Paper Menu >>
Journal Menu >>
 Vol.2, No.1, 16-22 (2011) Agricultural Sciences doi:10.4236/as.2011.2 1003 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/AS/ Mineral phosphate solubilization activity of gluconacetobacter diazotrophicus under P-limitation and plant root environment J. M. Crespo1, J. L. Boiardi1, M. F. Luna1,2* 1CINDEFI (UNLP-CONICET, CCT La Plata), Facultad de Ciencias Exactas, Universidad Nacional de La Plata, La Plata, Argentina; 2CIC PBA, Comision de Investigaciones Cientificas, Provincia de Buenos Aires, Argentina; *Corresponding Author: mafla@quimica.unlp.edu.ar Received 19 October 2010; revised 17 November 2010; accepted 24 November 2010 ABSTRACT The ability to solubilize insoluble inorganic pho- sphate compounds by Gluconacetobacter di- azotrophicus was studied using different cul- ture approaches. Qualitative plate assays using tricalcium phosphate as the sole P-source showed that G. diazotrophicus produced solu- bilization only when aldoses were used as the C-source. Extracellular aldose oxidation via a pyrroloquinoline quinone-linked glucose dehy- drogenase (PQQ-GDH) is the main pathway for glucose metabolism in G. diazotrophicus. In batch cultures with 5 g l-1 of hydroxyapatite as the P-source and glucose as the C-source, more than 98% of insoluble P was solubilized. No solubilization was observed neither using glyc- erol nor culturing a PQQ-GDH mutant of G. di- azotrophicus. Solubilizaton was not affected by adding 100 mmol l-1 of MES buffer. Continuous cultures of G. diazotrophicus sho wed significant activities of PQQ-GDH either under C or P limi- tation. An intense acidification in the root envi- ronment of tomato and wheat seedlings inocu- lated with a G. diazotrophicus PAL5 was ob- served. Seedlings inoculated with a PQQ-GDH mutant strain of G. diazotrophicus showed no acidification. Our results suggest that G. di- azotrophicus is an excellent candidate to be used as biofertilizer because in addition to the already described plant grow th-promoting abili- ties of this organism, it shows a significant mineral phosphate solubilization capacity. Keyw ords: Gluconacetobacter Diazotrophicus; Phosphate Solubilization; Glucose Dehydrogenase; Pqq; Biofertilizer 1. INTRODUCTION Phosphorus (P) is after Nitrogen, the most important nutrient limiting agricultural production. Soils are often abundant in insoluble P, either in organic or inorganic forms, but deficient in soluble phosphates essential for growth of most plants and microorganisms. Soluble forms of phosphate fertilizers are widely applied to ag- ricultural soils in order to circumvent P-deficiency but 75 to 90% of added P is rapidly precipitated as insoluble forms and becomes unavailable to plants [1]. Converting soil insoluble phosphates (both organic and inorganic) to a form available for plants is a necessary goal to achieve sustainable agricultural production. Several reports show the ability of different bacteria to solubilize inorganic phosphate compounds such as tricalcium phosphate, dicalcium phosphate, hydroxyapatite and rock phosphate [2]. Among the bacterial genera reported to express a mineral phosphate solubilization (MPS) phenotype are Pseudomonas, Bacillus, Rhizobium, Burkholderia, Ach- romobacter, Agrobacterium, Microccocus, Aereobacter, Flavobacterium and Erwinia [3]. A significant body of evidence has been developed to show that in gram- negative bacteria the expression of a direct extracellular oxidative pathway allows superior MPS capabilities [2]. Through this pathway (also called nonphosphorylating oxidation) glucose is oxidized to gluconic acid and 2-ketogluconic acid directly in the periplasmic space. These strong organic acids can dissolve poorly soluble calcium phosphates present in soils. On the other hand it has been reported that the buffering capacity of soils could limit P solubilization by microorganisms [4,5]. Gluconacetobacter diazotrophicus is a nitrogen-fixing endophytic bacterium able to colonize several plant spe- cies [6,7]. G. diazotrophicus promotes, besides N2-fixa- tion, other beneficial effects to plants such as phythor- mones production and