, 1366(1-2), 5-15. doi:10.1016/S0005-2728(98)00117-0 [30] Armstrong, J.S., Yang, H., Duan, W. and Whiteman, M. (2004) Cytochrome bc(1) regulates the mitochondrial permeability transition by two distinct pathways. The Journal of Biological Chemistry, 279(48), 50420-50428. doi:10.1074/jbc.M408882200 [31] Sakurai, K., Stoyanovsky, D.A., Fujimoto, Y. and Ceder- baum, A.I. (2000) Mitochondrial permeability transition induced by 1-hydroxyethyl radical. Free Radical Biology & Medicine, 28(2), 273-280. doi:10.1016/S0891-5849(99)00236-1 [32] Halestrap, A.P., Quinlan, P.T., Whipps, D.E. and Arm- ston, A.E. (1986) Regulation of the mitochondrial matrix volume in vivo and in vitro. The role of calcium. The Biochemical Journal, 236(3), 779-787. [33] Korge, P., Honda, H.M. and Weiss, J.N. (2005) K+-de- pendent regulation of matrix volume improves mito- chondrial function under conditions mimicking ische- mia-reperfusion. American Journal of Physiology. Heart and Circulatory Physiology, 289(1), H66-H77. doi:10.1152/ajpheart.01296.2004 [34] Crouser, E.D., Gadd, M.E., Julian, M.W., Huff, J.E., Broekemeier, K.M., Robbins, K.A. and Pfeiffer, D.R.  T. Ichimura et al. / Journal of Biophysical Chemistry 2 (2011) 10-18 Copyright © 2011 SciRes. http://www.scirp.org/journal/JBPC/Openly accessible at 18 (2003) Quantitation of cytochrome c release from rat liver mitochondria. Analytical Biochemistry, 317(1), 67-75. doi:10.1016/S0003-2697 (03)000 44-7 [35] Colombini, M. (1980) Structure and mode of action of a voltage dependent anion-selective channel (VDAC) lo- cated in the outer mitochondrial membrane. Annals of the New York Academy of Sciences, 341, 552-63. doi:10.1111/j.1749-6632.1980.tb47198.x [36] Zalman, L.S., Nikaido, H. and Kagawa, Y. (1980) Mito- chondrial outer membrane contains a protein producing nonspecific diffusion channels. The Journal of Biological Chemistry. 255(5), 1771-1774. [37] Shoshan-Barmatz, V., Israelson, A., Brdiczka, D. and Sheu, S.S. (2006) The voltage-dependent anion channel (VDAC): Function in intracellular signalling, cell life and cell death. Current Pharmaceutical Design, 12(18), 2249-2270. doi:10.2174/138161206777585111 [38] Gincel, D., Zaid, H. and Shoshan-Barmatz, V. (2001) Calcium binding and translocation by the voltage-dependent anion channel: A possible regulatory mechanism in mi- tochondrial function. The Biochemical Journal, 358(Pt1), 147-155. doi:10.1042/0264-6021:3580147 [39] Israelson, A., Abu-Hamad, S., Zaid, H., Nahon, E. and Shoshan-Barmatz, V. (2007) Localization of the voltage- dependent anion channel-1 Ca2+-binding sites. Cell Cal- cium, 41(3), 235-244. doi:10.1016/j.ceca.2006.06.005 [40] Tikunov, A., Johnson, C.B., Pediaditakis, P., Markevich, N., Macdonald, J.M., Lemasters, J.J. and Holmuhamedov, E. (2010) Closure of VDAC causes oxidative stress and accelerates the Ca(2+)-induced mitochondrial permeabil- ity transition in rat liver mitochondria. Archives of Bio- chemistry and Biophysics, 495(2), 174-181. doi:10.1016/j.abb.2010.01.008 [41] Gómez Dumm, C.L., Atwater, I., Epstein, P.N. and Gagliardino, J.J. (1994) Quantitative immunocytochemi- cal study of islet cell populations in diabetic calmodulin- transgenic mice. Virchows Archiv, 425(1), 73-77. [42] Epstein, P.N., Ribar, T.J., Decker, G.L., Yaney, G. and Means, A.R. (1992) Elevated beta-cell calmodulin pro- duces a unique insulin secretory defect in transgenic mice. Endocrinology, 130(3), 1387-1393. doi:10.1210/en.130.3.1387 [43] Gwiazda, K.S., Yang, T.L., Lin, Y. and Johnson, J.D. (2009) Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. American Journal of Physiology. Endocrinology and Metabolism, 296(4), E690-E701. doi:10.1152/ajpendo.90 525.200 8
|