 Open Journal of Physical Chemistry, 2013, 3, 138-149 Published Online November 2013 (http://www.scirp.org/journal/ojpc) http://dx.doi.org/10.4236/ojpc.2013.34017 Open Access OJPC Substituent, Temperature and Solvent Effects on the Keto-Enol EQUILIBRIUM in -Ketoamides: A Nuclear Magnetic Resonance Study Sergio L. Laurella, Manuel González Sierra, Jorge J. P. Furlong, Patricia E. Allegretti* Laboratorio LADECOR, División Química Orgánica, Departamento de Química, Facultad de Ciencias Exactas, Universidad Nacional de La Plata (UNLP), La Plata, Argentina Email: *pallegre@quimica.unlp.edu.ar Received August 2, 2013; revised September 1, 2013; accepted September 9, 2013 Copyright © 2013 Sergio L. Laurella et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Substituent, temperature and solvent effects on tautomeric equilibria in several β-ketoamides have been investigated by means of nuclear magnetic resonance spectroscopy (NMR). Keto-enol equilibrium predominates over the amide-imidol one. The relative stability of the individual tautomers and the corresponding equilibrium shifts are explained consider- ing electronic and steric effects and tautomer stabilization via internal hydrogen bonds. In solution, these compounds exist mainly as ketoamide and Z-enolamide tautomers, both presenting intramolecular hydrogen bonds. Keywords: β-Ketoamides; Keto-Enol Equilibrium; Nuclear Magnetic Resonance Spectroscopy 1. Introduction Keto-enol tautomerism in β-ketoesters, β-diketones and β-ketonitriles is a topic that has been extensively studied from several points of view and by means of a variety of experimental methods [1-3]. However, the occurrence of this phenomenon in β-ketoamides has not been studied deeply, with exception of a few previous works [4,5]. It is usual to describe them only as keto forms [6], although some of them have been demonstrated to exist as a tau- tomeric mixture where the enol form is the major tauto- mer. The importance of studying β-ketoamides arises from their versatility as intermediates in the synthesis of sev- eral heterocycles: 3-acyltetramic acids [7] (used in the total synthesis of tirandamycin and other related natural antibiotics [8]), pyrans [9], alkaloids [10], lactams and spirolactams [11], azetidin-2-ones [12], as well as several 3-hydroxyisothiazol bioisosteres of glutamic acid and analogs of the AMPA receptor agonist [13]. Moreover, some β-ketoamides have been converted into γ-ketoa- mides, a class of compounds related with a wide variety of biologically relevant systems [14]. The reactivity of β-ketoamides is related to their structure and their tautomeric equilibria; that is why it should be useful to determine their spectral behaviour in different conditions in order to study their tautomeric distribution. Hence, it is of practical and theoretical im- portance to investigate tautomeric equilibria in such sys- tems. Keto-enol tautomerism has attracted much interest during the last few decades. The fact that the equilibrium involved is sufficiently slow to permit keto and enol tautomeric forms to be detected by nuclear magnetic resonance (NMR) spectroscopy has allowed many inves- tigations on these processes [15]. The tautomeric equilibria of some β-ketobutanamides in solution were investigated by means of 1HNMR and 13CNMR. Their chemical shifts were compared with those of related β-hydroxybutanamides. Equilibrium po- pulations of the keto and enol forms were measured. Substituent effects on the chemical shifts and the equilib- rium populations were discussed [16]. Intramolecular hydrogen bonding is the main factor that governs the kinetics and influences the structure of keto-enol tautomerism in solution. Regarding β-ke- toamides, internal hydrogen bonding is possible to be established in several tautomeric forms. This point has been studied for a series of 3-oxo-2-phenylbutanamides [17]. *Corresponding author.  S. L. LAURELLA ET AL. 139 In the present work, effects of substituents, solvents and temperature on the equilibria among different tauto- meric forms in eleven β-ketoamides have been studied. Differential solvation effects, electron donor and accep- tor substituents and temperature variations should shift the protomeric tautomerism. 