biocontrol towards plant patho- gens [6]. Moreover, in some G. diazotrophicus strains the ability to promote P solubilization has been demon-  J. M. Crespo et al. / Agricultural Sciences 2 (2011) 16-2 2 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/AS/ 17 strated in vitro [8,5]. Considering all these characteris- tics G. diazotrophicus has been described as a plant growth-promoting bacterium. G. diazotrophicus possess a pyrroloquinoline quinone- linked glucose dehydrogenase (PQQ-GDH) responsible for the periplasmic conversion of aldoses into the corre- sponding aldonic acid [9]. This is considered the princi- pal pathway for glucose metabolism in this bacterium [10]. It has been reported that in the presence of aldoses and under N2-fixing conditions, G. diazotrophicus is able to express an enhanced production of energy linked to a fully active PQQ-GDH [11]. However, there is no in- formation about PQQ-GDH expression under P-limi- tation, a condition likely found in soils and it is not known whether buffering could affect MPS by G. di- azotrophicus. Moreover, it is not known whether this organism expresses an active PQQ-GDH in the ri- zosphere of plants, where the MPS activity needs to be expressed in order to provide soluble phosphate to plants. The objective of this study was to address the above mentioned issues and assess the potential of G. di- azotrophicus as a biofertilizer due to its MPS activity coupled to its well-known plant growth promoting abili- ties. 2. MATERIALS AND METHODS 2.1. Organism and Maintenance G. diazotrophicus strain PAL 5 (ATCC 49037), kindly provided by Dr. Caballero-Mellado, was maintained on agar slants on a potato medium [12]. Strain MF105, an already described PQQ-GDH negative mutant strain of G. diazotrophicus [10], was maintained on the same me- dium supplemented with streptomycin (400 µg ml-1). 2.2. Cultures and Growth Conditions Plate assays were carried out using the National Bo- tanical Research Institute's phosphate medium (NBRIP) [13] containing l-1: Ca3(PO4)2 (TCP), 5 g; MgCl2.6H2O, 5 g; MgSO4.7H2O, 0.25 g; KCl, 0.2 g; (NH4)2SO4, 0.1 g and 10 g of different carbon sources (Figure 1). Bacterial cultures of both strains of G. diazotrophicus with a con- centration of around 1.109 CFU ml-1 were centrifuged and resuspended in the same volume of saline solution pH 6.0. A volume of 50 µl of these cell suspensions was plated onto NBRIP medium. The plates were observed two days after incubation at 30 °C. Batch cultures were performed employing the NBRIP liquid medium with 10 g l-1 of glucose or glycerol as car- bon source, 5 g l-1 of hydroxyapatite (HY, Ca10(OH)2 (PO4)6) as the sole P source and 2.5 g l-1 of (NH4)2SO 4 (under non-BNF conditions). HY was replaced by K2HPO4 (2.0 g l-1) in experiments with soluble P. When the organism was grown under BNF conditions, (NH4)2- SO4 concentration was decreased to 0.132 g l-1 [12]. To evaluate the effect of buffering on MPS activity, NBRIP medium was strongly buffered with morpholineethane- sulfonic acid (MES) buffer 100 mmol l-1. Initial pH was adjusted at 6.0 by adding KOH 0.1 mol l-1 or HCl 0.1 mol l-1. Bacteria (both, wild type and mutant) were grown at 30 °C in 250 ml liquid NBRIP medium (two flasks per treatment) on a rotary shaker stirred at 200 or 100 rpm for non-BNF or BNF, respectively. Negative controls (no bacteria) were carried out to quantify the solublized P in the culture conditions regardless of mi- crobial activity. Chemostat cultures were carried out using the modi- fied LGIM medium described by Luna et al. (2000) [11] with glucose 10.0 g l-1 or 20.0 g l-1 and NaH2PO4.H2O 10 mmol l-1 or 0.5 mmol l-1 for C- or P-limitation respec- tively. (NH4)2SO4 (2.50 g l-1) was added to cultures grown under non-BNF conditions. Cultures under BNF were carried out without (NH4)2SO4 in the culture me- dium. The strategies to attain BNF conditions were de- scribed by Luna et al. (2000) [11]. G. diazotrophicus Pal 5 was grown at 30 °C in a 2-l LH (Incelltech 210) fer- mentation unit with a working volume of 1.0 l. The growth rate (dilution rate) was adjusted at 0.05 ± 0.001 h-1. The pH was automatically maintained at 5.5 ± 0.1 by addition of either 0.5 mol l-1 NaOH or 0.25 mol l-1 H2SO4. Foam formation was prevented by automatic addition of an antifoam agent. Cultures were flushed with air (20 to 25 l h-1). The dissolved oxygen concen- tration was continuously measured using an Ingold (Wilmington, MA) polarographic probe and maintained at the desired level of air saturation by varying the agita- tion speed of the impeller. Cultures were considered to be under steady-state conditions when biomass concen- tration and specific rate of oxygen consumption of cul- tures remained almost constant (varied less than 5 %), as previously described [11]. After modification in growth conditions, 5 to 10 volume changes were usually re- quired to re-obtain steady state. 2.3. Analyses Samples of batch (at 8-12 h intervals during 5 days) or continuous cultures (daily during 5-7 days) were taken for pH, absorbance, biomass dry weight, glucose and products quantification. Growth was estimated by meas- urement of the absorbance at 560 nm and biomass dry weight determined as previously reported [11]. Samples of batch cultures grown in medium with HY were di- luted 1:1 (v:v) using 0.1 mol l-1 HCl to dissolve the re- sidual insoluble phosphate and measured against a blank identically treated [14]. Samples were centrifuged 10 min at 10,000 g and the resulting supernatant was em- ployed to assay P, glucose, gluconic acid, and extracel-  J. M. Crespo et al. / Agricultural Sciences 2 (2011) 16-2 2 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/AS/ 18 lular polysaccharides (EPS). Glucose concentrations in media and supernatants were determined with a glucose oxidase enzymatic kit (Wiener, Argentina). Gluconate concentrations were assayed using a test-kit (Boehringer, Mannheim, Germany). EPS dry weight was determined by adding two volumes of ethanol to culture super- natants; after storing overnight at 4 °C precipitated ma- terial was collected by centrifugation (20 min at 7,000 g). The pellets were resuspended in distilled water and dried at 60 °C. Soluble P concentration was measured by the method described by Clesscerl et al. (1998) [15]. Oxy- gen and carbon dioxide concentrations in the emitted gases from the fermentor were determined using a para- magnetic oxygen analyzer (Servomex 1100A, Norwood, MA) and an infrared carbon dioxide analyzer (Horiba PIR 2000, Japan). Gas flow rates were measured with a bubble flow meter. Biomass yields, rates of oxygen consumption, and carbon dioxide production were cal- culated as previously described [11]. 2.4. Enzyme Assays PQQ-GDH and gluconate dehydrogenase (GaDH) ac- tivities were measured spectrophotometrically using 2,6-dichlorophenol-indophenol (DCIP) as the electron acceptor and glucose or gluconate respectively [16,17]. Samples (20 ml) from batch cultures were taken at ex- ponential phase, while glucose was still detectable, and centrifuged for 10 minutes at 12,000 g at 4 ºC. In che- mostat, once steady-state conditions were attained, an appropriate volume of culture (70 ml) was withdrawn and centrifuged as described above. Cells were washed twice in phosphate buffer 10 mmol l-1 (pH 6.0) contain- ing 5 mmol l-1 MgCl2 to a final concentration of 4.50 mg ml-1. This washed cells suspension (WCS) was em- ployed to determine the PQQ-GDH activity in whole cells. The final concentration of cells in the reaction mixture was 0.10 mg ml-1 dry weight. 2.5. Acid Production from Root Exudates Tomato (Lycopersicum esculentum cv. “platense itali- ano”) and wheat (Triticum aestevium cv. “baguette”) seeds were surface sterilized with 2 % sodium hypochlo- rite for 5 min followed by three washes with sterile water. Seeds were germinated onto water-agar plates (0.5 % agar) at 30 °C during 72 h. The seedlings were inocu- lated by immersion for 15 min in a G. diazotrophicus suspension (either PAL5 or MF105 centrifugated and resuspended as described for plates assays) and placed into tubes containing semi solid agarized Fähraeus me- dium [18] with 0.1 % methyl red as acid-base indicator. Uninoculated seedlings and a G. diazotrophicus suspen- sion were used as negative controls. 