2. Experimental 2.1. Synthesis of β-Ketoamides β-ketoamides were synthesized and purified according to literature procedures or their modified versions [18]. The compounds under study were identified by 1HNMR and 13CNMR in DMSO-d6, in which the peaks corresponding to the enol forms are depleted (Table 1). 2.2. NMR Measurements 1HNMR spectra in CDCl3 and DMSO-d6 were recorded with a Bruker 300 spectrometer, 300.13 MHz, grad Z and temperature control. The typical spectral conditions were as follows: spectral width 4000 Hz, acquisition time 2 s and 8 - 16 scans per spectrum. Digital resolution was 0.39 Hz per point, TMS was used as internal standard. Sample concentrations were 0.05 M. Spectra were taken at 25˚C, 35˚C and 45˚C. The content of long-lived tautomeric forms was calculated from the integrated peak intensities of hydoxyl and methine proton signals. 13C proton decoupled and gated decoupled spectra were recorded with a Varian Mercury Plus 200 spec- trometer operating at 4.5 T from DMSO-d6 solutions at 25˚C. The spectral conditions were the following: spec- tral width 10,559 Hz, acquisition times 1.303 s and 512 - 1000 scans per spectrum. 3. Results and Discussion Schemes 1 and 2 show the possible tautomeric structures for β-ketoamides I-III and IV-XI respectively. Each NMR spectrum is the result of the superposition of the spectra of the individual tautomers, since they are altogether in equilibrium. The only two tautomeric forms that could be identified in each spectrum were ketoamide and Z-enolamide (Scheme 3). The rest of the tautomeric forms could not be detected, and this fact indicates that they are absent or in very low concentration. The as- signment of the peaks to their corresponding protons was made keeping in mind the theoretical displacements. As an example, Figure 1 shows the 1HNMR spectrum of I (3-oxo-2-phenylbutanamide) in CDCl3 at 25˚C. Val- ues at the top correspond to the chemical shifts and the ones at the bottom, to the integration of each peak. In order to assign the 1HNMR signals to the corre- sponding tautomers, the peaks can be separated into to groups whose integration values show simple ratios. Peaks A, B, C and D appear to be in 3:1:1:1 ratio, while peaks X, Y and Z show 3:2:1 ratio. Table 2 shows the expected number of non-aromatic signals and their respective integration for each tautomer, considering that in some of them internal hydrogen bond is possible to be established. Thus, peaks X, Y ans Z can be assigned to the Z- enolamide hydrogens (CH3, NH2 and OH respectively), whereas peaks A, B, C and D can assigned to the ke- toamide hydrogens (CH3, CH, NH and NH respectively). The possibility of the latter to belong to ketoimidol or 2-enolimidol tautomers is discarded regarding previous studies which include theoretical calculations on these compounds [19]. Intramolecular hydrogen bonding is the main factor that governs the kinetics and influences the structure of keto-enol tautomerism in solution. In the case of keto- amides, the two tautomers of major concentration are capable of establishing internal hydrogen bonds (see Scheme 3). This stabilizing factor explains the following observations: 1) The high relative concentration of the involved tautomers, 2) The high value of δ observed for the hydroxyl pro- ton in the enolamide form (peak Z, Figure 1), 3) The two different δ values of the hydrogen atoms bonded to nitrogen in the ketoamide form (peaks C and D, Figure 1). Table 3 shows the 1H chemical shifts of the studied compounds in CDCl3 and DMSO-d6. In many cases, hy- drogens attached to N were observed as very broad and low peaks in DMSO-d6 (due to the fact that they estab- lish hydrogen bonds with the solvent causing a signify- cant broadening of the corresponding signals), so their chemical shift could not be stablished properly. Atom numbering is shown in Scheme 3. Table 4 shows the enol content present in each com- pound for both solvents. The integrated spectra made possible to calculate the enol ratio considering the peaks of H linked to C-2 (ketoamide tautomer) and the OH (Z-enolamide tautomer). Thus, enolic contents were cal- culated as follows: % enol = (OH integration)/(C-2 integration) Com- pounds I-VIII % enol = (OH integration)/((C-2 integration)/2) Com- pounds IX-XI Then the equilibrium constant (Keq = [enol]/[keto]) and the corresponding free energy differences at 25˚C (ΔG0 = −RT lnKeq) for the keto-enol equilibrium were determined (Table 3). The relative stability of individual tautomers and the corresponding equilibrium shifts are explained consider- ing several factors, such as electronic effects on the car- onyl group, stabilization by conjugation of the enol b Open Access OJPC  S. L. LAURELLA ET AL. Open Access OJPC 140 Table 1. 1HNMR and 13CNMR data for the selected -ketoamides (200MHz, DMSO-d6). COMPOUND 1H NMR (ppm) 13C NMR (ppm) COMPOUND 1H NMR (ppm) 13C NMR (ppm) O NH2 O 1 2 3 4 56 7 8 6 7 3-oxo-2-phenylbutanamide (I) 2.13 (s, 3H, 4) 4.58 (s, 1H, 2) 7.2-7.4 (m,5H, 6-7-8) 27.3 (4) 65.3 (2) 127.1 (8) 127.8 (6) 128.8 (7) 138.8 (5) 172.7 (1) 206.0 (3) O NH2 O 1 2 3 4 56 7 8 6 7 OCH3 9 2-(4-methoxyphenyl)-3-oxobutanam ide (II) 2.10 (s,3H, 4) 3.85 (s,3H 9) 4.51 (s,1H, 2) 6.87 (d,2H, 7) 7.12 (d,2H, 6) 27.1 (4) 56.5 (9) 62.1 (2) 114.4 (7) 130.1 (6) 131.1 (5) 159.1 (8) 171.0 (1) 205.5 (3) O NH2 O 1 2 3 4 56 7 8 6 7 Cl 2-(4-chlorophenyl)-3-oxobutanami de (III) 2.16 (s, 3H, 4) 4.65 (s, 1H, 2) 7.17 (d, 2H, 6) 7.37 (d, 2H, 7) 27.8 (4) 66.3 (2) 128.9 (7) 130.5 (6) 132.7 (8) 136.9 (5) 173.1 (1) 206.2 (3) O 1 2 3 4 5 6 76 55´ 6´ 7´ 4´ 5´ 6´ NH2 O 3-oxo-2,3-diphenylpropanamide (IV) 5.75 (s, 1H, 2) 7.2-7.9 (m, 10H, 5-5´-6-6´-7-7´) 60.3 (2) 127.5 (7´) 129.4 (6´) 128.0 (5´) 141.0 (4´) 128.2 (6) 128.9 (5) 132.7 (7) 172.1 (1) 195.6 (3) O 1 2 3 4 5 6 7 6 55´ 6´ 7´ 4´ 5´ 6´ H3CO 8 NH 2 O 3-(4-methoxyphenyl)-3-oxo-2-phe nylpropanamide (V) 3.83 (s, 3H, 8) 5.69 (s, 1H, 2) 7.1-7.9 (m, 10H, 5-5´-6-6´-7-7´) 60.9 (2) 55.6 (8) 114.3 (6) 127.2 (7´) 128.4 (5´) 129.0 (4) 129.3 (6´) 129.8 (5) 140.4 (4´) 165.2 (7) 172.5 (1) 194.1 (3) O 1 2 3 4 5 6 7 6 55´ 6´ 7´ 4´ 5´ 6´ Cl NH2 O 3-(4-chlorophenyl)-3-oxo-2-phenylp ropanamide (VI) 5.74 (s, 1H, 2) 7.2-8.0 (m, 10H, 5-5´-6-6´-7-7´) 61.5 (2) 127.5 (7´) 128.1 (5´) 128.7 (6) 129.2 (6´) 130.4 (5) 135.0 (4) 138.3 (7) 140.5 (4´) 173.2 (1) 195.3 (3) O 1 2 3 4 5 6 7 6 55´ 6´ 7´ 4´ 5´ 6´ OCH3 8 NH2 O 2-(4-methoxyphenyl)-3-oxo-3-phe nylpropanamide (VII) 3.73 (s, 3H, 8) 5.68 (s, 1H, 2) 7.1-7.9 (m, 10H, 5-5´-6-6´-7-7´) 60.5 (2) 55.3 (8) 114.8 (6´) 128.4 (6) 128.8 (5) 130.6 (5´) 132.7 (4´) 133.1 (7) 136.7 (4) 159.5 (7´) 171.1 (1) 193.2 (3) O 1 2 3 4 5 6 7 6 Cl 55´ 6´ 7´ 4´ 5´ 6´ NH2 O 2-(4-chlorophenyl)-3-oxo-3-phenylp ropanamide (VIII) 5.79 (s, 1H, 2) 7.3-7.9 (m, 10H, 5-5´-6-6´-7-7´) 61.7 (2) 128.2 (6) 128.7 (5) 129.3 (6´) 131.0 (5´) 133.0 (7´) 133.5 (7) 136.9 (4) 138.5 (4´) 173.0 (1) 195.3 (3) O 1 2 3 4 5 6 7 6 5 NH 2 O 3-oxo-3-phenylbutanamide (IX) 3.44 (s, 2H, 2) 7.16 (s, 2H, N) 7.5-7.9 (m, 5H, 5-6-7) 45.3 (2) 128.9 (5) 128.5 (6) 133.1 (7) 136.7 (4) 171.3 (1) 194.2 (3) O 1 2 3 4 5 6 7 6 5 H3CO 8 NH2 O 3-(4-methoxyphenyl)-3-oxopropana mide (X) 3.41 (s, 2H, 2) 3.83 (s, 3H, 8) 7.02 (s, 2H, N) 7.21 (d, 2H, 6) 7.83 (d, 2H, 5) 44.8 (2) 55.8 (8) 114.2 (6) 129.0 (4) 129.8 (5) 165.0 (7) 171.2 (1) 193.5 (3) O 1 2 3 4 5 6 7 6 5 Cl NH2 O 3-(4-chlorophenyl)-3-oxopropanam ide (XI) 3.55 (s, 2H, 2) 7.20 (s, 2H, N) 7.60 (d, 2H, 6) 7.88 (d, 2H, 5) 46.2 (2) 128.7 (6) 130.2 (5) 134.8 (4) 138.7 (7) 171.8 (1) 195.1 (3)  S. L. LAURELLA ET AL. 141 Scheme 1. Possible tautomeric structures for compounds I-III. Scheme 2. Possible tautomeric structures for compounds IV-XI. Scheme 3. Internal hydrogen bonds occurring in Z-enolamide and ketoamide tautomers. Table 2. Expected signal integration for non-aromatic hydrogens in compund I tautomers. Tautomer ketoamide 2-enolamide 3-enolamide ketoimidol 2-enolimidol 3-enolimidol Expected signal integration 3:1:1:1 3:2:1 2:1:1:1:1 3:1:1:1 3:1:1:1 1:1:1:1:1:1 double bond, steric effects introduced by bulky groups and tautomer stabilization via internal hydrogen bonds. Steric effects: The structure of Z-enolamide tautomers of compounds presenting two phenyl groups (compounds IV-VIII) exhibit greater steric repulsion than compounds having a phenyl and a methyl groups (compounds I-III), reducing the enol content in the former ones. In the case of ketoamide tautomer, this steric repulsion is reduced because of the rotation in the C-2 - C-3 bond, letting the two phenyl groups to get further from each other. On the other hand, bulky phenyl groups in C-2 posi- tion increase the enolic content (compare compounds IX-XI with I-III and IV-VIII). This fact is in concor- ance with previous studies [20,21]. d Open Access OJPC 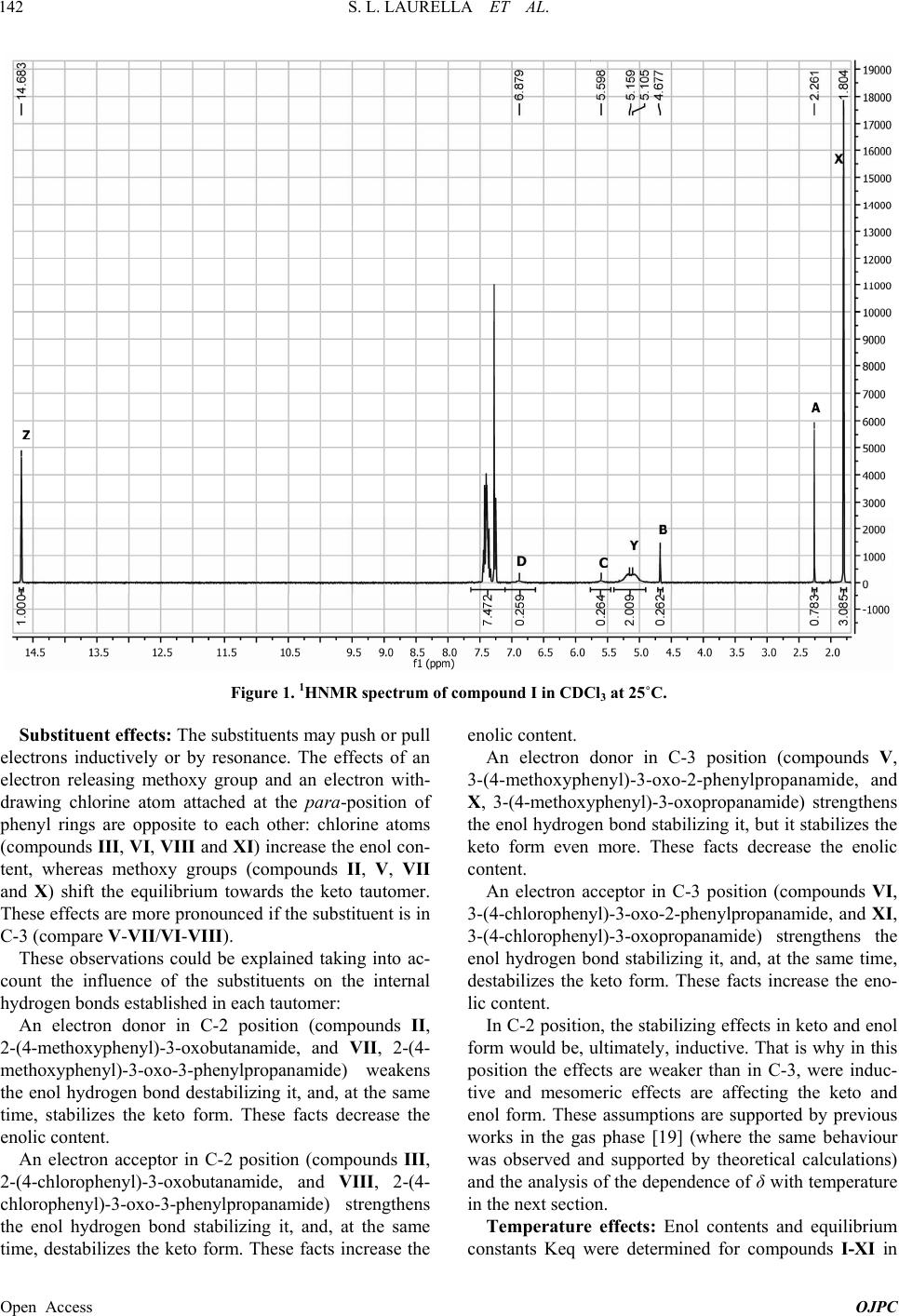 S. L. LAURELLA ET AL. 142 Figure 1. 