3. RESULTS Plate assays. Qualitative estimation of P solubilization was made in agar plates supplemented with TCP. The MPS phenotype was identified by the production of clearing zones of solubilization around the colony (Fig- ure 1). As it can be seen in Figure 1 appearance of clearing halos was dependent on the nature of the carbon source. Glucose, arabinose, galactose and xylose (all substrates of PQQ-GDH) showed an MPS (+) phenotype. With other carbon sources G. diazotrophicus showed growth but was not capable of solubilizing phosphates. When plates were inoculated with strain MF105 (PQQ-GDH (-) mutant) neither the PQQ-GDH substrates nor the other carbon sources showed a MPS (+) pheno- type. Batch cultures. Quantitative estimation of P solubili- zation was carried out in liquid medium cultures, since this approach is considered more accurate than plate assays [19]. Batch cultures performed with glucose and replacing the soluble P-source by HY, showed a very similar growth behavior to the one reported using solu- ble P [10]. Soluble P concentration in the media in- creased together with the gluconic acid. The culture pH dropped during the same period from 6.0 to 2.5 with a concomitant P solubilization that reached around 1,000 (a) (b) Bacterial strain Carbon source PAL5 MF105 Glucose + - Arabinose + - Galactose + - Xylose + - Lactose - - Maltose - - Gluconate - - Glycerol - - Fructose - - Figure 1. In vitro P solubilization by G. di- azotrophicus (a) PAL5 and (b) MF105. Clear- ing halo formation (+) and no halo formation (–) by G. diazotrophicus growing on NBRIP me- dium with TCP and different C-sources.  J. M. Crespo et al. / Agricultural Sciences 2 (2011) 16-2 2 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/AS/ 19 ppm (Figure 2). This amount represents more than 98% of the insoluble P contained in the HY added to the cul- ture medium (Figure 3). Once glucose was entirely oxi- dized (around 80 h of growth) soluble P decreased in the culture supernatants together with gluconate consump- tion. There was no difference between P solubilization levels under both BNF and non-BNF conditions (Figure 3). P concentration of negative controls (non inoculated) remained almost constant, between 2 and 8 mg l-1, along the experiment. It was observed that G. diazotrophicus exhibited a similar P-dissolving capability either in the absence or in the presence of buffer, even at a concentration of 100 mmol l-1 of MES (Figure 3). As already described for G. diazotrophicus growing with soluble P [10], PQQ-GDH was actively synthesized in glucose-containing batch cultures using HY as the time (h) 020406080100 120 140 160 P (mg l -1 ) 0 200 400 600 800 1000 1200 Gluconic acid (g l -1 ) 0 2 4 6 8 10 12 14 16 18 20 O.D. pH 0 1 2 3 4 5 6 7 Figure 2. Soluble P, gluconic acid, pH and O.D. measurements of G. diazotrophicus PAL5 cultures growing with glucose 20 g l-1, HY 5 g l-1 and BNF conditions. (○) soluble P; (□) gluconic acid; (●) O.D. and (■) pH. HY non-BN FHY BNFHY BNF (MES ) P Solubilization (%) 0 20 40 60 80 100 120 Figure 3. P solubilization by G. diazotrophicus PAL5 in cul- tures with glucose 20 g l-1, HY 5 g l-1 under both BNF and non-BNF condition and with MES buffer 100 mmol l-1. P solu- bilization percentage was determined dividing the maximum soluble P value obtained by the initial P amount in HY. sole P source, either under BNF or non-BNF conditions (Table 1). Cultures of G. diazotrophicus PAL5 using glycerol as the sole carbon source and cultures of strain MF105 with glucose, both with HY as the sole P-source, showed pH values over 6.0 all along cultures, indicating no acid production and levels of soluble P below 10 ppm (data not shown). Continuous cultures. G. diazotrophicus was grown under C- or P-limiting conditions in a chemostat using glucose as C-source. In order to check that growth was indeed C- (or P-limited), additions of the corresponding limiting substrate were made to the culture vessel. An immediate increase in the steady state biomass concen- tration was observed after the addition of either limiting substrate. Moreover, the residual concentration of the