1HNMR spectrum of compound I in CDCl3 at 25˚C. Substituent effects: The substituents may push or pull electrons inductively or by resonance. The effects of an electron releasing methoxy group and an electron with- drawing chlorine atom attached at the para-position of phenyl rings are opposite to each other: chlorine atoms (compounds III, VI, VIII and XI) increase the enol con- tent, whereas methoxy groups (compounds II, V, VII and X) shift the equilibrium towards the keto tautomer. These effects are more pronounced if the substituent is in C-3 (compare V-VII/VI-VIII). These observations could be explained taking into ac- count the influence of the substituents on the internal hydrogen bonds established in each tautomer: An electron donor in C-2 position (compounds II, 2-(4-methoxyphenyl)-3-oxobutanamide, and VII, 2-(4- methoxyphenyl)-3-oxo-3-phenylpropanamide) weakens the enol hydrogen bond destabilizing it, and, at the same time, stabilizes the keto form. These facts decrease the enolic content. An electron acceptor in C-2 position (compounds III, 2-(4-chlorophenyl)-3-oxobutanamide, and VIII, 2-(4- chlorophenyl)-3-oxo-3-phenylpropanamide) strengthens the enol hydrogen bond stabilizing it, and, at the same time, destabilizes the keto form. These facts increase the enolic content. An electron donor in C-3 position (compounds V, 3-(4-methoxyphenyl)-3-oxo-2-phenylpropanamide, and X, 3-(4-methoxyphenyl)-3-oxopropanamide) strengthens the enol hydrogen bond stabilizing it, but it stabilizes the keto form even more. These facts decrease the enolic content. An electron acceptor in C-3 position (compounds VI, 3-(4-chlorophenyl)-3-oxo-2-phenylpropanamide, and XI, 3-(4-chlorophenyl)-3-oxopropanamide) strengthens the enol hydrogen bond stabilizing it, and, at the same time, destabilizes the keto form. These facts increase the eno- lic content. In C-2 position, the stabilizing effects in keto and enol form would be, ultimately, inductive. That is why in this position the effects are weaker than in C-3, were induc- tive and mesomeric effects are affecting the keto and enol form. These assumptions are supported by previous works in the gas phase [19] (where the same behaviour was observed and supported by theoretical calculations) and the analysis of the dependence of δ with temperature in the next section. Temperature effects: Enol contents and equilibrium onstants Keq were determined for compounds I-XI in c Open Access OJPC  S. L. LAURELLA ET AL. 143 Table 3. 1H chemical shifts (δ, ppm) for compounds I-XI (atom numbering depicted in Scheme 3). Compound Solvent δH CDCl3 1.80 (C-4 enol); 2.26 (C-4 keto); 4.68 (C-2 keto); 5.10/5.16 (NH2 enol); 5.60/6.88 (NH2 keto); 7.2 - 7.5 (aromatics); 14.68 (OH enol). I DMSO-d6 1.66 (C-4 enol); 2.13 (C-4 keto); 4.58 (C-2 keto); 7.2 - 7.4 (aromatics); 15.7 (OH enol). CDCl3 1.79 (C-4 enol); 2.24 (C-4 keto); 3.82 (OCH3 keto); 3.84 (OCH3 enol); 4.61 (C-2 keto); 5.10/5.31 (NH2 enol); 5.73/6.81 (NH2 keto); 6.9 - 7.4 (aromatics); 14.63 (OH enol). II DMSO-d6 1.71 (C-4 enol); 2.10 (C-4 keto); 3.85 (OCH3 keto); 3.89 (OCH3 enol); 4.51 (C-2 keto); 6.8 - 7.2 (aromatics) 15.67 (OH enol). CDCl3 1.80 (C-4 enol); 2.26 (C-4 keto); 4.63 (C-2 keto); 5.02/5.21 (NH2 enol); 5.63/6.89 (NH2 keto); 7.2 - 7.5 (aromatics); 14.72 (OH enol). III DMSO-d6 1.66 (C-4 enol); 2.16 (C-4 keto); 4.65 (C-2 keto); 7.2 - 7.5 (aromatics); 15.79 (OH enol). CDCl3 5.30 (NH2 enol); 5.61 (C-2 keto); 5.53/6.98 (NH2 keto); 7.1 - 8.0 (aromatics); 15.25 (OH enol). IV DMSO-d6 5.75 (C-2 keto); 7.2 - 7.9 (aromatics) CDCl3 3.90 (OCH3 keto); 3.93 (OCH3 enol); 5.31 (NH2 enol); 5.49 (C-2 keto); 5.63/7.18 (NH2 keto); 6.9 - 8.0 (aromatics); 14.31 (OH enol). V DMSO-d6 3.83 (OCH3 keto); 5.69 (C-2 keto); 7.1 - 7.9 (aromatics) CDCl3 5.37/5.46 (NH2 enol); 5.54 (C-2 keto); 5.72/6.93 (NH2 keto); 7.0 - 8.0 (aromatics); 15.30 (OH enol). VI DMSO-d6 5.74 (C-2 keto); 7.2 - 8.0 (aromatics) CDCl3 3.76 (OCH3 keto); 3.76 (OCH3 enol); 5.38 (NH2 enol); 5.54 (C-2 keto); 5.66/6.91 (NH2 keto); 6.8 - 8.0 (aromatics); 15.22 (OH enol). VII DMSO-d6 3.73 (OCH3 keto); 5.68 (C-2 keto); 7.1 - 7.9 (aromatics) CDCl3 5.33 (NH2 