limiting substrate in the supernatants of steady state cul- tures was below the detection limits of the assays em- ployed (data not shown). In cultures grown under P-limitation, either with NH4 + or N2 as the N-source, biomass yields were lower than those observed in glucose-limited cultures and showed a significant increase of O2 consumption. Growth yields of C- or P-limited continuous cultures were not signifi- cantly affected by the nature of the N-source (NH4 + or N2), as already observed in a previous work [11] (Table 2). Table 1 In vitro PQQ-GDH activities of G. diazotrophicus PAL5 in batch cultures. Enzymatic activity HY 5 g l-1 non-BNF HY 5 g l-1 BNF HY 5 g l-1 BNF (MES 100 mmol l-1) PQQ-GDH476* ± 31.87 486* ± 30.70 503* ± 13.17 * Enzymatic activities are expressed as nmoles DCIP reduced min-1 mg protein-1 (assuming 60% protein content in the biomass). Data are mean of at least three repetitions. Table 2 Continuous cultures of G. diazotrophicus PAL5. C-limitation P-limitation non-BNF BNF non-BNF BNF Yx/s 29.2 34.7 13.9 14.9 QO2 8.44 7.69 11.03 10.37 EPS nd nd 3.6 1.41 Gluconic acidnd nd Nd 9.12 C-recovery 98.6 94.5 74.6 102.3 PQQ-GDH 360 406 343 361 GaDH 100.5 95.2 58.0 52.7 Yx/s is expressed as g biomass mol substrate-1; QO2 as mmol O2 g biomass-1 h-1; EPS as g l-1; gluconic acid as g l-1; C-recovery in %. PQQ-GDH and GaDH activities are expressed as nmol DCIP reduced min-1 mg protein-1 (assuming 60% protein content in the biomass). Data are mean of at least three repetitions. SD was never >10%. EPS was detected in supernatants of both P-limited 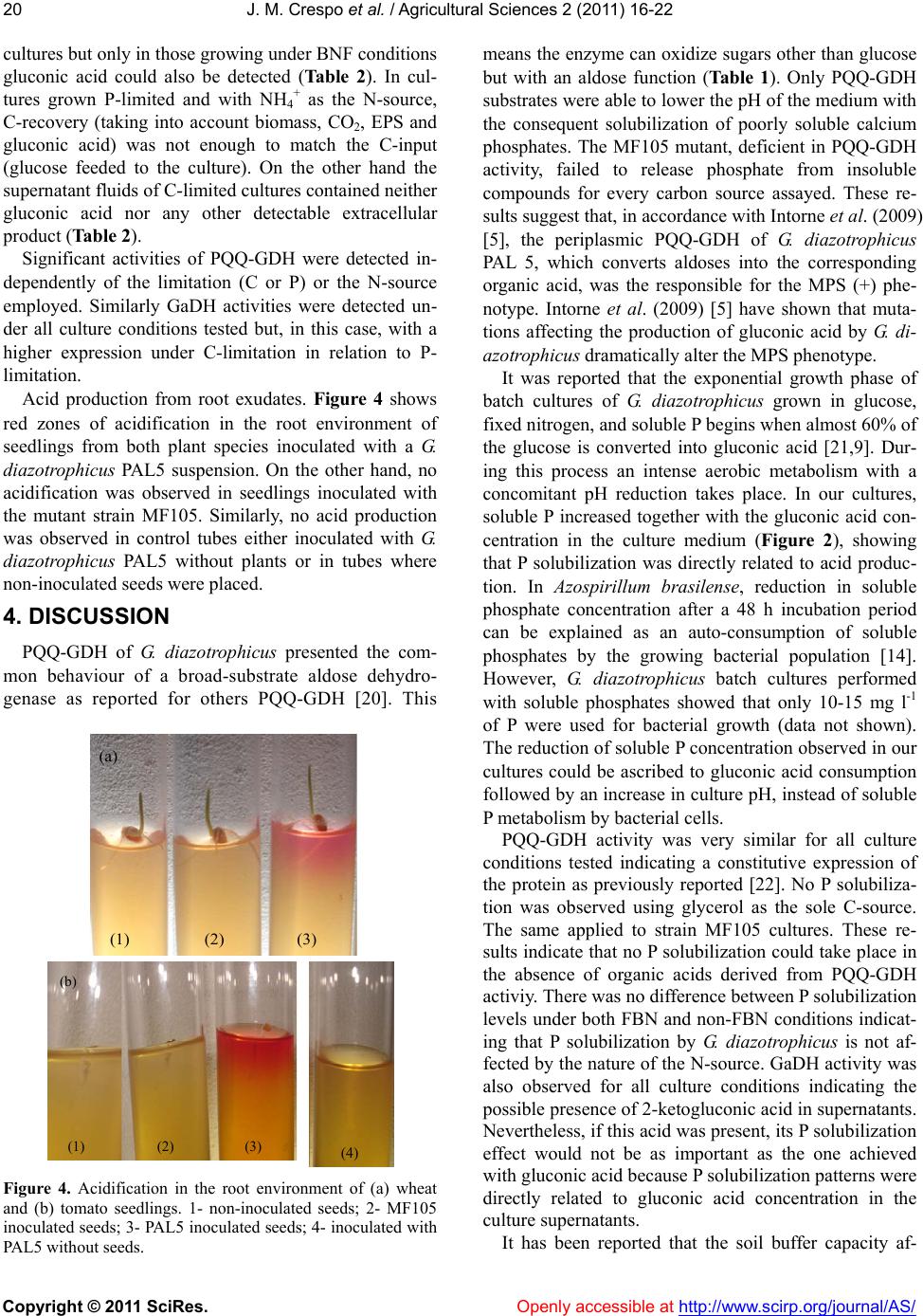 J. M. Crespo et al. / Agricultural Sciences 2 (2011) 16-2 2 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/AS/ 20 cultures but only in those growing under BNF conditions gluconic acid could also be detected (Table 2). In cul- tures grown P-limited and with NH4 + as the N-source, C-recovery (taking into account biomass, CO2, EPS and gluconic acid) was not enough to match the C-input (glucose feeded to the culture). On the other hand the supernatant fluids of C-limited cultures contained neither gluconic acid nor any other detectable extracellular product (Table 2). Significant activities of PQQ-GDH were detected in- dependently of the limitation (C or P) or the N-source employed. Similarly GaDH activities were detected un- der all culture conditions tested but, in this case, with a higher expression under C-limitation in relation to P- limitation. Acid production from root exudates. Figure 4 shows red zones of acidification in the root environment of seedlings from both plant species inoculated with a G. diazotrophicus PAL5 suspension. On the other hand, no acidification was observed in seedlings inoculated with the mutant strain MF105. Similarly, no acid production was observed in control tubes either inoculated with G. diazotrophicus PAL5 without plants or in tubes where non-inoculated seeds were placed. 4. DISCUSSION PQQ-GDH of G. diazotrophicus presented the com- mon behaviour of a broad-substrate aldose dehydro- genase as reported for others PQQ-GDH [20]. This (a) (1) (2) (3) (b) (1) (2) (3) (4) Figure 4. Acidification in the root environment of (a) wheat and (b) tomato seedlings. 1- non-inoculated seeds; 2- MF105 inoculated seeds; 3- PAL5 inoculated seeds; 4- inoculated with PAL5 without seeds. means the enzyme can oxidize sugars other than glucose but with an aldose function (Ta b l e 1 ). Only PQQ-GDH substrates were able to lower the pH of the medium with the consequent solubilization of poorly soluble calcium phosphates. The MF105 mutant, deficient in PQQ-GDH activity, failed to release phosphate from insoluble compounds for every carbon source assayed. These re- sults suggest that, in accordance with Intorne et al. (2009) [5], the periplasmic PQQ-GDH of G. diazotrophicus PAL 5, which converts aldoses into the corresponding organic acid, was the responsible for the MPS (+) phe- notype. Intorne et al. (2009) [5] have shown that muta- tions affecting the production of gluconic acid by G. di- azotrophicus dramatically alter the MPS phenotype. It was reported that the exponential growth phase of batch cultures of G. diazotrophicus grown in glucose, fixed nitrogen, and soluble P begins when almost 60% of the glucose is converted into gluconic acid [21,9]. Dur- ing this process an intense aerobic metabolism with a concomitant pH reduction takes place. In our cultures, soluble P increased together with the gluconic acid con- centration in the culture medium (Figure 2), showing that P solubilization was directly related to acid produc- tion. In Azospirillum brasilense, reduction in soluble phosphate concentration after a 48 h incubation period can be explained as an auto-consumption of soluble phosphates by the growing bacterial population [14]. However, G. diazotrophicus batch cultures performed with soluble phosphates showed that only 10-15 mg l-1 of P were used for bacterial growth (data not shown). The reduction of soluble P concentration observed in our cultures could be ascribed to gluconic acid consumption followed by an increase in culture pH, instead of soluble P metabolism by bacterial cells. PQQ-GDH activity was very similar for all culture conditions tested indicating a constitutive expression of the protein as previously reported [22]. No P solubiliza- tion was observed using glycerol as the sole C-source. The same applied to strain MF105 cultures. These re- sults indicate that no P solubilization could take place in the absence of organic acids derived from PQQ-GDH activiy. There was no difference between P solubilization levels under both FBN and non-FBN conditions indicat- ing that P solubilization by G. diazotrophicus is not af- fected by the nature of the N-source. GaDH activity was also observed for all culture conditions indicating the possible presence of 2-ketogluconic acid in supernatants. Nevertheless, if this acid was present, its P solubilization effect would not be as important as the one achieved with gluconic acid because P solubilization patterns were directly related to gluconic acid concentration in the culture supernatants. It has been reported that the soil buffer capacity af-  J. M. Crespo et al. / Agricultural Sciences 2 (2011) 16-2 2 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/AS/ 21 fects MPS by microorganisms [4]. However, in our ex- periments G. diazotrophicus was able to solubilize P in the presence of relatively high MES concentration indi- cating that buffering would not significantly affect the release of soluble P from HY. The lower growth yield values observed in chemostat under P-limitation, compared to those under C-limitation, were predictable because in C-limited grown microor- ganisms, catabolism is tightly coupled to anabolism and high biomass yields are achieved. On the other hand, cultures grown under C-excess conditions exhibit high rates of carbon consumption and low biomass yields and thus, have low energetic growth efficiency as indicated by an increased specific oxygen consumption rate. This behavior is generally coupled to the production of ex- tracellular products (overflow metabolism) [23]. In our case, significant concentrations of EPS (and gluconic acid under BNF) could be detected in culture super- natants when cells were grown under P-limitation and therefore, C-excess. Moreover, in P-limited cultures growing with N-fixed, another unknown extracellular product would have been released since C-recovery could account only for 75% of the consumed glucose. In spite of the extra energy expenditure for N2 fixation the biomass yields of cultures grown under BNF condi- tions were higher (in the case of C-limitation) or, at least, comparable (in the case of P-limitation) to those grown using N-fixed (Table 2). It has been reported that cul- tures of G. diazotrophicus grown in glucose (or mixtures of gluconic acid and xylose) under N2-fixing conditions express an improved growth energetic efficiency be- cause of a higher coupling of the respiratory chain. It was demonstrated that this was linked to the expression of an active aldose oxidation via PQQ-GDH [24]. Once again, it seems that the expression of an active PQQ- GDH and N2-fixation were the conditions required by G. diazotrophicus