enol); 5.58 (C-2 keto); 5.62/7.21 (NH2 keto); 7.0 - 8.0 (aromatics); 15.32 (OH enol). VIII DMSO-d6 5.79 (C-2 keto); 7.3 - 7.9 (aromatics) CDCl3 3.99 (C-2 keto); 5.26 (NH2 enol); 5.57 (C-2 enol); 5.55/7.02 (NH2 keto); 7.2 - 8.1 (aromatics); 14.22 (OH enol). IX DMSO-d6 3.90 (C-2 keto); 7.15 (NH2 enol); 5.78 (C-2 enol); 7.36 (NH2 keto); 7.4 - 8.1 (aromatics); 15.31 (OH enol). CDCl3 3.86 (OCH3 enol); 3.90 (OCH3 keto); 3.93 (C-2 keto); 5.49 (C-2 enol); 5.64/7.21 (NH2 keto); 6.9 - 8.0 (aromatics); 14.31 (OH enol). X DMSO-d6 3.78 (C-2 keto); 3.80 (OCH3 enol); 3.84 (OCH3 keto); 5.63 (C-2 enol); 7.0 - 8.0 (aromatics); 15.27 (OH enol). CDCl3 3.96 (C-2 keto); 5.34 (NH2 enol); 5.54 (C-2 enol); 5.68/7.00 (NH2 keto); 7.2 - 8.0 (aromatics); 14.28 (OH enol). XI DMSO-d6 3.86 (C-2 keto); 5.74 (C-2 enol); 7.1 - 8.0 (aromatics); 15.31 (OH enol). 1In compounds VII, VIII and X, the peak corresponding to the ketoamide NH2 overlaps the aromatic signals and its value could not be determined precisely. CDCl3 and DMSO-d6 at five different temperatures be- tween 25˚C and 45˚C. Equation 1 provides a simple method to determine ΔH and ΔS in keto-enol tautomeri- zation for the studied compounds. enol 1 ln ln keto GH kRTR TR S (1) Figures 2 and 3 show the lnK vs 1/T plot for β-ke- toamides I-XI in both solvents. The calculated slopes and y-intercepts from these graphics can be used to determine the enthalpy and entropy changes. Results are shown in Table 5. As it is expected, compounds bearing an electron re- leasing group (compounds II, V, VII and X), which have lower enolic contents (Table 3), show higher values of ΔH. Compounds attached to electron withdrawing groups (compounds III, VI, VIII and XI) have higher enolic contents and lower ΔH values. Solvent effects: Differential solvatation effects should shift the protomeric tautomerism. Data from Table 4 clearly demonstrate that an increase in the solvent polar- ity increases the proportions of keto forms for com- pounds I-VIII. In the case of compounds IX-XI, the ef- fect is the opposite. This effect can be explained consid- ering the values of ΔH and ΔS of each compound. Open Access OJPC 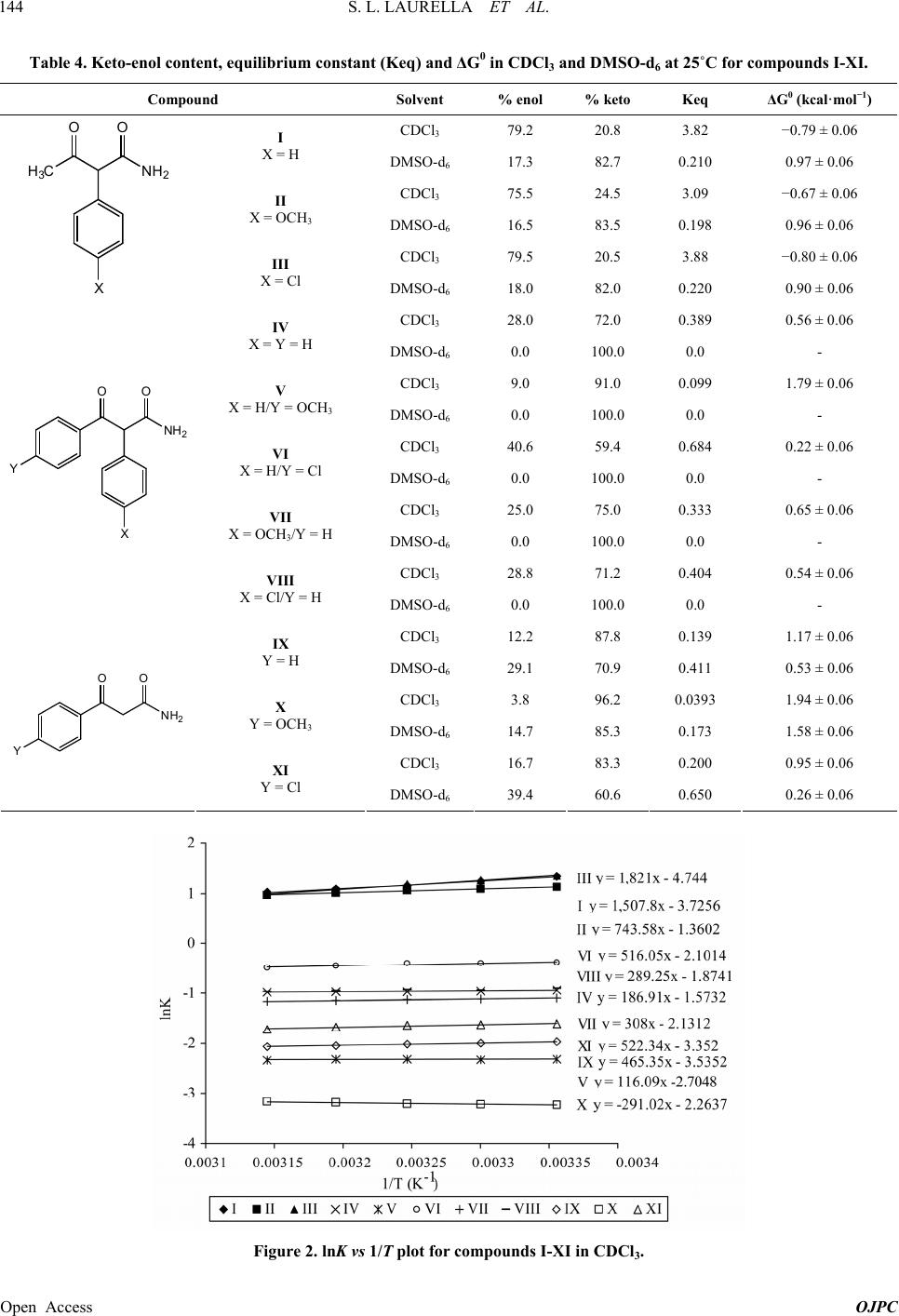 S. L. LAURELLA ET AL. 144 Table 4. Keto-enol content, equilibrium constant (Keq) and ΔG0 in CDCl3 and DMSO-d6 at 25˚C for compounds I-XI. Compound Solvent % enol % keto Keq ΔG0 (kcal·mol−1) CDCl3 79.2 20.8 3.82 −0.79 ± 0.06 I X = H DMSO-d6 17.3 82.7 0.210 0.97 ± 0.06 CDCl3 75.5 24.5 3.09 −0.67 ± 0.06 II X = OCH3 DMSO-d6 16.5 83.5 0.198 0.96 ± 0.06 CDCl3 79.5 20.5 3.88 −0.80 ± 0.06 H3C O NH2 O X III X = Cl DMSO-d6 18.0 82.0 0.220 0.90 ± 0.06 CDCl3 28.0 72.0 0.389 0.56 ± 0.06 IV X = Y = H DMSO-d6 0.0 100.0 0.0 - CDCl3 9.0 91.0 0.099 1.79 ± 0.06 V X = H/Y = OCH3 DMSO-d6 0.0 100.0 0.0 - CDCl3 40.6 59.4 0.684 0.22 ± 0.06 VI X = H/Y = Cl DMSO-d6 0.0 100.0 0.0 - CDCl3 25.0 75.0 0.333 0.65 ± 0.06 VII X = OCH3/Y = H DMSO-d6 0.0 100.0 0.0 - CDCl3 28.8 71.2 0.404 0.54 ± 0.06 O NH2 O X Y VIII X = Cl/Y = H DMSO-d6 0.0 100.0 0.0 - CDCl3 12.2 87.8 0.139 1.17 ± 0.06 IX Y = H DMSO-d6 29.1 70.9 0.411 0.53 ± 0.06 CDCl3 3.8 96.2 0.0393 1.94 ± 0.06 X Y = OCH3 DMSO-d6 14.7 85.3 0.173 1.58 ± 0.06 CDCl3 16.7 83.3 0.200 0.95 ± 0.06 O NH2 O Y XI Y = Cl DMSO-d6 39.4 60.6 0.650 0.26 ± 0.06 Figure 2. lnK vs 1/T plot for compounds I-XI in CDCl3. Open Access OJPC 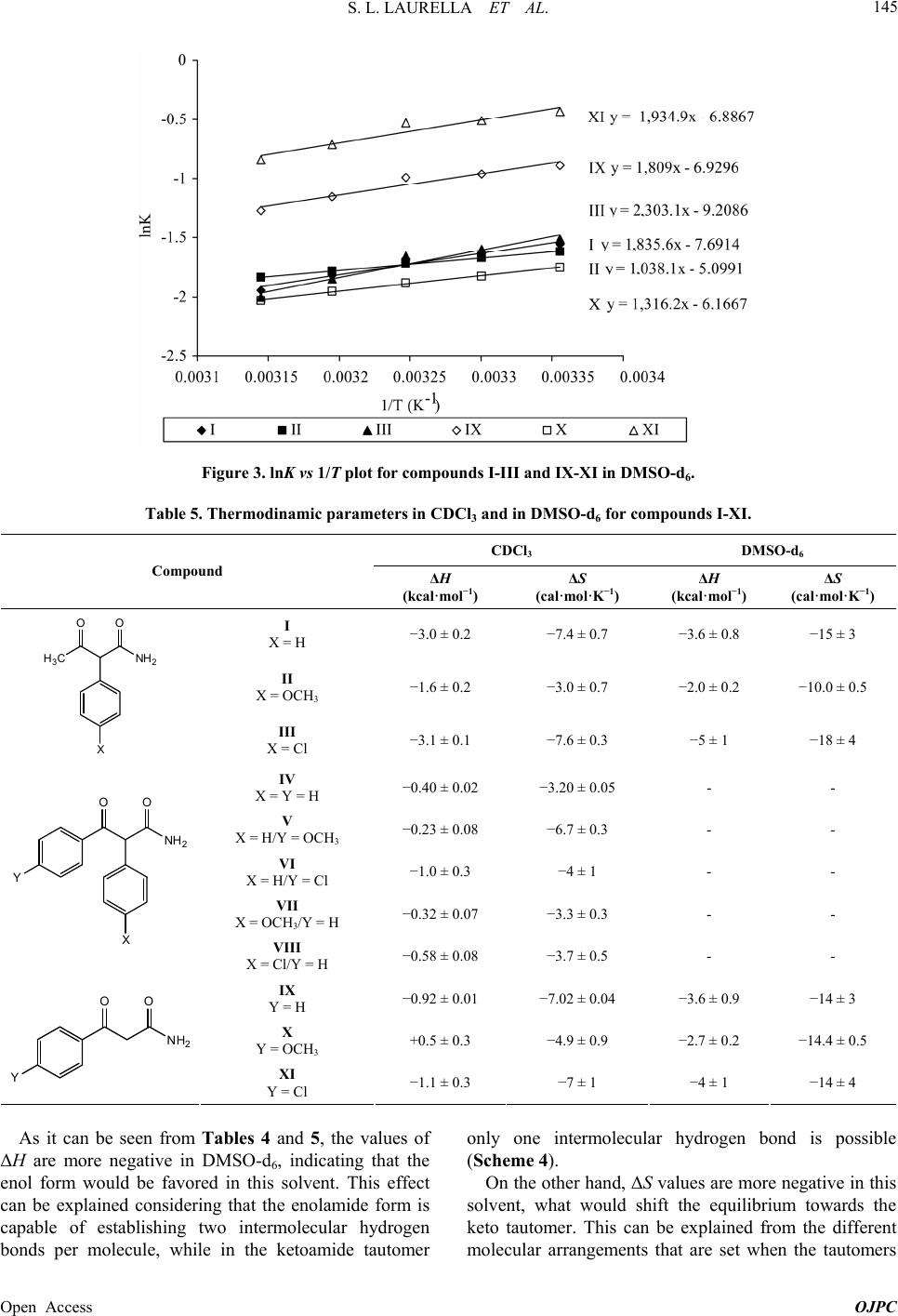 S. L. LAURELLA ET AL. 145 Figure 3. lnK vs 1/T plot for compounds I-III and IX-XI in DMSO-d6. Table 5. Thermodinamic parameters in CDCl3 and in DMSO-d6 for compounds I-XI. CDCl3 DMSO-d6 Compound ΔH (kcal·mol−1) ΔS (cal·mol·K−1) ΔH (kcal·mol−1) ΔS (cal·mol·K−1) I X = H −3.0 ± 0.2 −7.4 ± 0.7 −3.6 ± 0.8 −15 ± 3 II X = OCH3 −1.6 ± 0.2 −3.0 ± 0.7 −2.0 ± 0.2 −10.0 ± 0.5 H3C O NH2 O X III X = Cl −3.1 ± 0.1 −7.6 ± 0.3 −5 ± 1 −18 ± 4 IV X = Y = H −0.40 ± 0.02 −3.20 ± 0.05 - - V X = H/Y = OCH3 −0.23 ± 0.08 −6.7 ± 0.3 - - VI X = H/Y = Cl −1.0 ± 0.3 −4 ± 1 - - VII X = OCH3/Y = H −0.32 ± 0.07 −3.3 ± 0.3 - - O NH2 O X Y VIII X = Cl/Y = H −0.58 ± 0.08 −3.7 ± 0.5 - - IX Y = H −0.92 ± 0.01 −7.02 ± 0.04 −3.6 ± 0.9 −14 ± 3 X Y = OCH3 +0.5 ± 0.3 −4.9 ± 0.9 −2.7 ± 0.2 −14.4 ± 0.5 O NH2 O Y XI Y = Cl −1.1 ± 0.3 −7 ± 1 −4 ± 1 −14 ± 4 As it can be seen from Tables 4 and 5, the values of ΔH are more negative in DMSO-d6, indicating that the enol form would be favored in this solvent. This effect can be explained considering that the enolamide form is capable of establishing two intermolecular hydrogen bonds per molecule, while in the ketoamide tautomer only one intermolecular hydrogen bond is possible (Scheme 4). On the other hand, ΔS values are more negative in this solvent, what would shift the equilibrium towards the keto tautomer. This can be explained from the different molecular arrangements that are set when the tautomers Open Access OJPC  S. L. LAURELLA ET AL. 146 establish hydrogen bonds with DMSO-d6, with different degrees of molecular coordination order (Scheme 4). The result of these two contrary effects is explained by experimental determinations, and the overall equilibrium shift (which depends ultimately on ΔG, Table 4) indi- cates that in compounds I-VIII the entropic effect pre- dominates over the enthalpic effect. On the other hand, in compounds IX-XI the entropic effect is the one that rules the situation. The difference between these two opposite behaviors can be explained considering that in compounds I-VIII (in which R1 = Ar) the solvation of one NH hydrogen is sterically hindered by the phenyl group in C-2 position. In the case of compounds IX-XI (in which R1 = H) both NH hydrogens are easily solvated. These assumptions are supported by the different decrease in ΔH in DMSO-d6 respecting to CDCl3 (approximately a decrease of 1 Kcal/ mol in compounds I-VIII and 3 Kcal/mol in compounds IX-XI). Data obtained from these experiments suggest an en- thalpy-entropy compensation (since ΔH and ΔS seem to be lineally correlated ΔH = a·ΔS + b), but, at first sight, this correlation would not be strictly valid since ΔS and ΔH values were obtained from the same experiment [22]. However, linearity between lnK at two different tem- peratures (25˚C and 45˚C) is observed, as shown in Fig- ure 4. From the equation lnK(45˚C) = m·lnK(25˚C) + n, a simple deduction can be made to obtain ∆H = a·∆S + b (i.e. an enthalpy-entropy compensation), as follows: 21 ln ln mKn 22 11 GRTmGRT n 21 + RTSRmHRT mSRn 122112 21 1 m TTmTT SnRTTmTT aSb where T1 = 25˚C (298 K), T2 = 45˚C (318 K), K1 and K2 are the equilibrium constants at T1 and T2 respectively, and ΔH and ΔS are supposed to be constant within the Scheme 4. Tautomer-solvent interactions via hydrogen bond in DMSO-d6. Figure 4. lnK (45˚C) vs lnK (25˚C) plot for compounds I-XI in CDCl3. Open Access OJPC 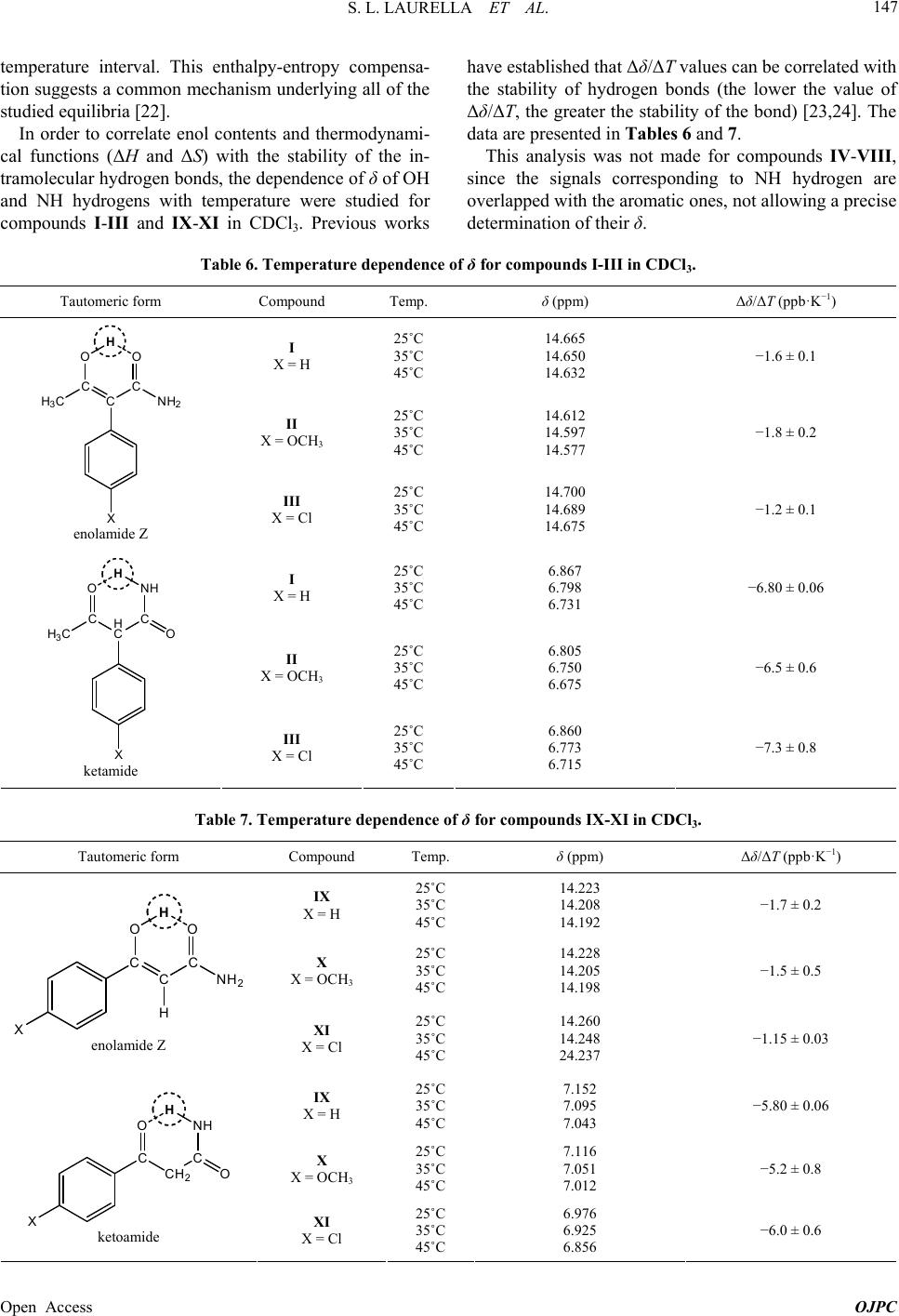 S. L. LAURELLA ET AL. 147 temperature interval. This enthalpy-entropy compensa- tion suggests a common mechanism underlying all of the studied equilibria [22]. In order to correlate enol contents and thermodynami- cal functions (ΔH and ΔS) with the stability of the in- tramolecular hydrogen bonds, the dependence of δ of OH and NH hydrogens with temperature were studied for compounds I-III and IX-XI in CDCl3. Previous works have established that Δδ/ΔT values can be correlated with the stability of hydrogen bonds (the lower the value of Δδ/ΔT, the greater the stability of the bond) [23,24]. The data are presented in Tables 6 and 7. This analysis was not made for compounds IV-VIII, since the signals corresponding to NH hydrogen are overlapped with the aromatic ones, not allowing a precise determination of their δ. Table 6. Temperature dependence of δ for compounds I-III in CDCl3. Tautomeric form Compound Temp. δ (ppm) Δδ/ΔT (ppb·K−1) I X = H 25˚C 35˚C 45˚C 14.665 14.650 14.632 −1.6 ± 0.1 II X = OCH3 25˚C 35˚C 45˚C 14.612 14.597 14.577 −1.8 ± 0.2 C CC NH2 O H3C OH X enolamide Z III X = Cl 25˚C 35˚C 45˚C 14.700 14.689 14.675 −1.2 ± 0.1 I X = H 25˚C 35˚C 45˚C 6.867 6.798 6.731 −6.80 ± 0.06 II X = OCH3 25˚C 35˚C 45˚C 6.805 6.750 6.675 −6.5 ± 0.6 H C CCO NH H3C OH X ketamide III X = Cl 25˚C 35˚C 45˚C 6.860 6.773 6.715 −7.3 ± 0.8 Table 7. Temperature dependence of δ for compounds IX-XI in CDCl3. Tautomeric form Compound Temp. δ (ppm) Δδ/ΔT (ppb·K−1) IX X = H 25˚C 35˚C 45˚C 14.223 14.208 14.192 −1.7 ± 0.2 X X = OCH3 25˚C 35˚C 45˚C 14.228 14.205 14.198 −1.5 ± 0.5 H C CC NH2 OO H X enolamide Z XI X = Cl 25˚C 35˚C 45˚C 14.260 14.248 24.237 −1.15 ± 0.03 IX X = H 25˚C 35˚C 45˚C 7.152 7.095 7.043 −5.80 ± 0.06 X X = OCH3 25˚C 35˚C 45˚C 7.116 7.051 7.012 −5.2 ± 0.8 CH2 CC O NHOH X ketoamide