cultures to direct the electron flow through a more efficient branch of the respiratory chain. In this case, the effect was observed under P-limitation. Table 2 indicates that PQQ-GDH is not induced by phosphate starvation. Nevertheless, the enzyme was ful- ly active in all cultures. Moreover, under P-limitation and N2-fixation significant concentrations of gluconic acid could be detected in the culture supernatants. Tubes containing seedlings developed from seeds that had been inoculated with G. diazotr ophicus PAL5 showed a significant area of acidification. This acidification can be ascribed to the production of organic acid/s by PQQ- GDH expression. This assumption is made on the basis that no acidification was observed around seedlings from non-inoculated seeds and from those inoculated with the mutant strain MF105, impaired in PQQ-GDH expression. The same was observed in tubes inoculated with G. d i - azotrophicus but without plants. Therefore the simulta- neous presence of G. diazotrophicus able to express PQQ-GDH and growing seedlings were necessary for acidification. Since root exudates of many plants, in- cluding the two used in this study, contain significant amounts of PQQ-GDH substrates [25,26], it is likely that the acidification areas observed in Figure 4 were caused by some aldonic acid produced by the inoculated G. di- azotrophicus cells. This result indicates that the presence of G. diazotrophicus in the root environment of plants allows the active expression of PQQ-GDH with the concomitant production of organic acids that are able to solubilize P from poorly soluble calcium phosphates, as shown. Taken together, the results show that G. diazotrophicus is able to actively express PQQ-GDH with the concomi- tant production of organic acids and consequent MPS activity. This activity was not affected by the buffering capacity of the environment. PQQ-GDH activity was expressed under conditions of P-limitation (either with N2 or NH4 + as N-source) and in the root environment of different plant species producing acids from the root exudates. Therefore, in addition to other plant growth promoting activities already described for G. diazotro- phicus [6], this organism expresses a MPS (+) phenotype which allows its consideration as a promising species for being used as a biofertilizer. 5. Acknowledgements We are thankful to Dr. Jesús Caballero-Mellado (Centro de Ciencias Genómicas, Universidad Nacional Autónoma de México) for providing G. diazotrophicus strain PAL5. REFERENCES [1] Goldstein, A.H. (1986) Bacterial mineral phosphate so- lubilization: Historical perspective and future prospects. American Journal of Alternative Agricultur e, 1, 57-65. [2] Sashidhar, B. and Podile, A.R. (2010) Mineral phosphate solubilization by rhizosphere bacteria and scope for ma- nipulation of the direct oxidation pathway involving glucose dehydrogenase. Journal of Applied Microbiology, 109, 1-12. [3] Rodriguez, H. and Fraga, R. (1999) Phosphate solubiliz- ing bacteria and their role in plant growth promotion. Biotechnology Advan ces, 17, 319-339. doi:10.1016/S0734-9750(99)00014-2 [4] Gyaneshwar, P., Kumar, G.N., Parekh, L.J. and Poole, P.S. (2002) Role of soil microorganisms in improving P nutri- tion of plants. Plant and Soil, 245, 83-93. doi:10.1023/A:1020663916259 [5] Intorne, A.C., de Oliveira, M.V., Lima, M.L., da Silva, J.F., Olivares, F.L. and de Souza Filho, G.A. (2009) Iden- tifcation and characterization of Gluconacetobacter di- azotrophicus mutants defective in the solubilization of phosphorus and zinc. Archives of Microbiology, 191, 477-483. doi:10.1007/s00203-009-0472-0  J. M. Crespo et al. / Agricultural Sciences 2 (2011) 16-2 2 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/AS/ 22 [6] Pedraza, R.O. (2008) Recent advances in nitrogen-fixing acetic acid bacteria. International Journal of Food Mi- crobiology, 125, 25-35. doi:10.1016/j.ijfoodmicro.2007.11.079 [7] Luna, M.F., Galar, M.L., Aprea, J., Molinari M.L. and Boiardi, J.L. (2010) Colonization of sorghum and wheat by seed inoculation with Gluconacetobacter diazotro- phicus. Biotechnology Letters, 32, 