XI X = Cl 25˚C 35˚C 45˚C 6.976 6.925 6.856 −6.0 ± 0.6 Open Access OJPC  S. L. LAURELLA ET AL. 148 The calculated Δδ/ΔT values agree with the ones ob- tained in previous works for similar compounds (Δδ/ΔT < 4 ppb·K−1 for O―H•••O and Δδ/ΔT < 9 ppb·K−1 for N―H•••O) [23,24], corroborating that the hydrogen bonds are, indeed, intramolecular. Taking into account data from Tables 6 and 7, it can be concluded that they are consistent with the previous assumption regarding the effect of the internal hydrogen bonds on the stability of the tautomers. In other words, compounds showing lower values of Δδ/ΔTOH enol and higher values of Δδ/ΔTNH keto are also the ones that have higher enolic content and vice versa. 4. Conclusions It has been demonstrated that keto-enol tautomerism in β-ketoamides (studied by NMR spectroscopy, a very useful technique for the determination of tautomeric spe- cies in solution) is strongly dependent on the solvents, temperature and substituents. Intramolecular hydrogen bond seems to be the main factor in stabilizing the dif- ferent tautomeric forms. In solution, these compounds exist mainly as ke- toamide and Z-enolamide tautomers, both presenting internal hydrogen bonds. The rest of the possible tautomers (ketoimidol, E-enolamide, 3-enolamide, eno- limidol) show very low concentration (at least lower than the detection limit) or probably do not exist in neutral solution. In all cases, the equilibrium is shifted to the Z-enola- mide form by several factors: 1) Electron withdrawing substituents attached to aro- matic rings (e.g. chlorine). 2) A temperature decrease, since the equilibria are exothermic (except for compound X). 3) Bulky groups in C-2 position. This effect is less important when two phenyl groups in C-2 and C-3 posi- tions are present, because of the steric hindrance. 4) The presence of hydrogen bond acceptor solvents (e.g. DMSO), but only when there are no bulky groups in C-2. Otherwise, hydrogen bond acceptor solvents de- crease the enol content. All these factors increase the enolamide content ap- parently by stabilizing the Z-enolamide tautomers (streng- thening the internal O―H•••O = C hydrogen bond) and/or destabilizing the ketoamide tautomer (weakening the internal C = O•••H―N hydrogen bond). 5. Acknowledgements We are indebted to the Facultad de Ciencias Exactas, Universidad Nacional de La Plata for financial support, to Agencia Nacional de Promoción Científica y Tecno- lógica, República Argentina and the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET). REFERENCES [1] J. W. Bunting and J. P. Kanter, “Acidity and Tautomerism of β-Keto-Esters and Amides in Aqueous-Solution,” Jour- nal of the American Chemical Society, Vol. 115, No. 25, 1993, pp. 11705-11715. http://dx.doi.org/10.1021/ja00078a008 [2] K. Yamasaki and O. Kajimoto, “Solvent Effect in Super- critical Fluids: Keto-Enol Equilibria of Acetylacetone and Ethyl Acetoacetate,” Chemical Physics Letters, Vol. 172, No. 3-4, 1990, pp. 271-274. http://dx.doi.org/10.1016/0009-2614(90)85401-W [3] D. L. Ruiz, A. G. Albesa, A. Ponzinibbio, P. E. Allegretti and M. M. Schiavoni, “Solvent Effects on Tautomerics Equilibria in β-Ketonitriles: NMR and Theoretical Stu- dies,” Journal of Physical Organic Chemistry, Vol. 23, No. 10, 2010, pp. 985-994. http://dx.doi.org/10.1002/poc.1764 [4] M. J. Hynes and E. M. Clarke, “Kinetics of Enolisation of β-Ketoamides,” Journal of the Chemical Society, Perkin Transactions 2, No. 4, 1994, pp. 901-904. [5] H. Wengenroth and H. Meier, “(E)- und (Z)-Enole von β-Ketocarbonsäureamiden,” Chemische Berichte, Vol. 123, No. 6, 1990, pp. 1403-1409. http://dx.doi.org/10.1002/cber.19901230633 [6] M. M. Naoum and G. R. Saad, “Solvent Effect on Tauto- meric Equilibria and Dipole Moments of Some Alpha Substituted Benzoylacetanilides,” Journal of Solution Chemistry, Vol. 17, No. 1, 1998, pp. 67-76. http://dx.doi.org/10.1007/BF00651854 [7] R. E. Valters, F. Fülöp and D. Korbonits, “Recent Devel- opments in Ring-Chain Tautomerism. II. Intramolecular Reversible Addition Reactions to the C=N, C=N, C=C, and C=C Groups,” Advances in Heterocyclic Chemistry, Vol. 66, 1996, p. 1. http://dx.doi.org/10.1016/S0065-2725(08)60304-9 [8] L. Lázár and F. Fülöp, “Recent Developments in the Ring-Chain Tautomerism of 1,3-Heterocycles,” European Journal of Organic Chemistry, Vol. 2003, No. 16, 2003, pp. 3025-3042. [9] L. Lázár, A. Göblyös, F. Evanics, G. Bernáth and F. Fülöp, “Ring-Chain Tautomerism of 2-Aryl-Substituted Imida- zolidines,” Tetrahedron, Vol. 54, No. 44, 1998, pp. 13639-13644. http://dx.doi.org/10.1016/S0040-4020(98)00840-0 [10] A. Göblyös, L. Lázár, F. Evanics and F. Fülöp, “Ring- Chain Tautomerism of 2-Aryl-Substituted Imidazoli- dines,” Heterocycles, Vol. 51, No. 10, 1999, pp. 2431- 2438. http://dx.doi.org/10.3987/COM-99-8624 [11] K. N. Zelenin, V. V. Alekseyev, I. V. Ukraintsev and I. V. Tselinsky, “2-Substituted Hexahydropyrimidines and Their Tautomerism,” Organic Preparations and Procedures In- ternational, Vol. 30, No. 1, 1998, pp. 53-61. http://dx.doi.org/10.1080/00304949809355259 [12] A. Göblyös, L. Lázár and F. Fülöp, “Ring-Chain Tautomer- ism of 2-Aryl-Substituted-Hexahydropyrimidines and Tet- Open Access OJPC  S. L. LAURELLA ET AL. 149 rahydroquinazolines,” Tetrahedron, Vol. 58, No. 5, 2002, pp. 1011-1016. http://dx.doi.org/10.1016/S0040-4020(01)01196-6 [13] O. Maloshitskaya, J. Sinkkonen, V. V. Ovcharenko, K. N. Zelenin and K. Pihlaja, “Chain-Ring-Chain Tautomerism in 2-Aryl-Substituted Hexahydropyrimidines and 1H-2,3- Dihydroperimidines. Does It Appear?” Tetrahedron, Vol. 60, No. 32, 2004, pp. 6913-6921. http://dx.doi.org/10.1016/j.tet.2004.05.092 [14] J. Sinkkonen, K. N. Zelenin, A. K. Potapov, I. V. Lagoda, V. V. Alekseyev and K. Pihlaja, “Ring-Chain Tautomer- ism in 2-Substituted 1,2,3,4-Tetrahydroquinazolines A1H, 13C and 15N NMR Study,” Tetrahedron, Vol. 59, No. 11, 2003, pp. 1939-1950. http://dx.doi.org/10.1016/S0040-4020(03)00148-0 [15] R. M. Claramunt, C. López, M. D. Santa María, D. Sanz and J. Elguero, “The Use of NMR Spectroscopy to Study Tautomerism,” Progress in Nuclear Magnetic Resonance Spectroscopy, Vol. 49, No. 3-4, 2006, pp. 169-206. http://dx.doi.org/10.1016/j.pnmrs.2006.07.001 [16] M. T. Barros, C. F. G. C. Geraldes, C. D. Mavcock and M. I. Silva, “A Proton and 13C NMR Study of Keto-Enol Tautomerism of Some β-Ketoamides,” Journal of Mole- cular Structure, Vol. 142, 1986, pp. 345-349. http://dx.doi.org/10.1016/0022-2860(86)85150-X [17] S. Laurella, M. González Sierra, J. Furlong and P. Alle- gretti, “Analysis of Tautomerism in β-Ketobuanamides by Nuclear Magnetic Resonance: Substituent, Temperature and Solvent Effects,” Journal of Applied Solution Chem- istry and Modeling, Vol. 1, 2012, pp. 6-12. [18] C. R. Hauser and C. J. Eby, “Cyclization of β-Ketonitriles or β-Ketoamides with Ketones by Polyphosphoric Acid to Form Substituted 2-Pyridones,” Journal of the American Chemical Society, Vol. 79, No. 3, 1957, pp. 728-731. [19] S. L. Laurella, C. V. Latorre, R. C. Dietrich, J. J. P. Fur- long and P. E. Allegretti, “Tautomeric Equilibria Analysis of β-Ketoamides by Mass Spectrometry,” Journal of Phy- sical Organic Chemistry, Vol. 25, No. 12, 2012, pp. 1365- 1373. [20] Z. Rappoport and S. Biali, “Stable Simple Enols. 9. Solid State Structures and Conformations of Several Simple Enols and Their Keto Tautomers,” Journal of the Ameri- can Chemical Society, Vol. 105, 1985, pp. 1701-1709. [21] A. R. Miller, “Sterically Stabilized Enols: A Study Em- ploying the Internal Rotational Barriers of the Destabi- lized Ketones,” The Journal of Organic Chemistry, Vol. 41, No. 22, 1976, pp. 3599-3602. http://dx.doi.org/10.1021/jo00884a026 [22] E. B. Starikov and B. Nordén, “Entropy-Enthalpy Com- pensation as a Fundamental Concept and Analysis Tool for Systematical Experimental Data,” Chemical Physics Letters, Vol. 538, 2012, pp. 118-120. http://dx.doi.org/10.1016/j.cplett.2012.04.028 [23] S. H. Gellman, G. P. Dado, G. Liang and B. R. Adams, “Conformation-Directing Effects of a Single Intramole- cular Amide-Amide Hydrogen Bond: Variable-Tempera- ture NMR and IR Studies on a Homologous Diamide Se- ries,” Journal of the American Chemical Society, Vol. 113, No. 4, 1991 pp. 1164-1173. http://dx.doi.org/10.1021/ja00004a016 [24] A. Lämmermann, I. Szatmári, F. Fülöp and E. Kleinpeter, “Inter- or Intramolecular N···H−O or N−H···O Hydrogen Bonding in 1,3-Amino-α/β-naphthols: An Experimental NMR and Computational Study,” The Journal of Physical Chemistry A, Vol. 113, No. 21, 2009, pp. 6197-6205. http://dx.doi.org/10.1021/jp902731n Open Access OJPC
|