1071-1076. doi:10.1007/s10529-010-0256-2 [8] Maheshkumar, K.S., Krishnaraj, P.U. and Alagwadi, A.R. (1999) Mineral solubilising activity of Acetobacter di- azotrophicus, a bacterium associated with sugarcane. Current Science, 76, 874-875. [9] Galar, M.L. and Boiardi, J.L. (1995) Evidence for a membrane-bound pyrroloquinoline quinone-linked glu- cose dehydrogenase in Acetobacter diazotrophicus. Ap- plied Microbiology and Biotechnology, 43, 713-716. doi:10.1007/BF00164778 [10] Luna, M.F., Bernardelli, C.E., Galar, M.L. and Boiardi, J.L. (2006) Glucose metabolism in batch and continuous cultures of Gluconacetobacter diazotrophicus PAL 3. Current Microbiology, 52, 163-168. doi:10.1007/s00284-005-4563-0 [11] Luna, M.F., Mignone, C.F. and Boiardi, J.L. (2000) The carbon source influences the energetic efficiency of the respiratory chain of N2-fixing Acetobacter diazotrophicus. Applied Microbiology and Biotechnology, 54, 564-569. doi:10.1007/s002530000425 [12] Stephan, M.P., Oliveira, M., Teixeira, K.R.S., Martínez-Drets, G. and Döbereiner, J. (1991) Physiology and dinitrogen fixation of Acetobacter diazotrophicus. FEMS Microbiology Letters, 77, 67-72. doi:10.1111/j.1574-6968.1991.tb04323.x [13] Nautiyal, C.S. (1999) An effcient microbiological growth medium for screening phosphate solubilizing microor- ganisms,” Microbiology Letters, 170, 265-270. doi:10.1111/j.1574-6968.1999.tb13383.x [14] Rodriguez, H., Gonzalez, T. and Selman, G. (2000) Ex- pression of a mineral phosphate solubilizing gene from Erwinia herbicola in two rhizobacterial strains. Journal of Biotechnology, 84, 155-161. doi:10.1016/S0168-1656(00)00347-3 [15] Clesscerl, L.S., Greenberg, A.E. and Eaton, A.D. (1998) Standard methods for the examination of water and wastewater. 20th Edition, APHA-AWWA-WEF, Wash- ington, DC. [16] Matsushita, K. and Ameyama, M. (1982) D-Glucose dehydrogenase from Pseudomonas fluorescens, mem- brane-bound. Methods in Enzymology, 89, 149-155. doi:10.1016/S0076-6879(82)89026-5 [17] Matsushita, K., Shinagawa, E. and Ameyama, M. (1982) D-gluconate dehydrogenase from bacteria, 2-keto-D- gluconate yielding, membrane bound. Methods in Enzy- mology, 89, 187-193. doi:10.1016/S0076-6879(82)89033-2 [18] Fähraeus, G. (1957) The infection of clove root hairs by nodule bacteria studied by simple glass slide technique. Journal of General Microbiology, 16, 347-381 [19] Mehta, S. and Nautiyal, C.S. (2001) An efficient method for qualitative screening of phosphate solubilizing bacte- ria. Current Microbiology, 43, 51-55. doi:10.1007/s002840010259 [20] Olsthoorn, A.J. and Duine, J.A. (1998) On the mecha- nism and specificity of soluble, quinoprotein glucose dehydrogenase in the oxidation of aldose sugars. Bio- chemistry, 37, 13854-13861. doi:10.1021/bi9808868 [21] Attwood, M.M., van Dijken, J.P. and Pronk, J.T. (1991) Glucose metabolism and gluconic acid production by Acetobacter diazotrophicus. Journal of Fermentation and Bioengineering, 72, 101-105. doi:10.1016/0922-338X(91)90317-A [22] Luna, M.F. and Boiardi, J.L. (2008) Growth yields and glucose metabolism of N2-fixing Gluconacetobacter di- azotrophicus at different culture pH values. World Jour- nal of Microbiology and Biotechnology, 24, 587-590. doi:10.1007/s11274-007-9507-3 [23] Russell, J.B., and Cook, G.M. (1995) Energetics of bac- terial growth: balance of anabolic and catabolic reactions. Microbiology and Molecular Biology Reviews, 59, 48-62. [24] Luna, M.F., Bernardelli, C.E., Mignone, C.F. and Boiardi, J.L. (2002) Energy generation by extracellular aldose oxidation in N2-fixing Gluconacetobacter diazotrophicus. Applied and Environmental Microbiology, 64, 2054-2056. doi:10.1128/AEM.68.4.2054-2056.2002 [25] Lugtenberg, B.J.J. Kravchenko, L.V. and Simons, M. (1999) Tomato seed and root exudate sugars: composi- tion, utilization by Pseudomonas biocontrol strains and role in rhizosphere colonization. Environmental Microbi- ology, 1, 439-446. doi:10.1046/j.1462-2920.1999.00054.x [26] Wang, P., Bi, S., Wang, S. and Ding, Q. (2006) Variation of wheat root exudates under aluminum stress. Journal of Agricultural and Food Chemistry, 54, 10040-10046. doi:10.1021/jf061249o |

